Abstract
Background: In hepatic ischaemia/reperfusion injury, activated liver macrophages (Kupffer cells) are dominantly regulated by a transcription factor, nuclear factor κB (NFκB), with respect to expression of inflammatory cytokines, acute phase response proteins, and cell adhesion molecules.
Aims: We assessed whether inactivation of NFκB in the liver could attenuate total hepatic warm ischaemia/reperfusion injury.
Methods: We studied rats with hepatic overexpression of inhibitor κBα super-repressor (IκBα SR) caused by a transgene introduced using an adenoviral vector. Hepatic ischaemia/reperfusion injury was induced under warm conditions by total occlusion of hepatoduodenal ligament structures for 20 minutes, followed by reperfusion. Controls included uninfected and control virus (AdLacZ) infected rats.
Results: IκBα SR was overexpressed in Kupffer cells as well as in hepatocytes, blocking nuclear translocation of NFκB (p65) into the nucleus after reperfusion. Gene transfection with IκBα SR, but not with LacZ, markedly attenuated ischaemia/reperfusion injury, suppressing inducible nitric oxide synthase and nitrotyrosine expression in the liver. Moreover, no remarkable hepatocyte apoptosis was detected under IκBα SR overexpression.
Conclusions: Adenoviral transfer of the IκBα SR gene in the liver ameliorates short term warm ischaemia/reperfusion injury, possibly through attenuation of hepatic macrophage activation.
Keywords: Kupffer cells, rats, ischaemia/reperfusion injury, nuclear factor κB, repressor gene transfection
Warm hepatic ischaemia/reperfusion injury often occurs during certain surgical manipulations, such as when Pringle’s manoeuvre is performed to reduce blood loss during tumour resection.1 Relatively brief ischaemia primes cells for damage, but cell injury may occur after the ischaemic liver is reperfused.2 After reperfusion, reactive oxygen species (ROS) play a critical role in inducing inflammatory responses and cellular damage.3,4 Inflammation, including ongoing neutrophil recruitment and microcirculatory disturbance, further accelerates cellular damage to result in necrotic cell death.5,6
Kupffer cells, the resident macrophages of the liver, are activated after ischaemia/reperfusion stress, and have been recognised to produce ROS, proinflammatory cytokines, chemokines, and other mediators.5,7 Pharmacological inactivation of Kupffer cells has been reported to suppress hepatic ischaemia/reperfusion injury, suggesting pathogenetic involvement of these macrophages.8,9,10 A transcription factor, nuclear factor κB (NFκB), has been determined to be crucial in the cascade bringing about Kupffer cell activation.11–13 ROS produced by Kupffer cells have been proposed to activate DNA binding activity of NFκB in these cells,14–16 although such activation of NFκB by ROS remains controversial.17 Moreover, stimulation of Kupffer cells with endotoxin, which may participate in total ischaemia/reperfusion injury of the liver, leads to increased DNA binding of NFκB through the Toll-like receptor 4, myeloid differentiation factor 88, and interleukin 1 receptor associated kinases.18–20 Thus inactivation of NFκB in Kupffer cells might be of clinical value in blocking injurious events in total hepatic ischaemia/reperfusion.
Several recent studies have proposed that apoptotic cell death may affect both hepatocytes and sinusoidal endothelial cells after either warm or cold ischaemia/reperfusion.21–23 Through release of tumour necrosis factor α (TNF-α), Kupffer cells and platelets reportedly induce apoptosis in these target cells.24 However, some reports have indicated that apoptosis in such cells never involves more than 2% of liver cells when strict morphological criteria for apoptosis are employed.25 NFκB participates importantly in promoting cell survival by regulating several antiapoptotic factors, including inhibitors of apoptosis.26,27 Inactivation of NFκB resulting from overexpression of inhibitor κBα super-repressor (IκBα SR) renders hepatocytes susceptible to various proapoptotic stimuli.28–31 Such blockade of NFκB activity in hepatocytes could lead to massive cell death after hepatic ischaemia/reperfusion if the dominant mechanism of cell death after reperfusion is apoptosis.
In the present study, we overexpressed dominant negative IκBα SR in the liver using an adenoviral vector (Ad5IκB) capable of transfecting both hepatocytes and Kupffer cells. We examined whether the consequent blockade of NFκB DNA binding activity in the liver protected against or accelerated short term warm total ischaemia/reperfusion injury. We employed a rat model of total hepatic ischaemia/reperfusion that closely simulated a common surgical context of such injury (Pringle’s manoeuvre). We note that the model may not be suitable for study of pure hepatic ischaemia/reperfusion injury as endotoxin translocation from the congested intestine could occur during the ischaemic period.
MATERIALS AND METHODS
Recombinant adenoviruses
The recombinant adenovirus (Ad5IκB) encoding a haemagglutinin (HA) tagged cDNA of the dominant negative forms of human IκB (IκBα S32A/S36A) was generated as reported elsewhere.31 Ad5LacZ, encoding the Escherichia coli β-galactosidase gene, was used as a control adenovirus. Adenoviral stock was amplified in HEK293 cells (CRL1573.ATCC; Manassas, Virginia, USA) and purified by double caesium gradient, as described previously, and plaque tittered.31 HEK293 cells were incubated in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum (Dainippon Pharmaceutical, Japan) and penicillin (100 IU/ml)/streptomycin (100 μg/ml) (Meiji Seika, Japan) at 37°C. When the cells reached confluence they were infected with Ad5IκB or Ad5LacZ at a multiplicity of infection of 200 for 48–72 hours in Dulbecco’s modified Eagle’s medium with 5% fetal bovine serum. Adenoviruses were dialysed in 1000 ml of dialysis buffer (phosphate buffered saline 10% glycerol) overnight at 4°C before use.
Animal protocols and hepatic ischaemia/reperfusion procedure
All animals were handled according to the method approved under the institutional guidelines outlined in the Guide for Use and Care of Laboratory Animals of Kyoto University Graduate School of Medicine. Male Sprague-Dawley rats with a starting weight of 240∼255 g (7–8 weeks old) were used. Recombinant adenoviruses were administered through their tail veins in a volume of 250 μl (5×109 pfu/body) with 27 G needles. No viruses were injected in uninfected control rats.
Seventy two hours after infection, rats were anaesthetised by intraperitoneal injection of 0.1 μl/g Nembutal (pentobarbital sodium 50 mg/ml; Dainippon Pharmaceutical). After laparotomy, whole hepatic ischaemia was induced clamping the hepatic artery, portal vein, and bile duct for 20 minutes without any decompression of the splanchnic circulation, resembling a clinical situation (Pringle’s manoeuvre). After 20 minutes, these vessels were unclamped leading to reperfusion of the liver. This model is sublethal and exhibits less liver injury compared with that previously published.32,33 Because adenoviral infection per se possibly induces transient liver injury due to its immunogeneity, we performed the ischaemia/reperfusion procedure at 72 hours when transient liver injury induced by adenovirus should have returned to near normal.
Small amounts of blood (0.4 ml) were collected from the inferior vena cava at 10 and 40 minutes after reperfusion, and liver tissues and blood samples were taken when the animals were sacrificed at 180 minutes. In some rats, liver tissues and blood samples were collected at 12 or 24 hours after reperfusion when the animals were sacrificed. At least four rats in each group were analysed at each time point. Serum separated from these samples was used for enzymatic measurement of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH). Serum concentration of TNF-α in each animal was also measured by means of an ELISA kit (Genzyme, Cambridge, Massachusetts, USA). Samples of the liver were snap frozen in liquid nitrogen or mounted in Tissue Tec (Sakura Finetechnical Co., Tokyo, Japan) and stored at −80°C for immunohistochemistry. Some of the tissues were fixed in 10% buffered formalin for subsequent histological analysis (haematoxylin-eosin staining).
Histological assessment
Liver injury was accessed using liver specimens stained with haematoxylin-eosin. The extent of sinusoidal congestion, cytoplasmic vacuolisation, and liver necrosis was semiquantitatively assessed, respectively, according to a scoring criteria previously published.34 Namely, congestion and vacuolisation were evaluated as follows: none = 0, minimal = 1, mild = 2, moderate = 3, and severe = 4. Liver necrosis was scored as follows: none = 0, single cell necrosis = 1, up to 30% lobular necrosis = 2, up to 60% lobular necrosis = 3, and more than 60% lobular necrosis = 4. Scoring was performed in five independent high power fields on each sample, and mean values were represented. Blind analysis was performed on all samples. Infiltration of neutrophils into the liver was also estimated by means of naphthol AS-D chloroacetate esterase staining.35 The number of esterase positive polymorphonuclear cells was counted in 10 high power fields (×400) in each sample, and mean values were calculated.
X-gal staining analysis and immunofluorescence
Efficiency of gene transfer after adenoviral infection was assessed with X-gal staining of liver tissues from rats infected with Ad5LacZ at 72 hours. Frozen sections from the liver were evaluated for β-galactosidase activity by incubation in X-gal solution (3.3 mM K4Fe(CN)6·3H2O, 3.3 mM K3Fe(CN)6, 1 mM MgCl2, 0.2%X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside); Roche Diagnostics, Switzerland).
To access whether liver macrophages were transfected with Ad5LacZ more accurately, immunofluorescent staining against β-galactosidase and surface antigen of macrophages was performed. Frozen liver samples were cut into cryostat sections 5 μm in thickness, and fixed in acetone for 10 minutes. After washing with phosphate buffered saline, sections were incubated with mouse antisurface antigen of macrophages monoclonal antibody (ED-1, 1: 100 dilution; Chemicon International, Temecula, California, USA) and rabbit anti-β-galactosidase polyclonal antibody (1:200; ICN Pharmaceuticals, Inc., Aurora, Ohio, USA) for 60 minutes. Slides were washed with phosphate buffered saline and then incubated for 30 minutes with FITC conjugated goat antimouse IgG (1:50 dilution; Southern Biotechnology Associates, Inc., Birmingham, UK) and Texas red conjugated goat antirabbit IgG. (1: 50 dilution; Southern Biotechnology Associates, Inc.). Localisation of β-galactosidase and liver tissue macrophages, and Kupffer cells was analysed using a confocal laser scanning microscope LSM510 (Carl Zeiss, Jena, Germany).
Western blot analysis for IκBα and iNOS
Rats were infected with Ad5IkB (2.5×109 pfu/body) for 72 hours. Expression of dominant negative IκB protein was assessed in 20 μg of whole liver homogenates using rabbit anti-IκBα antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, California, USA). Equal amounts of lysates were electrophoresed on 15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. After blocking, the membrane was incubated with the first antibody at 4°C overnight and then with the horseradish peroxidase linked goat antirabbit secondary antibody at 1:1000 dilution (Santa Cruz Biotechnology). Chemiluminescence was detected with an ECL kit, as previously described. Equal protein loading was confirmed by staining the gels with the Coomasie stain solution (Bio-Rad Laboratories, Hercules, California, USA). For western blot of inducible nitric oxide synthase (iNOS), rabbit anti-iNOS antibody (1:1000 dilution; sc-8310, Santa Cruz Biotechnology) was used, and subsequent procedures were performed as described above.
Immunohistochemistry for NFκB, HA tagged IκBα SR, 4-HNE, and nitrotyrosine
Paraffin embedded sections were pretreated by microwaving for 20 minutes with 1% bovine serum albumin in 0.05 M Tris HCl, pH 7.6. After blocking of endogenous peroxidase with peroxidase blocking agent, sections were incubated for 60 minutes at 37°C with goat polyclonal antibody against NFκB p65 (1:100 dilution; c-20:sc-372G; Santa Cruz Biotechnology). After being washed, sections were incubated for 60 minutes with biotin conjugated rabbit antibody against goat (1:200 dilution; AP106-b; Chemicon) labelled with peroxidase streptavidin and examined, after being incubated with chromogen conjugated DAB substrate for 90 seconds.
For detecting the HA tagged IκBα, mouse monoclonal anti-HA antibody (5 µl/ml, clone 12CA5; Boehringer Mannheim, Indianapolis, Indiana, USA) was used as the primary antibody, and sections were stained as described above.
Expression of nitrotyrosine and 4-hydroxy-2′-nonenal (HNE) adducts in the liver were analysed by immunohistochemistry using polyclonal antinitrotyrosine antibody (AB5411, 1:100; Chemicon) and monoclonal anti-4-HNE antibody (JaICA, MHN-20, 1:5; Shizuoka, Japan) as the primary antibody, respectively.
Reverse transcriptase-polymerase chain reaction (RT-PCR) assay for TNF-α
Messenger RNA was extracted from frozen liver tissue with a mRNA purification kit (Amersham Biosciences Corp., Piscataway, New Jersey, USA). The concentration of mRNA was ascertained by UV spectrophotometer at 260 nm and 10 μg of mRNA were used to synthesise first strand cDNA with the First Strand cDNA kit (Amersham Pharmacia). Rat TNF-α was amplified using the primer pair (each 100 μM, Bp, 5′-CAC GCT CTT CTG TCT ACT GA-3′, forward; 5′-GGA CTC CGT GAT GTC TAA GT-3′, reverse) in a 50 μl PCR reaction containing 1.5 mM MgCl2, 50 mM KCl, 10 mM Tris HCl, pH 8.3, and 1.4 μl deoxynucleotides (10 mM each) using 3 μl cDNA template. Dilutions of cDNA were amplified for 30 cycles at 94°C for 20 seconds, 56°C for 60 seconds, and 72°C for 60 seconds. β-Actin probe was used as the integrity control, using the primers synthesised from conserved coding sequences (265 bp, 5′-TCC TAT GTG GGT GAC GAG GC-3′, forward; 5′-TAC ATG GCT GGG GTG TTG AA-3′, reverse). PCR products were electrophoresed on 2% agarose gel, stained with ethidium bromide, and visualised under UV transillumination.
Gel mobility shift assay for NFκB
For the gel mobility shift assay for NFκB binding activity, nuclear extracted protein was collected as described elsewhere.33 Double stranded NFκB specific oligodeoxynucleotide probe containing two tandemly positioned NFκB binding sites (GGG ATT TCC C) were labelled with [α-32P] dCTP by Klenow fragment. Nuclear extract (3 μg) was incubated with a 300 fmol probe in a total of 25 μl of binding buffer (10 nM HEPES,3 50 mM KCl, 1 mM EDTA, 5 mM MgCl2, 10% glycerol, and 2 μg of poly-dIdC) for 20 minutes at room temperature. For the competition assay, a 50-fold molar excess of unlabelled oligodeoxynucleotide probe was added to nuclear extract for 15 minutes before addition of the labelled probe. The supershift assay was performed by preincubating with 1 μg of anti-p65 or anti-p50 antibodies (Santa Cruz Biotechnologies) for 60 minutes at 4°C before addition of the labelled probe. After incubation, samples were fractionated on a 4% polyacrylamide gel in 25 mM Tris Cl (pH 8.5), 190 mM glycine, and 1 mM EDTA. The gel was subsequently dried and visualised by autoradiography.
In situ detection of apoptosis
Formalin fixed paraffin embedded liver sections (5 µm) were deparaffinised in xylene and rehydrated through graded ethanols. After blocking of endogenous peroxidase with peroxidase blocking agent, sections were incubated with protease K (20 μg/ml) for 10 minutes. Then, sections were incubated with a goat anti-single strand DNA polyclonal antibody (1:400 dilution; A4506; Dako Cytomation, Kyoto, Japan), which detects apoptotic cells in situ, for 60 minutes at room temperature. After being washed, sections were incubated for 30 minuets with biotin conjugated rabbit antibody against goat (1:200 dilution; AP106-b; Chemicon) labelled with peroxidase streptavidin, and with chromogen conjugated DAB substrate for 90 seconds. Counterstaining was performed with methyl green.
Statistical analyses
Data are expressed as means (SD), and the statistical significance of differences among groups was assessed by the Student’s t test or Mann-Whitney test, as appropriate. A p value less than 0.05 was regarded as statistically significant.
RESULTS
Efficiency and targets of adenoviral gene transfer in vivo
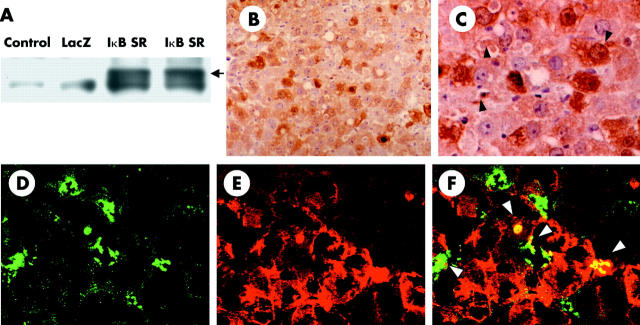
Efficiency of adenoviral gene transfer in vivo was determined by western blotting with antibodies against IκBα and immunostaining of HA tagged IκBα SR or β-galactosidase. As reported previously, western blotting using anti-IκBα antibody demonstrated high expression of HA tagged IκBα S32A/S36A only in liver homogenates from Ad5IkB infected rats, not those from uninfected or Ad5LacZ infected rats (fig 1A ▶). Immunohistochemistry using anti-HA antibody directed against HA tagged IκBα SR demonstrated that more than 80% of liver cells expressed IκBα SR (fig 1B ▶, C). This efficiency was similar to that of Ad5LacZ, which was determined by X-gal staining (data not shown). More detailed morphological analysis revealed that non-parenchymal cells of the liver as well as hepatocytes were expressing IκBα SR (fig 1C ▶, arrowheads). To further determine whether liver macrophages were infected with recombinant adenoviral vectors, immunofluorescent analysis of frozen liver tissues was carried out. Immunofluorescence against β-galactosidase and macrophage specific surface antigen revealed that approximately 60% of liver macrophages as well as about 80% of hepatocytes were immunoreactive for β-galactosidase (fig 1D ▶–F). Only a few sinusoidal endothelial cells or hepatic stellate cells were reactive for β-galactosidase according to immunofluorescence using an antirat platelet endothelial cell adhesion molecule 1 monoclonal antibody or a rabbit antirat desmin polyclonal antibody (data not shown).
Figure 1.
Efficiency of adenovirus mediated gene transfer. (A) Extracts from liver homogenate (20 μg) from uninfected, Ad5LacZ infected, and Ad5IκB infected animals were examined by western blot against inhibitor κBα (IκBα). Black arrow indicates haemagglutinin (HA) tagged IκBα super-repressor (IκB SR). (B, C) Immunohistochemistry of HA tagged IκBα SR (B ×200, C ×400). Black arrowheads indicate HA positive non-parenchymal cells in the liver. Immunofluorescent staining of surface antigen of macrophages (ED-1; D) and β-galactosidase (E), and their superimposed image (F) are presented (×400). Each datum is representative of four separate experiments.
Attenuation of short term warm ischaemia/reperfusion injury in the liver by IκBα SR
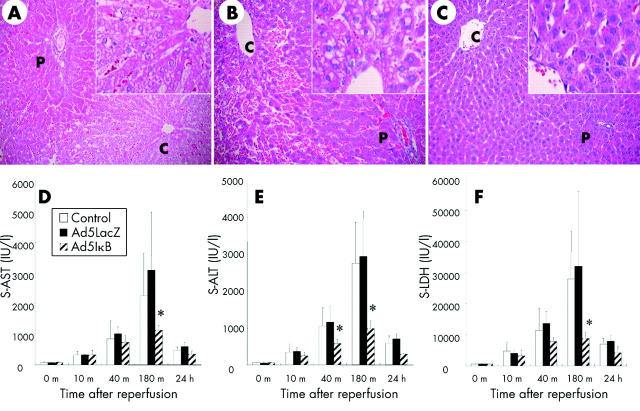
In the present study, all rats in each group survived after reperfusion, possibly reflecting the short term nature of ischaemia. At 180 minutes after reperfusion, histological examination of uninfected and Ad5LacZ infected liver tissues demonstrated ballooning, and to some extent necrosis, of hepatocytes; these findings were most evident around the central veins (fig 2A ▶, B). Inactivation of NFκB in the liver by overexpression of IκBα SR markedly attenuated these post reperfusion histological changes (fig 2C ▶). Semiquantitative scores for sinusoidal congestion, cytoplasmic vacuolisation, and hepatocytic necrosis at three hours were 1.4 (0.5), 1.1 (0.4), and 1.2 (0.5) (mean (SD)) in uninfected rats, and 1.7 (0.7), 1.3 (0.5), and 1.3 (0.6) in Ad5LacZ infected animals, respectively; these scores were 0.4 (0.5), 0.6 (0.6), and 0.4 (0.5) (mean (SD)) in Ad5IκB infected rats, all showing significant attenuation (p<0.05). Serum concentrations of AST, ALT, and LDH gradually increased in uninfected and Ad5LacZ infected groups and then returned to near normal values by 24 hours. No significant differences in serum concentrations of these liver enzymes were observed between these two groups (fig 2D ▶–F). The transient increases in serum AST, ALT, and LDH were significantly attenuated in Ad5IκB infected rats at various time points. These results indicate that inactivation of NFκB in the liver by adenoviral gene transfer of IκBα SR could ameliorate short term warm ischaemia/reperfusion injury.
Figure 2.
Histological analysis of the liver and liver function tests after ischaemia/reperfusion. Haematoxylin-eosin staining on liver tissues from uninfected (A), Ad5LacZ infected (B), and Ad5IκB infected (C) rats 180 minutes after ischaemia/reperfusion. Necrosis in hepatocytes around central veins and infiltration of inflammatory cells, which were observed in uninfected and Ad5LacZ infected rats, were almost totally blocked in Ad5IκB infected animals. Each picture is representative of six individual animals in each group. Serum (s) aspartate aminotransferase (AST) (D), alanine aminotransferase (ALT) (E), and lactate dehydrogenase (LDH) (F) levels were measured in uninfected, Ad5LacZ infected, and Ad5IκB infected rats at each time point indicated (n = 6 respectively). After reperfusion, the increase in AST, ALT, and LDH in Ad5IκB infected animals was significantly lower than those in uninfected and Ad5LacZ infected animals at the indicated times (*p<0.05).
Inhibition of nuclear translocation of NFκB and its DNA binding activity in the liver after I/R by transduction of IκBα SR
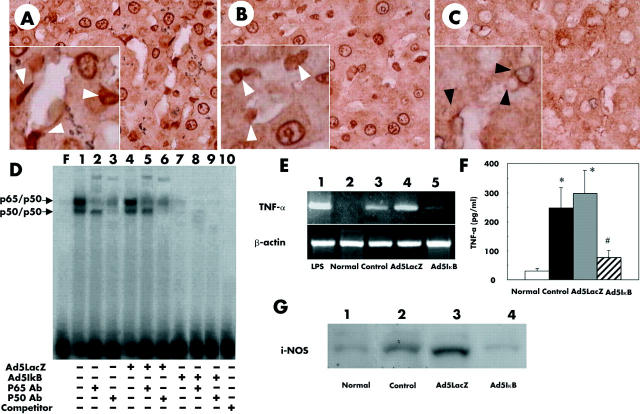
Immunohistochemistry for NFκB (p65) revealed that p65 had translocated to the nucleus in hepatocytes as well as in non-parenchymal liver cells (NPC) at three hours after reperfusion in uninfected or Ad5LacZ infected rats (fig 3A ▶, B). Adenoviral gene transfer of IkBα SR markedly suppressed this nuclear translocation of p65 in hepatocytes as well as in NPC (fig 3C ▶). The percentage of NPC in which nuclear translocation of NFκB was detected was 85% in uninfected, 87% in Ad5LacZ infected, and 15% in Ad5IkB infected livers. Transgenes of LacZ or IκBα SR had no effect on distribution of p65 in the liver before ischaemia/reperfusion (data not shown). The gel mobility shift assay for NFκB demonstrated that gene transfer of IκBα SR dramatically suppressed DNA binding activity of NFκB after ischaemia/reperfusion while its DNA binding activity had increased markedly at three hours after reperfusion in uninfected or Ad5LacZ infected liver (fig 3D ▶).
Figure 3.
Uninfected (A), Ad5LacZ infected (B), and Ad5IκB infected (C) livers with ischaemia/reperfusion (×400) underwent immunohistochemical staining of nuclear factor κB (NFκB) p65. After reperfusion, a large number of nuclei were stained with the antibody in uninfected (A) and Ad5LacZ infected (B) livers. In Ad5IκB induced livers, only a small number of positive nuclei were detected (C). High power views showed that a large number of nuclei in non-parenchymal liver cells (NPCs, white arrowheads) as well as in hepatocytes were positive for p65 in uninfected (A, inset) and Ad5LacZ infected (B, inset) livers. In contrast, in the Ad5IκB infected liver, the nuclei of approximately 85% of hepatocytes were negative for p65 staining and only about 15% of NPCs were positive for p65 (C, inset). Black arrowheads indicate nucleus of NPCs, which were negative for p65. (D) Gel shift assay for NFκB. Nuclear extracts from fresh liver samples were assayed for NFκB DNA binding activity by electrophoretic mobility shift assay using a radiolabelled consensus NFκB site probe, as described in materials and methods. A 50-fold cold competitor was added to lane 10. Antibodies against p65 (RelA) or p50 were used for the supershift assay, respectively (α-p65 lanes 2, 5, and 8; α-p50 lanes 3, 6, and 9). Data are representative of three separate experiments. (E) Messenger RNA expression of tumour necrosis factor α (TNF-α). After reperfusion, expression of TNF-α mRNA was strongly induced in uninfected and Ad5LacZ infected animals (lanes 3 and 4). In Ad5IκB infected animals (lane 5), expression of TNF-α mRNA was remarkably suppressed. Results are representative of three separate experiments. cDNA taken from rat liver injected with lipopolysaccharide (LPS) (10 mg/kg) for two hours was used as a positive control (lane 1). (F) Serum concentration of TNF-α was also determined in each animal by means of ELISA at three hours after reperfusion. (G) Western blot for inducible nitric oxide synthase (iNOS) protein. After reperfusion, increased amounts of iNOS were observed in uninfected (lane 2) and Ad5LacZ infected (lane 3) animals. In Ad5IκB infected animals (lane 4), the amount of iNOS was significantly smaller than in the other groups. Results are representative of three separate experiments. Normal, untreated normal rat liver.
We also analysed mRNA and protein expression of TNF-α and protein expression of inducible NOS (iNOS), whose genes are a target for NFκB, in the liver after reperfusion. TNF-α mRNA was barely detected in uninjured normal livers while its expression was markedly increased after reperfusion in uninfected and Ad5LacZ infected livers (fig 3E ▶, lanes 2–4). Overexpression of IκBα SR in the liver clearly blocked this increase even after reperfusion (fig 3E ▶, lane 5). Serum concentrations of TNF-α at three hours after reperfusion, which were determined by ELISA, were similar to those of mRNA (fig 3F ▶). Protein expression of iNOS markedly increased after reperfusion in uninfected and Ad5LacZ infected livers while IκBα SR dramatically suppressed iNOS expression following reperfusion (fig 3F ▶). These data suggest that transfer of IκBα SR effectively suppresses NFκB activation after reperfusion in non-parenchymal hepatic cells as well as in hepatocytes.
Attenuation of neutrophil infiltration into the liver by IkBα SR
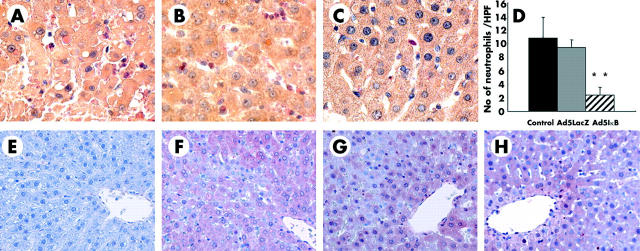
Infiltration of neutrophils, which play pivotal roles in inflammatory injury, was assessed in the liver by naphthol AS-D chloroacetate esterase staining. The number of infiltrating neutrophils obviously increased after reperfusion (three hours) in uninfected and Ad5LacZ infected livers (fig 4A ▶, B) while that in Ad5IkBα SR infected rats was not prominent, even after reperfusion (fig 4C ▶). This effect was confirmed by counting esterase positive polymorphonuclear cells under microscopic high power fields (fig 4D ▶).
Figure 4.
Infiltration of neutrophils was estimated by means of naphthol AS-D chloroacetate esterase staining in uninfected (A), Ad5LacZ infected (B), and Ad5IκB infected (C) livers. (D) Number of esterase positive polymorphonuclear cells was counted in 10 high power fields (×400) in each animal; **p<0.01. (E–H) Immnunohistochemistry of 4-hydroxy-2′-nonenal (HNE) adducts in the liver. HNE adducts were not detected in untreated normal rat livers (E) while significant amounts of HNE adducts were detected after reperfusion in uninfected (F), Ad5LacZ infected (G), and Ad5IκB infected (H) livers, particularly surrounding central veins (×200). Each picture is representative of six individual animals in each group.
Lack of attenuation by IκBα SR of lipid peroxidation after reperfusion
As a marker of lipid peroxidation resulting from oxidative stress in the liver after reperfusion, we used immunohistochemistry to detect HNE adducts in liver tissues. HNE adducts were not demonstrated in untreated normal rat livers (fig 4E ▶) while significant amounts of HNE adducts were detected after reperfusion in untreated, Ad5LacZ infected, and Ad5IκB infected livers, particularly surrounding central veins (fig 4F ▶–H). No difference in the distribution pattern of HNE adducts in the liver was observed between these three groups. These data indicate that inactivation of NFκB in the liver did not block lipid peroxidation by oxidative stress after ischaemia/reperfusion.
Blockade of nitrotyrosine production after reperfusion by overexpressed IκBα SR
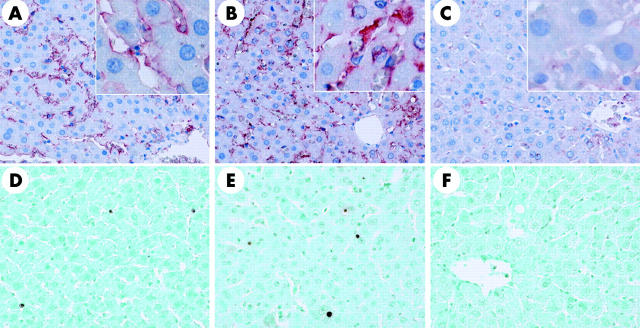
We assessed tyrosine nitration, an index of nitrosylation of proteins by peroxynitrite and/or other free radicals, because iNOS expression was affected after reperfusion. Interestingly, nitrotyrosine adducts were detected preferentially along the sinusoidal area but not in hepatocytes, after short term ischaemia/reperfusion in uninfected and Ad5LacZ infected livers (fig 5A ▶, B). This increase in nitrotyrosine expression along sinusoids was dramatically suppressed in Ad5IκB infected livers, suggesting that IκBα SR attenuated ischaemia/reperfusion injury through blockade of nitric oxide production by non-parenchymal hepatic cells (fig 5C ▶).
Figure 5.
Immnunohistochemistry of nitrotyrosine (A–C) and single strand DNA (ssDNA) (D–F) in the liver. Nitrotyrosine adducts were detected preferentially along the sinusoidal area, but not in hepatocytes, after short term ischaemia/reperfusion in uninfected (A) and Ad5LacZ infected (B) livers. This increase in nitrotyrosine expression along sinusoids was dramatically suppressed in Ad5IκB infected livers (C) (×200). A relatively small number of ssDNA containing nuclei were detected along the sinusoidal area at three hours after reperfusion in uninfected (D) and Ad5LacZ infected (E) livers, while occasionally such nuclei were also detected among hepatocytes. Gene transfer of IκBα SR suppressed the appearance of ssDNA containing cells after reperfusion (F) (×200). Each picture is representative of six individual animals in each group.
Suppression by IκBα SR of non-parenchymal cell apoptosis after reperfusion
As the appearance of apoptotic cells in hepatic ischaemia/reperfusion injury remains controversial, we assessed apoptosis in the liver after reperfusion in our model. Using an antibody against single stranded DNA (ssDNA), a relatively small number of apoptotic cells were detected along the sinusoidal area at three hours after reperfusion in uninfected and Ad5LacZ infected livers, while occasionally apoptosis was also detected among hepatocytes (fig 5D ▶, E). Gene transfer of IκBα SR suppressed the appearance of ssDNA positive cells after reperfusion (fig 5F ▶), even though over 80% of hepatocytes were transfected with Ad5IκB according to anti-HA immunostaining (fig 1B ▶, C). Immunohistological analysis at 12 hours after reperfusion detected ssDNA in a few non-parenchymal cells, but not in hepatocytes, even in uninfected and Ad5LacZ infected livers (data not shown). These data suggest that proapoptotic signalling is not the main cause of reperfusion injury, at least after reperfusion following short term warm ischaemia.
DISCUSSION
In pathogenetic sequences underlying hepatic ischaemia/reperfusion injury, activated liver macrophages (Kupffer cells) have been assigned critical roles.5–7 During the cascade leading to activation of Kupffer cells, DNA binding activity of NFκB, a key regulator of genes encoding inflammatory cytokines, acute phase response proteins, and cell adhesion molecules,36,37 is upregulated through several mechanisms, including pathways dependent on oxidative stress or endotoxin.14–16,18 In the present study, we demonstrated that inactivation of NFκB in the liver using adenoviral gene transfer of IκBα SR effectively blocked a short term warm ischaemia/reperfusion injury that was transient and sublethal, showing a good resemblance to liver injury observed clinically after an intraoperative manipulation, Pringle’s manoeuvre.
Immunohistochemical analysis demonstrated that the adenoviral gene transfer method used in the present study (dose 5×109 pfu/rat) successfully delivered LacZ or IκBα SR into both parenchymal and non-parenchymal cells, particularly Kupffer cells (fig 1B ▶–F). Overexpressed IκBα SR effectively blocked nuclear translocation of NFκB (p65) in both cell types after reperfusion (fig 3C ▶), abolishing increased p65 DNA binding activity that was observed in uninfected or Ad5LacZ infected rats (fig 3D ▶). This effect resulted in marked suppression of genes whose expression is regulated by NFκB, such as TNF-α and iNOS, even after reperfusion (fig 3E ▶–G). Considering that a variety of inflammatory cytokines regulated by NFκB are produced predominantly by non-parenchymal cells in the liver,11,16 our results suggest that inactivation of Kupffer cells through blocking NFκB from DNA binding is possibly the major mechanism underlying the protective effect of IκBα SR observed in the present study.
Among potential mechanisms contributing to hepatic ischaemia/reperfusion injury,6 formation of ROS and reactive nitrogen species after reperfusion has been recognised as a critical factor.3,4,38,39 Generally, SOD scavenges superoxide to form oxygen and hydrogen peroxide, which is further detoxified via catalase to produce water and oxygen. An excessive amount of hydrogen peroxide, however, can undergo a one electron reduction with Fe (II) to form Fe (III) and the highly toxic hydroxyl radical, a very reactive species that rapidly induces lipid peroxidation.40 In the present study, inactivation of NFκB in parenchymal and non-parenchymal hepatic cells did not affect lipid peroxidation after reperfusion (fig 4E ▶–H). This lipid peroxidation after reperfusion possibly accounts for the incomplete nature of suppression of transient increases in serum AST, ALT, and LDH in Ad5IκB infected livers (fig 2D ▶ to F), even though they appeared nearly intact by haematoxylin-eosin staining (fig 2C ▶).
Nitric oxide is another bioregulatory molecule produced in the liver after reperfusion; production involves upregulated expression of iNOS which has been implicated in the pathogenesis of ischaemia/reperfusion injury.6,38 While nitric oxide can directly affect cell signalling, it also forms peroxynitrite, a highly reactive nitrogen species produced by reaction with ROS that carries out nitration of tyrosine residues in proteins. In the present study, iNOS expression was blocked dramatically via NFκB inactivation (fig 3G ▶), which should suppress nitric oxide synthesis even after reperfusion of Ad5IκB infected livers. If nitric oxide synthesis is blocked, formation of peroxynitrite should be attenuated. This hypothesis was supported by suppressed expression of nitrotyrosine after reperfusion in these livers (fig 5C ▶). Thus we conclude that suppression of iNOS induction by IκBα SR in non-parenchymal cells, possibly Kupffer cells, can account for part of the beneficial effect from IκBα SR gene transfer in the present study.
While the generally accepted mechanism of hepatic reperfusion injury is cell damage involving oncotic necrosis,41 several recent reports have proposed that apoptotic cell death during hepatic ischaemia/reperfusion also may participate in the mechanism of the injury.21–23 NFκB is known as an antiapoptotic transcription factor in the liver31,42 and its blockade results in frequent occurrence of apoptosis of hepatocytes under proapoptotic stimuli.28–31 In the present study, we blocked NFκB activation after reperfusion in both parenchymal and non-parenchymal cells by transfer of IκBα SR. However, we observed no remarkable appearance of apoptotic cells in Ad5IκB infected livers even at 12 hours after reperfusion, while a few apoptotic cells, mainly non-parenchymal cells, were detected in uninfected and Ad5LacZ infected livers. Because the ssDNA staining might not be a definitive tool for detecting apoptotic cells, we cannot conclude that apoptotic cell death does not participate in the mechanism underlying reperfusion injury in short term warm total hepatic ischaemia. From our data, however, we speculate that apoptotic cell death does not play a major role in our model. Inactivation of NFκB in donor livers was found to increase histologically evident tissue injury and apoptosis after experimental liver transplantation representing cold hepatic ischaemia/reperfusion.43 Differences in animal models and duration of ischaemia may account for the disagreement between that report and our study.
In summary, we successfully attenuated hepatic reperfusion injury after short term warm total ischaemia by delivering the IκBα SR gene to the rat liver. Because NFκB may be required for proliferation of intact hepatocytes,31 non-parenchymal cell selective as opposed to non-selective inactivation of NFκB should be more beneficial if the strategy of the present study were applied to clinical situations. Moreover, relatively short term inactivation of NFκB limited to non-parenchymal cells would be safer than adenoviral gene expression of IκBα SR for 1–2 weeks. For example, Kupffer cell selective transfer of NFκB decoy, which binds NFκB and blocks its translocation to the nucleus,11 may come to represent a clinically important way of preventing ischaemia/reperfusion injury during hepatic surgery.
Acknowledgments
The authors thank Miss Keiko Mitani for technical assistance.
This study was supported in part by Grant-in-Aid for Scientific Research (B) (12470262, 14370394 and 16390385) from the Japan Society for the Promotion of Science (JSPS) and Grant-in-Aid for Exploratory Research (14657313) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to YI.
Abbreviations
NFκB, nuclear factor κB
IκB, inhibitor κB
IκBα SR, IκBα super-repressor
ROS, reactive oxygen species
TNF-α, tumour necrosis factor α
HA, haemagglutinin
AST, aspartate aminotransferase
ALT, alanine aminotransferase
LDH, lactate dehydrogenase
iNOS, inducible nitric oxide synthase
HNE, 4-hydroxy-2′-nonenal
RT-PCR, reverse transcriptase-polymerase chain reaction
NPC, non-parenchymal liver cells
ssDNA, single stranded DNA
Conflict of interest: None declared.
REFERENCES
- 1.Sugawara Y, Kubota K, Ogura T, et al. Increased nitric oxide production in the liver in the perioperative period of partial hepatectomy with Pringle’s maneuver. J Hepatol 1998;28:212–20. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell-Kenkel JC, Currin RT, Tanaka Y, et al. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology 1989;10:292–9. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol 1991;260:G355–62. [DOI] [PubMed] [Google Scholar]
- 4.Lentsch AB, Kato A, Yoshidome H, et al. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000;32:169–73. [DOI] [PubMed] [Google Scholar]
- 5.Liu P, McGuire GM, Fisher MA, et al. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock 1995;3:56–62. [PubMed] [Google Scholar]
- 6.Jaeschke H . Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 2003;284:G15–26. [DOI] [PubMed] [Google Scholar]
- 7.Lichtman SN, Lemasters JJ. Role of cytokines and cytokine-producing cells in reperfusion injury to the liver. Semin Liver Dis 1999;19:171–87. [DOI] [PubMed] [Google Scholar]
- 8.Bremer C, Bradford BU, Hunt KJ, et al. Role of Kupffer cells in the pathogenesis of hepatic reperfusion injury. Am J Physiol 1994;267:G630–6. [DOI] [PubMed] [Google Scholar]
- 9.Zhong Z, Qu W, Connor HD, et al. Inactivation of Kupffer cells minimizes reperfusion injury in fat-loaded livers from ethanol-treated rats. Transplant Proc 1995;27:528–30. [PubMed] [Google Scholar]
- 10.Mosher B, Dean R, Harkema J, et al. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res 2001;99:201–10. [DOI] [PubMed] [Google Scholar]
- 11.Ogushi I, Iimuro Y, Seki E, et al. Nuclear factor kappa B decoy oligodeoxynucleotides prevent endotoxin-induced fatal liver failure in a murine model. Hepatology 2003;38:335–44. [DOI] [PubMed] [Google Scholar]
- 12.Luckey SW, Taylor M, Sampey BP, et al. 4-Hydroxynonenal decreases interleukin-6 expression and protein production in primary rat Kupffer cells by inhibiting nuclear factor-kappaB activation. J Pharmacol Exp Ther 2002;302:296–303. [DOI] [PubMed] [Google Scholar]
- 13.Essani NA, McGuire GM, Manning AM, et al. Endotoxin-induced activation of the nuclear transcription factor kappa B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J Immunol 1996;156:2956–63. [PubMed] [Google Scholar]
- 14.Bowie A, O’Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol 2000;59:13–23. [DOI] [PubMed] [Google Scholar]
- 15.Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol 2000;32:508–15. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler MD, Yamashina S, Froh M, et al. Adenoviral gene delivery can inactivate Kupffer cells: role of oxidants in NF-kappaB activation and cytokine production. J Leukoc Biol 2001;69:622–30. [PubMed] [Google Scholar]
- 17.Hayakawa M, Miyashita H, Sakamoto I, et al. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J 2003;22:3356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 1999;162:3749–52. [PubMed] [Google Scholar]
- 19.O’Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today 2000;21:206–9. [DOI] [PubMed] [Google Scholar]
- 20.Zhang FX, Kirschning CJ, Mancinelli R, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem 1999;274:7611–14. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki H, Matsuno T, Tanaka N, et al. Activation of apoptosis during the reperfusion phase after rat liver ischemia. Transplant Proc 1996;28:1908–9. [PubMed] [Google Scholar]
- 22.Gao W, Bentley RC, Madden JF, et al. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology 1998;27:1652–60. [DOI] [PubMed] [Google Scholar]
- 23.Kohli V, Selzner M, Madden JF, et al. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation 1999;67:1099–105. [DOI] [PubMed] [Google Scholar]
- 24.Sindram D, Porte RJ, Hoffman MR, et al. Synergism between platelets and leukocytes in inducing endothelial cell apoptosis in the cold ischemic rat liver: a Kupffer cell-mediated injury. Faseb J 2001;15:1230–2. [DOI] [PubMed] [Google Scholar]
- 25.Gujral JS, Bucci TJ, Farhood A, et al. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology 2001;33:397–405. [DOI] [PubMed] [Google Scholar]
- 26.Wang CY, Mayo MW, Korneluk RG, et al. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 1998;281:1680–3. [DOI] [PubMed] [Google Scholar]
- 27.Lee R, Collins T. Nuclear factor-kappaB and cell survival: IAPs call for support. Circ Res 2001;88:262–4. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Bialik S, Jones BE, et al. NF-kappaB inactivation converts a hepatocyte cell line TNF-alpha response from proliferation to apoptosis. Am J Physiol 1998;275:C1058–66. [DOI] [PubMed] [Google Scholar]
- 29.Hatano E, Bradham CA, Stark A, et al. The mitochondrial permeability transition augments Fas-induced apoptosis in mouse hepatocytes. J Biol Chem 2000;275:11814–23. [DOI] [PubMed] [Google Scholar]
- 30.Jones BE, Lo CR, Liu H, et al. Hepatocytes sensitized to tumor necrosis factor-alpha cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. J Biol Chem 2000;275:705–12. [DOI] [PubMed] [Google Scholar]
- 31.Iimuro Y, Nishiura T, Hellerbrand C, et al. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest 1998;101:802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamagami K, Yamamoto Y, Ishikawa Y, et al. Effects of geranyl-geranyl-acetone administration before heat shock preconditioning for conferring tolerance against ischemia-reperfusion injury in rat livers. J Lab Clin Med 2000;135:465–75. [DOI] [PubMed] [Google Scholar]
- 33.Uchinami H, Yamamoto Y, Kume M, et al. Effect of heat shock preconditioning on NF-kappaB/I-kappaB pathway during I/R injury of the rat liver. Am J Physiol Gastrointest Liver Physiol 2002;282:G962–71. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ. Role of neutrophils during the first 24 hours after liver ischemia and reperfusion injury. Transplant Proc 1994;26:3695–700. [PubMed] [Google Scholar]
- 35.Yam LT, Li CY, Crosby WH. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol 1971;55:283–90. [DOI] [PubMed] [Google Scholar]
- 36.Muller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology 1993;187:233–56. [DOI] [PubMed] [Google Scholar]
- 37.Bradham CA, Plumpe J, Manns MP, et al. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol 1998;275:G387–92. [DOI] [PubMed] [Google Scholar]
- 38.Hon WM, Lee KH, Khoo HE, et al. Nitric oxide in liver diseases: friend, foe, or just passerby? Role of nitric oxide in inflammation. Ann N Y Acad Sci 2002;962:275–95. [DOI] [PubMed] [Google Scholar]
- 39.Laroux FS, Pavlick KP, Hines IN, et al. Role of nitric oxide in inflammation. Acta Physiol Scand 2001;173:113–18. [DOI] [PubMed] [Google Scholar]
- 40.Mathews WR, Guido DM, Fisher MA, et al. Lipid peroxidation as molecular mechanism of liver cell injury during reperfusion after ischemia. Free Radic Biol Med 1994;16:763–70. [DOI] [PubMed] [Google Scholar]
- 41.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 2003;125:1246–57. [DOI] [PubMed] [Google Scholar]
- 42.Beg AA, Sha WC, Bronson RT, et al. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 1995;376:167–70. [DOI] [PubMed] [Google Scholar]
- 43.Bradham CA, Schemmer P, Stachlewitz RF, et al. Activation of nuclear factor-kappaB during orthotopic liver transplantation in rats is protective and does not require Kupffer cells. Liver Transpl Surg 1999;5:282–93. [DOI] [PubMed] [Google Scholar]