Abstract
Background and aims: In the pancreas, myofibroblasts (MFBs) were shown to play an important role in the cellular response during inflammation and injury. However, there is only fragmentary information concerning the fate of these cells in pancreas regeneration and fibrosis development.
Methods: Explant cultures of rat pancreatic tissue were used as a model to follow cellular dynamics and phenotype conversion of pancreatic MFBs in vitro. For detailed biochemical analyses a pancreatic fibroblast cell line (long culture fibroblast (LCF)) was generated from MFBs in a long term culture. Cerulein induced acute pancreatitis and dibutyltin dichloride induced pancreas fibrosis were used as experimental models for acute and chronic fibrogenic reactions, respectively.
Results: In the explant culture, pancreatic MFBs which derived from fat storing fibroblastic cells underwent apoptosis or converted again to fibroblasts. The phenotype switch to fibroblasts was associated with translocation of p21Cip1/WAF1 from the nucleus into the cytoplasm. Molecular analyses in LCFs revealed subsequent binding to and inhibition of the activities of Rho kinase 2 and apoptosis signal regulating kinase 1. In the experimentally established pancreas fibrosis, fibroblasts with cytoplasmic expression of p21Cip1/WAF1 were distributed throughout fibrotic bands whereas in experimental acute pancreatitis MFBs with nuclear expression of p21Cip1/WAF1 dominated.
Conclusions: The results indicate that pancreatic MFBs are transient and suggest that intracellular localisation of p21Cip1/WAF1 can contribute to the phenotype conversion of these cells to fibroblasts in culture and experimental injury.
Keywords: pancreas, myofibroblast, p21Cip1/WAF1, fibrosis, explant culture
Fibrosis is a striking pathological feature representative of chronic pancreatitis of different aetiologies.1–3 In contrast, synthesis and deposition of extracellular matrix (ECM) proteins that follow reiterated pancreatic injury and that are transient in experimental acute pancreatitis are referred to as part of the tissue repair mechanisms, which are essential in pancreas regeneration.4–6 To therapeutically interfere with these mechanisms, it is important to identify the target cell types that are involved in tissue repair and fibrosis development.
Recently it became evident that fibroblast cells, also termed stellate cells when they exhibit a characteristic morphology and contain lipid, play a key role in pancreas fibrogenesis.7,8,9,10,11,12,13 Under conditions of pancreatic injury and in primary culture, they undergo a change in phenotype to myofibroblasts (MFBs), called activation.8,10,11 This phenotype conversion to MFBs is accompanied by the presence of α smooth muscle actin (α-SMA) containing stress fibres, overexpression of desmin and platelet derived growth factor receptor type beta (PDGFRB), as well as by excessive synthesis of ECM, metalloproteinases, and their inhibitors.8,10–14 Despite morphological characterisation and analyses of cytoskeletal components of MFBs in primary culture, to date we have no comprehensive information about the fate of these cells in pancreatic regeneration and fibrosis development. While recent studies provided evidence that MFBs may drop out by apoptosis,15–18 to date there are few indications for a probable conversion of MFBs to fibroblasts during pancreatic and liver fibrosis development.19–21 Concerning possible mechanisms of how cells become resistant to apoptosis and perform a phenotype conversion, studies with monocytes and neurones have revealed translocation of the cell cycle inhibitory protein p21Cip1/WAF1 into the cytoplasm.22–24 This protein, when located in cytoplasm, acted as an inhibitor of stress fibre formation and apoptosis by forming a complex with Rho kinase 2 (Rock2) and apoptosis signal regulating kinase 1 (ASK1). Here, we used a rat explant culture system that mimics conditions in vivo rather than isolated cells to follow the cellular dynamics and phenotype conversions of pancreatic MFBs. To demonstrate the relevance of our results in vivo, we used models of cerulein induced acute pancreatitis and dibutyltin dichloride (DBTC) induced pancreatic fibrosis in rats.
METHODS
Antibodies and reagents
Mouse monoclonal anti-α-SMA (clone 1A4), anti-vimentin (clone V9), anti-desmin (clone DEU 10), anti-glial fibrillary acidic protein (GFAP, clone GA5), and anti-myosin light chain (MLC, clone MY21), and rabbit polyclonal anti-fibronectin (Fn) (F3648), anti-c-jun N terminal kinase 1/c-jun N terminal kinase 2 (JNK1/JNK2) (J4500) antibody, and phalloidin TRITC labelled were all from Sigma Chemical Co (St Louis, Missouri, USA). Rabbit polyclonal anti-PDGFRB (958), anti-Rock2 (H85), and anti-ASK1 (H300), and mouse monoclonal anti-pp38 (D8), anti-p21Cip1/WAF1 (F5), and anti- death receptor 5 (DR5) (M20) antibody were from Santa Cruz Biotechnology (Santa Cruz, California, USA). Mouse monoclonal anti-CD 95L (F 37720) was from Calbiochem (San Diego, California, USA). Rabbit polyclonal anti-collagen 1 (Col I) (11346) was from Rockland (Gilbertsville, Pennsylvania, USA). Rabbit polyclonal anti-pMLC 2 (Nr 3674) and mouse monoclonal anti-pSAPK/JNK (No 9255) were from Cell Signaling (Beverly, Massachusetts, USA). For immunofluorescence, Alexa Fluor 488 conjugated goat anti-mouse IgG and Alexa 594 conjugated chicken anti-rabbit Ig (Molecular Probes, Eugene, Oregon, USA) were used as secondary antibodies. Monoclonal anti-rabbit Ig and monoclonal anti-mouse Ig, conjugated with AP (Dako A/S, Glostrup, Denmark) were used as secondary antibodies for immunoblotting. BrdU labelling and detection kit I and the in situ cell death detection kit were from Roche (Mannheim, Germany). All trans-retinol (cat No R 7632), myelin basic protein (M 2016), and trypan blue (T 8154) were from Sigma. JNK inhibitor I (L form, cell permeable, No 420116) was from Calbiochem.
Animals
Male Wistar rats (90 days old, weighing 260–270 g, water ad libitum) were obtained from an outbreeding colony of the University of Rostock.
Explant culture
Rats were anaesthetised using 5-ethyl-5-isoamylbartituric acid (sodium salts), the pancreas was removed, and after mechanical cutting of the tissue into small blocks the pieces were placed into Col I (Sigma) coated Petri dishes (size 94 mm) and cultured in Dulbecco’s modified Eagle’s medium (Invitogen, Karlsruhe, Germany) supplemented with 10% fetal calf serum at 37°C in a 5% CO2 atmosphere.
Long term subculture and generation of a fibroblast cell line (LCF)
Myofibroblasts which were obtained on day 9 of the explant cultures were further cultivated at low density on plastic under the same conditions during a period of one year (25 passages) to generate a cell line with a stable fibroblast phenotype. These cells were termed long culture fibroblasts (LCFs). Soft agar cloning of these cells was negative and excluded malignant transformation.
Morphological analysis and detection of vitamin A uptake by LCFs
Cells of three independent explant cultures were morphologically examined daily over a period of 21 days under an inverted microscope (Axiovert 35) using phase contrast illumination. The presence of vitamin A in cells outgrowing from explants was detected by the fast fading of green autofluorescence of retinoids excited with 328 nm UV light. In the LCFs, the presence of vitamin A was detected after incubation with 5 μM of all trans-retinol over four days prior to the test.
Immunofluorescence analyses
Immunostaining of α-SMA, vimentin, desmin, GFAP, PDGFRB, p21Cip1/WAF1, Rock2, and ASK1 was performed as described previously.22,25 Fluorescence was analysed using a confocal laser scanning microscope (LSM-410, Carl Zeiss, Jena, Germany) equipped with a 63× oil immersion objective. Immunostaining for each detected protein was repeated three times in three independent explant and LCF cell cultures.
Proliferation and apoptosis assays
For analyses of proliferation and apoptosis, the BrdU incorporation assay and the TUNEL reaction were used, respectively. BrdU labelling and detection kit I and the in situ cell death detection kit were used according to the manufacturer’s instructions.
Western analyses
Immunoblots were performed from total cell lysates and from immunoprecipitates of cells on day 9 of the explant cultures (MFBs) and LCFs. Analyses were performed for Fn, Col I, PDGFRB, α-SMA, desmin, GFAP, CD 95L, DR-5, p21Cip1/WAF1, phospho-MLC, MLC, phospho-p38, p38, phospho-JNK1/2, JNK1/2, Rock2, and ASK1. For total cell lysates, adherent cells were lysed using a detergent containing buffer (62.5 mM Tris HCl, pH 6.8, 5 mM EDTA, 2% sodium dodecyl sulphate, 10% glycerol, 2% β-mercaptoethanol). Equal amounts of total cellular protein or of immunoprecipitates were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then transblotted to PVDF membranes. Membranes were incubated with appropriate primary antibodies overnight at 4°C followed by AP conjugated secondary antibody. Immunoblotting for each detected protein was repeated three times using lysates from three independent explants and LCF cell cultures.
Cellular fractionation
Briefly, cells were lysed in buffer A (10 mM HEPES, 1.5 mM MgCl2, 50 mM KCl, 0.2 mM phenylmethylsulphonyl fluoride, and protease inhibitor cocktail) and after incubation on ice for 10 minutes cells were sonicated by ultrasound. Cellular fractionation was carried out as described previously.26
Immuno- and coimmunoprecipitation
Immuno- and coimmunoprecipitation were performed using cytoplasmic and nuclear fractions. Briefly, lysates containing 350 μg of total cellular protein were precleared by incubation with protein A/G agarose for 10 minutes at 4°C and proteins were immunoprecipitated by incubation with appropriate antibodies (3 μg antibodies per 350 μg of total cellular protein) overnight at 4°C followed by protein A/G agarose for two hours at 4°C. Pellets were washed twice with lysis buffer and then with kinase buffer (50 mM Tris, 50 mM NaCI, 10 mM MgCI2, 25 mM β-glycerophosphate, 2 mM DTT, 0.2 mM PMSF). Complexes were disrupted in 2× Laemmli buffer and resolved in 10% gels. Immunoblotting for each detected protein was repeated three times using lysates from three independent cytoplasmic and nuclear fractions of MFB and LCF cells.
In vitro kinase assay
Rock2 and ASK1 proteins were immunoprecipitated from cytoplasmic fractions as described above. Beads were washed three times in kinase buffer and incubated in the presence of 10 μCi 32P-ATP and 2.5 μg myelin basic protein (MBP). Reactions were incubated for 30 minutes at 30°C. The reaction products were spotted onto P81 phosphocellulose paper discs, dried overnight at room temperature, and quantified using a scintillation counter. Reactions for each immunoprecipitated protein were repeated three times from three independent cytoplasmic fractions of MFB and LCF cells.
Induction of oxidative stress and trypan blue staining for plasma membrane integrity
MFBs and LCFs (0.5×106) were cultured for 48 hours and then incubated with 200 μM H2O2 in culture medium for six and 12 hours followed by further culture for 12 hours without H2O2. To test the role of JNK activity in the apoptotic sensitivity to oxidative stress, cells were incubated with JNK inhibitor (1 μM) for three hours and then washed, followed by incubation with H2O2. MFBs and LCFs without H2O2 treatment or treated only with JNK inhibitor were used as controls. Detached cells as well as adherent cells were stained with trypan blue solution, as described previously.27
In vivo models and histochemistry
Cerulein induced pancreatitis in rats was used as a model of acute pancreatic disease and generated as described previously.28 DBTC induced pancreatic fibrosis in rats was used as a model for established pancreatitis, and was generated as described previously.29
For immunohistochemistry, cryosections were fixed with 4% paraformaldehyde and then permeabilised with 0.1% Triton X-100 followed by incubation with anti-p21Cip1/WAF1 and anti-α-SMA antibodies for two hours. As secondary antibody, a rabbit anti-mouse AP conjugated antibody was used. After washing, FAST RED visualisation agent was added. Finally, the tissue was counterstained with haematoxylin. Cryosections incubated with a secondary antibody alone were used as negative controls (not shown).
RESULTS
Phenotype conversions of pancreatic fibroblast cells in primary explant cultures
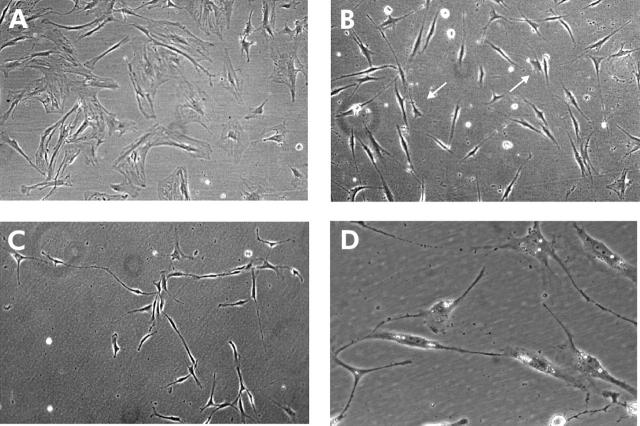
Cells that grew out from the explants were examined daily over a period of 21 days, with particular interest in cellular dynamics and phenotype differentiation. After 24 hours in culture, the first cells were growing out from the explants (fig 1A ▶–D). These cells had a fibroblastic shape and contained numerous lipid droplets in cytoplasm. During the following days, proliferating cells which incorporated BrdU in the S phase of the cell cycle were distributed throughout the population (data not shown). In the immunofluorescence experiments, fibroblasts on day 4 weakly expressed α-SMA and GFAP in a granular pattern, mainly localised in the perinuclear region (fig 3A, 3G ▶ ▶). After day 6, on the periphery, flattened cells with a great volume of cytoplasm and without processes dominated (fig 1D ▶). These cells were characterised as MFBs due to α-SMA filaments in cytoplasm (see below), which were organised in parallel and distributed along the cell axis.
Figure 1.

Phenotype conversions of outgrowing cells in the explant culture. (A) Fat storing cells grew out after 24 hours. (B) Fat droplets in cytoplasm, short cellular processes, and triangular shape were characteristic of the cells on day 3. (C) Elongated spindle shaped cells derived from triangular cells established a migration pattern on day 5. Some of them still have fat droplets in cytoplasm (arrows). (D) On day 6, cells had well developed stress fibres (myofibroblasts (MFBs)) (arrow). All magnification 320×. (E, F) On days 12 and 13, at the periphery, morphological conversion of MFB cells to fibroblasts was initiated. Cells acquired a fibroblast-like shape and 3–4 cytoplasmic processes (arrows). Magnification 100×. (G) On day 17, rounded up and condensed cells appeared to undergo apoptosis and were distributed over the whole culture. Magnification 320×. (H) On day 19, fibroblasts were present, which were morphologically similar to the fibroblasts in normal tissue. Magnification 100×. Different magnifications were used to emphasise special phenotype features—namely, fat droplets, stress fibres, and apoptotic condensation.
Figure 3.
Differential expression of cytoskeletal proteins and platelet derived growth factor receptor type beta (PDGFRB) in the explant culture. (A) Weak and dot-like distribution of α smooth muscle actin (α-SMA) on day 4 and on day 19 (C). (B) High expression of α-SMA in stress fibres on day 9 (control as in (F)). Filamentous organisation of vimentin (D) and desmin (E) in myofibroblast cells on day 9 ((F) control with a secondary Alexa Fluor 488 labelled antibody alone). (G) Granular expression of glial fibrillary acidic protein (GFAP) on day 3 and on day 19 (I). (H) Filamentous expression of GFAP on day 9 (control as in (F)). Expression of PDGFRB on days 3 (J), 9 (K), and 19 (L). Note the enhanced expression on day 9 (control as in (F)).
In MFBs analysed in the immunofluorescence experiments on day 9, α-SMA was expressed in stress fibres and colocalised with F-actin (fig 3B ▶). Cells expressed filamentous vimentin and desmin (fig 3D ▶–F) and PDGFRB was upregulated (fig 3K ▶). From day 12 to 21, we again observed a change in the cellular phenotype on the periphery of the cell population (fig 1E ▶-H). MFBs converted to cells that revealed a fibroblast phenotype with a decreased volume of cytoplasm and gradual package of stress fibres into 2–3 cytoplasmic processes (fig 1E ▶, F). These fibroblastic cells appeared morphologically identical to the pancreatic fibroblasts on day 4 but did not contain lipid droplets (fig 1H ▶). When analysed in the immunofluorescence experiments, these cells on day 19 showed weak and granular expression of α-SMA (fig 3C ▶), GFAP (fig 3I ▶), and decreased expression of PDGFRB (fig 3 ▶L). In parallel, the majority of MFBs rounded up, condensed, and could be characterised as apoptotic cells on day 17 by the TUNEL assay (fig 1G ▶, fig 2 ▶). These findings demonstrate recovery of pancreatic fibroblasts from MFBs in explant culture, which was accompanied by MFB apoptosis.
Figure 2.

Apoptosis of cells in the explant culture. (A) Apoptotic cells detected by TUNEL reaction were distributed throughout the whole cell colony on day 17. (B) Control staining with the secondary FITC labelled antibody alone.
Long term subculture of pancreatic MFBs and generation of LCFs
To evaluate phenotype stability of the cells in vitro, pancreatic MFBs and fibroblasts were transferred from explant culture into tissue culture flasks on day 9 and 19, respectively. Due to the massive apoptosis of MFBs, only a few fibroblasts were generated on day 19 that could be harvested which maintained their phenotype on plastic for two days (fig 4B ▶) followed by activation and conversion to MFBs. MFBs from day 9 (fig 4A ▶) maintained their phenotype for 24 passages and then converted to fibroblasts (fig 4C ▶). These fibroblasts (LCF) exhibited a stable phenotype during further subcultivation. In LCFs, uptake of vitamin A and its storage in fat droplets were detected after incubation with trans-retinol (fig 4D ▶). Immunofluorescence revealed the same characteristics as found in fibroblasts of the explant culture: granular expression of α-SMA, desmin, and GFAP as well as downregulation of PDGFRB (fig 5 ▶). In western blots, LCFs revealed a decrease in expression of α-SMA, desmin, GFAP, PDGFRB, and ECM proteins compared with MFBs (fig 6 ▶). In addition, we found strong downregulation of CD95L and DR5 protein expression (fig 6 ▶) in LCFs compared with MFBs, which suggests a lower sensitivity of the fibroblasts to apoptosis.
Figure 4.
Generation of fibroblastic cells (long culture fibroblast (LCF) cell line) after passaging and subculture of myofibroblast (MFB) cells on plastic. (A) MFBs on day 9 of the explant culture transferred on plastic. (B) Fibroblasts on day 19 of the explant culture transferred on plastic. (C) Morphology of LCF cells which were generated in passage 25 of the MFBs isolated from the explant culture on day 9. All magnifications 100×. (D) Storage of vitamin A in the form of fat droplets in cytoplasm of the LCF cells after two days of incubation with 5 μM retinol. Magnification 320× was used to emphasise fat droplet formation.
Figure 5.
Immunofluorescence analysis of cytoplasmic proteins and platelet derived growth factor receptor type beta (PDGFRB) in the long culture fibroblast (LCF) cells. Weak and granular expression of α-smooth muscle actin (A), glial fibrillary acidic protein (B), desmin (C), and PDGFRB (D) were assessed (control as in fig 3F ▶).
Figure 6.

Western analysis of protein expression in myofibroblast (MFB) (1) and long culture fibroblast (LCF) cells (2). Note the marked downregulation in expression of cytoskeletal, cell surface, and extracellular matrix proteins in LCF cells compared with MFB cells. Immunoblots for each detected protein were repeated three times using lysates from three independent explant and LCF cell cultures. Col, collagen; DR, death receptor; FN, fibronectin; GFAP, glial fibrillary acidic protein; PDGFRB, platelet derived growth factor receptor type beta; α-SMA, smooth muscle actin.
Pancreatic MFBs display nuclear while fibroblasts show cytoplasmic localisation of p21Cip1/WAF1
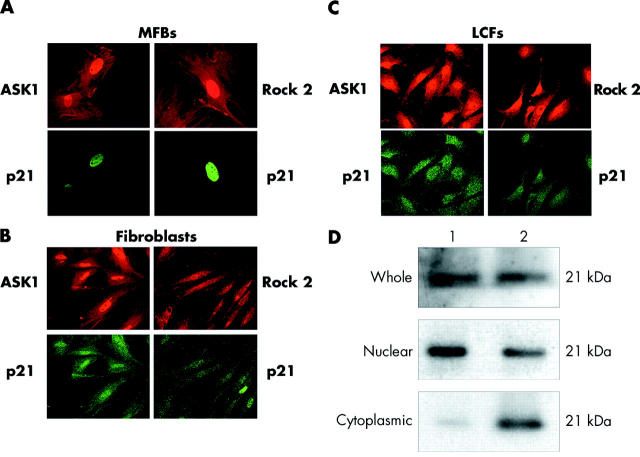
To further evaluate possible mechanisms that could be responsible for inhibition of apoptosis and phenotype conversion of pancreatic MFBs to fibroblasts, we hypothesised a pathway that involves p21Cip1/WAF1 and could negatively regulate stress fibre formation and apoptotic sensitivity.22–24 Using immunofluorescence, we found that MFBs expressed p21Cip1/WAF1 exclusively in the nucleus whereas both fibroblasts on day 19 in the explant culture and LCFs contained p21Cip1/WAF1 also in the cytoplasm (fig 7 ▶). In LCFs, cytoplasmic localisation was confirmed in western blots (fig 7 ▶). These findings suggest that phenotype conversion of MFBs to fibroblasts was associated with translocation of p21Cip1/WAF1 from the nucleus into the cytoplasm.
Figure 7.
Intracellular localisation of p21Cip1/WAF1, Rho associated kinase 2 (Rock2), and apoptosis signal regulating kinase 1 (ASK1) in fibroblasts on day 19 (B), myofibroblasts (MFBs) on day 9 of the explant culture (A), and long culture fibroblasts (LCFs) (C). In MFBs, p21Cip1/WAF1 was exclusively expressed in the nucleus whereas in both fibroblasts on day 19 and in LCFs, p21Cip1/WAF1 localised together with Rock2 and ASK1 in cytoplasm. (D) Western blot of p21Cip1/WAF1 in the whole cell lysate, nuclear, and cytoplasmic fractions of MFB (1) and LCF (2) cells. Immunostainings and immunoblots for each detected protein were repeated three times using cells and cell lysates from three independent explants, MFBs, and LCF cell cultures.
Cytoplasmic p21Cip1/WAF1 interacts with Rock2 and ASK1 in LCFs which correlates with inhibition of the activities of Rock2 and ASK1
To further support a role for cytoplasmic p21Cip1/WAF1 in the conversion of MFBs to fibroblasts, we tested its association with Rock2 and ASK1. Rock2 is one of the three downstream mediators of GTP-Rho and plays an important role in stress fibres and focal adhesion formation through phosphorylation of the MLC.30 ASK1 is a mitogen activated protein kinase kinase kinase and involved in the cellular response to Fas, tumour necrosis factor receptor, and oxidative stress stimulation through activation of the JNK1/2 and p38 pathways.31 As detected by immunofluorescence, Rock2 and ASK1 were localised together with p21Cip/WAF1 in the cytoplasm of both LCFs and fibroblasts on day 19 of the explant culture but not in MFBs (fig 7 ▶). Furthermore, p21Cip1/WAF1 was only detected in immunoprecipitates of Rock2 and ASK1 from cytoplasmic fractions of LCFs and not from MFBs (fig 8 ▶) which shows that p21Cip1/WAF1 binds to Rock2 and ASK1 in the cytoplasm of LCFs. To investigate the effect of p21Cip1/WAF1 on phosphorylation activity, cytoplasmic immunoprecipitates of Rock2 and ASK1 were subjected to a kinase assay using MBP, a previously reported suitable substrate for Rock2 and ASK1.32–34 We measured decreased kinase activities of both Rock2 and ASK1 immunoprecipitates in LCFs compared with MFBs (fig 8 ▶). These results suggest that cytoplasmic p21Cip1/WAF1 in LCFs forms a complex with Rock2 and ASK1, which correlates with inhibition of phosphorylation activities of both tested serine-threonine kinases.
Figure 8.
Cytoplasmic p21Cip1/WAF1 interacts with apoptosis signal regulating kinase 1 (ASK1) and Rho associated kinase 2 (Rock2) that corresponds with changes in their activity. (Top) p21Cip1/WAF1 (WB, western blot) was coimmunoprecipitated with ASK1 and Rock2 (IP, immunoprecipitate) in the cytoplasmic fractions obtained from long culture fibroblast (LCF) cells (2) but not from myofibroblast (MFB) cells (1). (Bottom) Activities of Rock2 and ASK1 measured towards myelin basic protein were significantly reduced in LCF cells (2) compared with MFBs (1). The percentage was quantified compared with counts per minutes in MFB cells. Expression of both kinases was determined with Coomassie blue staining to normalise the relative activities. Data represent means (SD) of three independent experiments (in each, six samples were measured).
Cytoplasmic p21Cip1/WAF1 correlates with reduced phosphorylation of MLC, p38 and JNK1/2 in LCFs
To see whether decreased kinase activities of Rock2 and ASK1 immunoprecipitates, which we found in LCFs, were associated with lower activation of downstream proteins in the stress activated pathways, we examined phosphorylation of MLC, p38, and JNK1/2. We revealed decreased phosphorylation of these proteins in LCFs compared with MFBs, whereas levels of protein expression were identical in both cell phenotypes (fig 9 ▶). These findings suggest that cytoplasmic expression of p21Cip1/WAF1 correlates with a decrease in MLC, p38, and JNK1/2 phosphorylation in LCFs.
Figure 9.
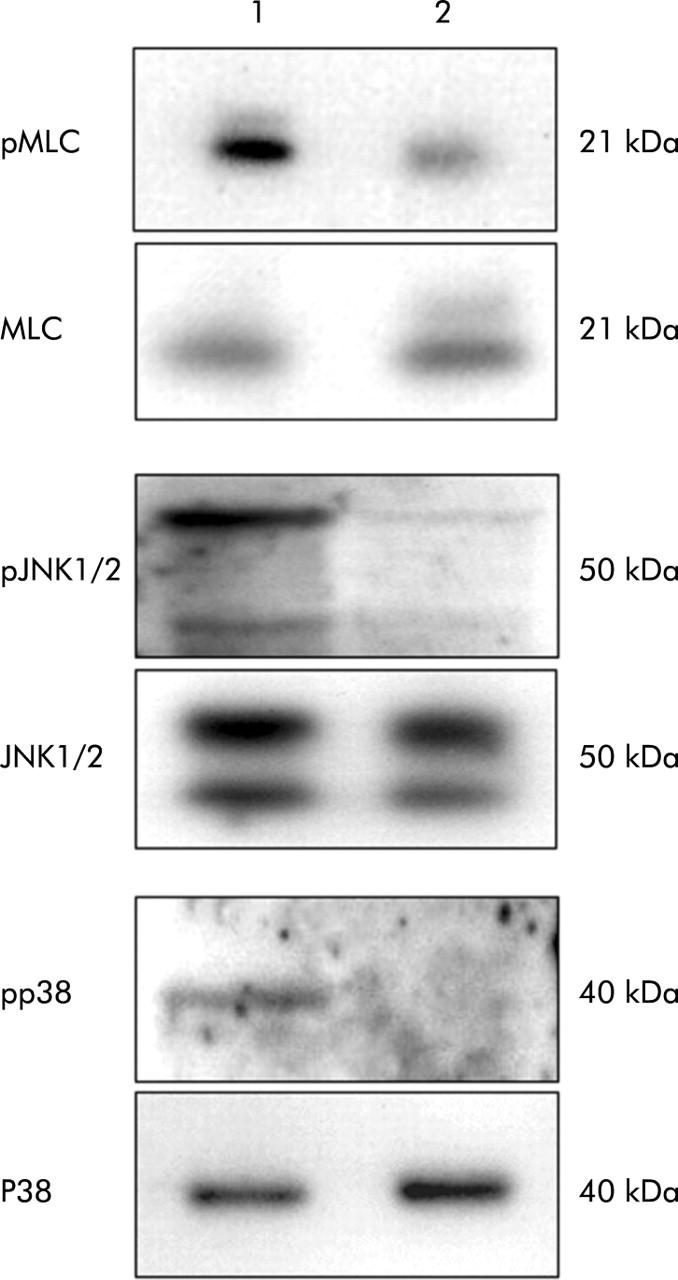
Decreased phosphorylation of myosin light chain (MLC), c-jun N terminal kinase 1/2 (JNK1/2), and p38 was detected in long culture fibroblast (LCF) cells (2) compared with myofibroblast (MFB) cells (1). Levels of protein expression were identical in both cell phenotypes. Immunoblots for each detected protein were repeated three times using lysates from three independent MFBs and LCF cell cultures.
Different sensitivities to oxidative stress in MFBs and LCFs which depend on JNK1/2 activation
To investigate whether the apoptotic sensitivity of pancreatic MFBs in culture can be explained by increased ASK1 activity which is followed by downstream activation of JNK1/2, we incubated MFBs and LCFs with 200 μM H2O2 to induce oxidative stress which was shown to induce ASK1 activation and induction of apoptosis in several cell types.35 We found an increased proportion of cells that detached from the substrate due to H2O2 in MFBs compared with LCFs (fig 10 ▶). Trypan blue staining of cells revealed a permeable membrane. Furthermore, we inhibited activation of JNK1/2 using a concentration of inhibitor proposed by the manufacturer which revealed reduced sensitivity of MFBs to oxidative stress. LCFs remained unaffected. This indicates that this apoptotic sensitivity depends on JNK1/2 activation in MFBs (fig 10 ▶). Together, these findings suggest decreased apoptotic sensitivity in LCF cells compared with MFBs due to the low activity of ASK1 and reduced activation of JNK1/2 (see results above).
Figure 10.
Resistance of long culture fibroblasts (LCFs) to oxidative stress (H2O2 treatment) for six hours compared with stress sensitive myofibroblasts (MFBs). Reduced sensitivity to oxidative stress of MFBs was induced by c-jun N terminal kinase (JNK) inhibition. As controls, cells were not treated (control) or treated with JNK inhibitor alone (JNK inhibitor). Two independent experiments (1, 2) for six hours of H2O2 treatment were performed. Similar results were obtained with treatment for 12 hours (data not shown). Dead cells were expressed as percentage of trypan blue positive cells.
Pancreatic MFBs and fibroblasts show different p21Cip1/WAF1 localisation in experimental acute pancreatitis and pancreas fibrosis
To test the hypothesis that the phenotype conversion from MFBs to fibroblasts combined with cytoplasmic expression of p21Cip1/WAF1 that we have found in cell culture also occurs in pancreatic fibrosis, we examined expression of α-SMA and p21Cip1/WAF1 in cryosections from normal rat pancreas, experimental cerulein induced acute pancreatitis, and DBTC induced established pancreatic fibrosis (fig 11 ▶). Consistent with our findings in vitro, interstitial cells in cerulein induced acute pancreatitis demonstrated prominent anti-α-SMA immunoreactivity and nuclear localisation of p21Cip1/WAF1 whereas weak anti-α-SMA immunoreactivity and cytoplasmic expression of p21Cip1/WAF1 were detected in fibroblasts distributed throughout fibrotic bands in DBTC induced established pancreatic fibrosis. In the normal pancreas, only a few fibroblasts were positive for p21Cip1/WAF1 which revealed cytoplasmic expression.
Figure 11.

Intracellular localisation of p21Cip1/WAF1 and α smooth muscle actin (α-SMA) expression in fibroblasts in quiescent tissue (A, E) and during the course of cerulein induced acute pancreatitis 48 hours (B, F) and 96 hours (C, G) after induction of dibutyltin dichloride (DBTC) induced pancreatic fibrosis (D, H) (both proteins in red). (A, E) Fibroblasts were negative for α-SMA in normal pancreas whereas a few showed cytoplasmic expression of p21Cip1/WAF1. Magnification 160×. (B, F) De novo expression of α-SMA and p21Cip1/WAF1 in the cytoplasm of fibroblasts 48 hours after induction of experimental acute pancreatitis. Magnification 100×. (C, G) Prominent expression of α-SMA and nuclear localisation of p21Cip1/WAF1 in interstitial cells 96 hours after induction of experimental acute pancreatitis. Magnification 100× (histology of the pancreas on day 10 after induction of experimental acute pancreatitis was virtually the same as in normal tissue (not shown)). (D, H) Weak and scant expression of α-SMA and cytoplasmic localisation of p21Cip1/WAF1 in fibroblasts distributed throughout fibrotic bands 60 days after induction of DBTC induced pancreatic fibrosis. Magnification 100×.
DISCUSSION
The principle finding of this study was that in a culture model, pancreatic MFBs were transient and disappeared by apoptosis or converted to fibroblasts. This phenotype conversion to fibroblasts was documented by corresponding changes in morphology, expression, and organisation of cytoskeletal proteins, cell surface receptors, and ECM proteins, and by a lower apoptotic sensitivity. Moreover, our histochemical results in the cerulein induced model of acute pancreatic disease and in the DBTC induced model of established pancreatic fibrosis revealed the relevance of our in vitro findings for tissue reactions in vivo. Prominent α-SMA expression as a main histopathological marker of MFB transdifferentiation was found exclusively in cryosections from experimental acute pancreatitis whereas in established pancreatic fibrosis, weak α-SMA immunoreactivity or even immunonegativity was a characteristic feature.
Given that recovery of fibroblasts from pancreatic MFBs is characterised by significant alterations in the cytoskeleton and increase in resistance to apoptotic stimuli, we believe that a pathway activated during this phenotype conversion negatively regulates stress fibre formation and apoptotic sensitivity. It was previously reported that p21Cip1/WAF1, an inhibitor of cell cycle progression, exhibits such functions in different cell types through its ectopic cytoplasmic localisation.22–24 In fact, the present study showed that pancreatic MFB to fibroblast conversion in culture was combined with p21Cip1/WAF1 translocation from the nucleus to the cytoplasm. Investigating the molecular mechanisms in more detail, in LCFs we found a complex of cytoplasmic p21Cip1/WAF1 with Rock2 and ASK1 which correlated with decreased phosphorylation activities of these proteins in a kinase assay. Moreover, cytoplasmic expression of p21Cip1/WAF1 in LCFs correlated with hypophosphorylation of MLC, JNK1/2, and p38 compared with their state of phosphorylation in MFBs, where nuclear expression of p21Cip1/WAF1 was found. Reduced activation of these signalling proteins was shown to mediate stress fibres/focal adhesion disintegration, and block continuous activation of MFBs.30,36–38 Furthermore, in LCFs, cytoplasmic expression of p21Cip1/WAF1, decrease in ASK1 activity, and inhibition of JNK phosphorylation correlated with resistance to apoptotic stimuli by oxidative stress. The mechanisms which regulate cellular localisation of p21Cip1/WAF1 in different cell types are not yet clear. Theories include phosphorylation of p21Cip1/WAF1 by Akt, which may either induce direct translocation of p21Cip1/WAF1 from the nucleus to the cytoplasm26 or inhibit its degradation, which may contribute to accumulation of p21Cip1/WAF1 in the cytoplasm after translocation.39–41
Based on our in vitro findings as well as our results in the animal models, we propose a plausible model of how translocation of p21Cip1/WAF1 can influence phenotype conversion of pancreatic MFBs in culture and during experimental injury. In the normal pancreas, only a few fibroblasts are positive for p21Cip1/WAF1 and show exclusive cytoplasmic localisation. Activation of fibroblasts and subsequent conversion to MFBs is accompanied by de novo cytoplasmic expression of p21Cip1/WAF1 and its translocation into the nucleus followed by activation of Rock2 and ASK1, increase in apoptotic sensitivity, and inhibition of cell cycle progression. MFBs with nuclear expression of p21Cip1/WAF1 were characteristic in the cerulein induced model for acute pancreatitis. Furthermore, while a significant number of MFBs disappeared by apoptosis, some converted to fibroblasts which was mediated by translocation of p21Cip1/WAF1 from the nucleus to the cytoplasm with subsequent binding to and inhibition of Rock2 and ASK1. Fibroblasts with cytoplasmic expression of p21Cip1/WAF1 were characteristic in the DBTC model for established pancreatic fibrosis. These cells may be derived from MFBs as we have found in vitro. Apart from MFBs which were rare in the established DBTC model, these fibroblasts could contribute towards maintaining pancreatic fibrosis and may be of pathophysiological relevance in chronic pancreatitis where continuous oxidative stress was shown to contribute to fibrosis development.42
Taken together, our results provide evidence that pancreatic MFBs are transient and p21Cip1/WAF1, by its intracellular localisation, can serve as an endogenous regulator of these cells in culture and during experimental injury.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (GRK 19/03). We would like to thank Dr Heike Weber and Inge Weber for providing the cryosections from experimental acute pancreatitis and pancreas fibrosis, Dr Robert Jaster for help in the interpretation of experimental data, Petra Wolf for providing the animals, and Christa Lukat and Annelie Peters for technical assistance.
Abbreviations
ASK, apoptosis signal regulating kinase
Col, collagen
DBTC, dibutyltin dichloride
DR, death receptor
ECM, extracellular matrix
Fn, fibronectin
GFAP, glial fibrillary acidic protein
JNK, c-jun N terminal kinase
LCF, long culture fibroblast
MBP, myelin basic protein
MFB, myofibroblast
MLC, myosin light chain
PDGFRB, platelet derived growth factor receptor type beta
Rock, Rho associated kinase
SMA, smooth muscle actin
Conflict of interest: None declared.
REFERENCES
- 1.Kloppel G, Maillet B. Chronic pancreatitis: evolution of the disease. Hepatogastroenterology 1991;38:408–12. [PubMed] [Google Scholar]
- 2.Kennedy RH, Bockman DE, Uscanga L, et al. Pancreatic extracellular matrix alterations in chronic pancreatitis. Pancreas 1987;2:61–71. [DOI] [PubMed] [Google Scholar]
- 3.Gress TM, Müller-Pilasch F, Lerch MM, et al. Balance of expression of genes coding for extracellular matrix proteins and extracellular matrix degradating proteases in chronic pancreatitis. Z Gastroenterol 1994;32:221–5. [PubMed] [Google Scholar]
- 4.Elsasser HP, Haake T, Grimmig M, et al. Repetitive cerulein-induced pancreatitis and pancreas fibrosis in the rat. Pancreas 1992;7:385–90. [DOI] [PubMed] [Google Scholar]
- 5.Morisset J, Morisset S, Lauzon K, et al. Pancreatic inflammation, apoptosis and growth: sequential events after partial pancreatectomy in pigs. Pancreas 2002;21:321–4. [DOI] [PubMed] [Google Scholar]
- 6.Menke A, Adler G. TGFbeta-induced fibrogenesis of the pancreas. Int J Gastointest Cancer 2002;31:41–6. [DOI] [PubMed] [Google Scholar]
- 7.Morohoshi T, Kanda M. Periacinar fibroblastoid cell: Its action in early stage of alcoholic pancreatitis. J Bil Tract Pancreas 1985;6:1205–11. [Google Scholar]
- 8.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol 1999;155:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells RG, Crawford JM. Pancreatic stellate cells: the new stars of chronic pancreatitis? Gastroenterology 1998;115:491–3. [DOI] [PubMed] [Google Scholar]
- 10.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation and culture. Gut 1998;43:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachem MG, Schneider E, Gross H, et al. Identification, culture and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421–32. [DOI] [PubMed] [Google Scholar]
- 12.Kato Y, Inoue H, Fujiyama Y, et al. Morphological identification and collagen synthesis of periacinar fibroblastoid cells isolated and cultured from rat pancreatic acini. J Gastroenterol 1996;31:565–71. [DOI] [PubMed] [Google Scholar]
- 13.Saotome T, Inoue H, Fujiyama M, et al. Morphological and immunocytochemical identification of periacinar fibroblast-like cells derived from human pancreatic acini. Pancreas 1997;14:373–82. [DOI] [PubMed] [Google Scholar]
- 14.Phillips PA, McCarroll JA, Park S, et al. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 2003;52:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klonowski-Stumpe H, Fischer R, Reinehr R, et al. Apoptosis in activated rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2002;283:819–26. [DOI] [PubMed] [Google Scholar]
- 16.Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 1998;102:538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issa R, Williams E, Trim N, et al. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 2001;48:548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taimr P, Higuchi H, Kocova E, et al. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology 2003;37:87–95. [DOI] [PubMed] [Google Scholar]
- 19.Suda K . Pathogenesis and progression of human pancreatic fibrosis. Med Electron Microsc 2000;33:200–6. [DOI] [PubMed] [Google Scholar]
- 20.Tashiro M, Nakamura H, Taguchi M, et al. Oleic acid-induced pancreatitis alters expression of transforming growth factor-beta 1 and extracellular matrix components in rats. Pancreas 2003;26:197–204. [DOI] [PubMed] [Google Scholar]
- 21.Levy MT, Mccaughan GW, Marinos G, et al. Intrahepatic expression of the hepatic stellate cell marker fibroblast activation protein correlates with the degree of fibrosis in hepatitis C virus infection. Liver 2002;22:93–101. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Yamashita T, Asada M, et al. Cytoplasmic p21Cip1/WAF1 regulates neurite remodeling by inhibiting Rho-kinase activity. J Cell Biol 2002;158:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asada M, Yamada T, Fukumuro K, et al. p21CIP1/WAF1 is important for differentiation and survival of U937 cells. Leukemia 1998;12:1944–50. [DOI] [PubMed] [Google Scholar]
- 24.Asada M, Yamada T, Ichijo H, et al. Apoptosis inhibitory activity of cytoplasmic p21Cip1/WAF1 in monocytic differentiation. EMBO J 1999;18:1223–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rychly J . Epithelial cell integrins. In: Wise C, ed. Methods in molecular biology. Totowa, NJ: Humana Press Inc, 2002:169–77. [DOI] [PubMed]
- 26.Zhou BP, Liao Y, Xia W, et al. Cytoplasmic localization of p21Cip1/WAF1 by AKT-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 2001;3:245–52. [DOI] [PubMed] [Google Scholar]
- 27.Lohr M, Muller P, Karle P, et al. Targeted chemotherapy by intratumour injection of encapsulated cells engineered to produce CYP2B1, an ifosfamide activating cytochrome P450. Gene Ther 1998;5:1070–8. [DOI] [PubMed] [Google Scholar]
- 28.Willemer S, Elsasser HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res 1992;24:29–39. [DOI] [PubMed] [Google Scholar]
- 29.Sparmann G, Merkord J, Jaschke A, et al. Pancreatic fibrosis in experimental pancreatitis induced by dibutyltin dichloride. Gastroenterology 1997;112:1762–5. [DOI] [PubMed] [Google Scholar]
- 30.Riento K, Ridley A. Rocks: multifunctional kinases in cell behaviour. Nature 2003;4:446–56. [DOI] [PubMed] [Google Scholar]
- 31.Ichijo H, Nishida E, Irie K, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signalling pathways. Science 1997;275:90–4. [DOI] [PubMed] [Google Scholar]
- 32.Ko YG, Kang YS, Park H, et al. Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm. J Biol Chem 2001;276:39103–6. [DOI] [PubMed] [Google Scholar]
- 33.Ishizaki T, Maekawa M, Fujisawa K, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 1996;15:1885–93. [PMC free article] [PubMed] [Google Scholar]
- 34.Galva V, Logvinova A, Sperandio S, et al. Type 1 insulin-like growth factor receptor (IGF-IR) signaling inhibits apoptosis signal-regulating kinase 1 (ASK1). J Biol Chem 2003;278:13325–32. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Matsuzawa A, Nishitoh H, et al. Role of MAPKKK ASK1 in stress-induced cell death. Cell Struct Func 2003;28:23–9. [DOI] [PubMed] [Google Scholar]
- 36.Masamune A, Satoh M, Kikuta K, et al. Inhibition of p38 mitogen-activated kinase blocks activation of rat pancreatic stellate cells. J Pharmacol Exp Ther 2003;304:8–14. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto S, Gon Y, Takeshita I, et al. Transforming growth factor beta1 induces phenotype modulation of human lung fibroblasts to myofibroblasts through a c-Jun-NH2-terminal kinase dependent pathway. Am J Respir Crit Care Med 2001;163:152–7. [DOI] [PubMed] [Google Scholar]
- 38.Preaux AM, D’ortho MP, Bralet MP, et al. Apoptosis of human hepatic myofibroblasts promotes activation of matrix metalloproteinase-2. Hepatology 2002;36:615–22. [DOI] [PubMed] [Google Scholar]
- 39.Rossig L, Jadidi AS, Urbich C, et al. AKT-dependent phosphorylation of p21Cip1/WAF1 regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol 2001;21:5644–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossig L, Badorff C, Holzmann Y, et al. Glycogen synthase kinase-3 couples AKT-dependent signalling to the regulation of p21Cip1/WAF1 degradation. J Biol Chem 2002;277:9684–9. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Dowbenko D, Lasky L. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 2002;277:11352–61. [DOI] [PubMed] [Google Scholar]
- 42.Matsumura N, Ochi K, Ichimura M, et al. Study of free radicals and pancreatic fibrosis induced by repeated injections of superoxide dismutase inhibitor. Pancreas 2001;22:53–7. [DOI] [PubMed] [Google Scholar]