Abstract
Some chronic pain conditions are maintained or enhanced by sympathetic activity. In animal models of pathological pain, abnormal sprouting of sympathetic fibers around large- and medium-size sensory neurons is observed in dorsal root ganglia (DRG). Large and medium size cells are also more likely to be spontaneously active, suggesting that sprouting may be related to neuron activity. We previously showed that sprouting could be reduced by systemic or locally applied lidocaine. In the complete sciatic nerve transection model in rats, spontaneous activity initially originates in the injury site; later, the DRG become the major source of spontaneous activity. In this study, spontaneous activity reaching the DRG soma was reduced by early nerve blockade (local perfusion of the transected nerve with TTX for the first 7 days after injury). This significantly reduced sympathetic sprouting. Conversely, increasing spontaneous activity by local nerve perfusion with K+ channel blockers increased sprouting. The hyperexcitability and spontaneous activity of DRG neurons observed in this model were also significantly reduced by early nerve blockade. These effects of early nerve blockade on sprouting, excitability, and spontaneous activity were all observed 4 to 5 weeks after the end of early nerve blockade, indicating that the early period of spontaneous activity in the injured nerve is critical for establishing the more long-lasting pathologies observed in the DRG. Individual spontaneously active neurons, labeled with fluorescent dye, were 5–6 times more likely than quiescent cells to be co-localized with sympathetic fibers, suggesting a highly localized correlation of activity and sprouting.
Keywords: ectopic discharge, basket formation, local anesthetics
Introduction
In some chronic pain patients, pain and hyperalgesia are maintained by efferent noradrenergic sympathetic activity and circulating catecholamines (sympathetically maintained pain, SMP) (Roberts 1986), and may be partly responsive to sympathetic blockade, while in others the pain is sympathetically independent (Campbell et al. 1988). SMP may be a component of many painful conditions such as complex regional pain syndrome (CRPS), phantom pain, neuralgias, and herpes zoster. The lack of understanding of the neurophysiological mechanisms by which the sympathetic system invades the peripheral sensory system has hindered progress in the treatment of these painful conditions.
Clinical observations and animal studies have shown that coupling of the sympathetic nervous system and the sensitized sensory nervous system is important for development of SMP (Janig et al. 1996). An abnormally enhanced communication between these two systems may occur under a variety of pathologic conditions. For example, sympathetic stimulation may excite sensory neurons in animals with inflamed peripheral tissue or after peripheral nerve injury (Devor et al. 1994; Xie et al. 1995). Chemical or surgical sympathectomy relieves allodynia and hyperalgesia, and improves chronic pain behavior in several animal models (Choi et al. 1994; Kim et al. 1993; Kinnman and Levine 1995; Malmberg and Basbaum 1998; Seltzer and Shir 1991). These observations suggest that increased activity of the sympathetic nervous system may contribute to the enhanced sensitivity to painful stimuli observed in chronic pain models, although the behavioral relevance of sympathetic activity in animal models of pain has been somewhat controversial [e.g., (Habler 2000; Kim et al. 1999)].
Sympathetic-sensory coupling may occur either centrally or peripherally. The dorsal root ganglion (DRG) has been identified as one important site for peripheral sympathetic-sensory coupling (McLachlan et al. 1993). Within the normal DRG, sympathetic axons are only found accompanying blood vessels (Kummer et al. 1990). Following peripheral nerve injury, sympathetic efferent fibers extensively sprout into both DRG and spinal nerves. Sprouting fibers sometimes form distinctive basketlike webs -- sympathetic “baskets” or tyrosine hydroxylase [TH]-immunoreactivity [IR] rings wrapping around medium and large DRG neurons (Chung et al. 1996; Lee et al. 1998; McLachlan et al. 1993; Ramer and Bisby 1997). Pain induced by localized inflammation of the DRG or mechanical compression of the DRG in the absence of nerve injury can also be accompanied by sympathetic sprouting in the DRG (Chien et al. 2005; Xie et al. in press). Most recently, sympathetic sprouting was observed in the glabrous skin after partial nerve injury (Grelik et al. 2005; Yen et al. 2006).
Abnormal spontaneous activity is also a common feature of many different animal pain models (Amir 1999; Govrin-Lippmann and Devor 1978; Hu and Xing 1998; Liu et al. 2000; Song et al. 1999; Study and Kral 1996; Xie et al. in press). Large and medium diameter cells, which preferentially acquire sympathetic baskets, also show a much higher incidence of spontaneous activity in various neuropathic pain models. This suggested the possibility that spontaneous activity might be one cause of sympathetic sprouting. In support of this idea, we recently found (Zhang et al. 2004) that systemic application of the local anesthetic lidocaine could reduce sympathetic sprouting in two different pain models, the neuroma model (sciatic axotomy) originally described by Wall (Wall et al. 1974), and the spinal nerve ligation model (Kim and Chung 1992). The inhibition of sprouting outlasted the duration of lidocaine application by at least 7 days. Sprouting could also be reduced by local application of lidocaine to the nerve trunk proximal to the injury site in the sciatic axotomy model. In the present study, we examined in more detail the possible connection between spontaneous activity and sympathetic sprouting in the sciatic axotomy model. We determined the effects on sympathetic sprouting of either enhancing the spontaneous activity originating at the injury site, or of preventing spontaneous activity from reaching the DRG by local nerve blockade with tetrodotoxin (TTX). We also conducted histological analyses on identified individual neurons, to determine whether spontaneously active cells were more likely to be the target of sympathetic sprouts.
Methods
Surgery
All the surgical procedures were reviewed and approved by the University of Arkansas for Medical Sciences and University of Cincinnati Institutional Animal Care and Use Committee (IACUC).
Surgical procedure for complete sciatic nerve transection
Young female Sprague-Dawley rats weighing 80–100 g at the time of surgery were anesthetized with pentobarbital sodium (40 mg/kg ip). The right sciatic nerve was exposed at mid-thigh level, tightly ligated (3-0 silk suture) and transected 5 mm distally from the ligature as described previously (Wall et al. 1974). The incision was then closed in layers and a single dose of penicillin (8000 IU) was given to all rats.
Surgical procedure for implantation of osmotic minipumps for local delivery of chemicals to the nerve trunk
At the time of sciatic nerve ligation/transection, osmotic minipumps (Model 2002, Durect Corporation, Cupertino, CA, USA) preloaded with artificial cerebrospinal fluid (ACSF, in mM: NaCl 130, NaHCO3 24, KCl 3.5, NaH2PO4 1.25, MgCl2 1.2, CaCl2 1.2, and Dextrose 10, pH=7.3), along with TTX (780 μM in ACSF), tetraethylammonium chloride (TEA; 10 mM in ACSF) or 4-aminopyridine (4-AP;10 mM in ACSF) (Sigma Chemical Co., St Louis, MO, USA) were implanted subcutaneously after 4 hr incubation in saline at 37°C. The flow rate was 1 μl/h for 7 days (i.e., a total of 130 nanomoles of TTX or 1.7 μmoles of TEA or 4-AP was delivered over the course of 7 days). To block the injured sciatic nerve, the minipump filled with TTX was attached to a piece of silicone tubing (50–60 mm in length, 0.64 mm ID ×1.19 mm OD; Dow Corning Corporation Midland, MI, USA) which was led to the sciatic nerve about 5–10 mm proximal to the ligature. The silicone tubing was slit over the final 10 mm. The two halves of the slit end of the tubing were placed surrounding the sciatic nerve and tightened together at the very end with 6–0 silk suture as previously described (Xie et al. 2005). The TTX pump was in place before the actual transection of the nerve and hence the procedure included blockade of the injury discharge. In control experiments, the TTX pump was placed on the uninjured contralateral sciatic nerve to rule out the possibility of a systemic effect on sympathetic sprouting or abnormal activity by TTX. To enhance the firing of the injured axons, the injury site of the sciatic nerve was infused with osmotic pump preloaded with TEA or 4-AP solution through a fine PE tubing which was inserted into the sciatic nerve through the transected stump. The sciatic nerve and the inserted tubing were then tightly ligated with 3-0 silk suture at 5 mm proximal to the injury site.
In vitro microelectrode intracellular recording
At postoperative days (POD) 35–49, intracellular recording was performed on sensory neurons in whole DRG preparations isolated from normal and axotomized rats. As described in previous publications (Liu et al. 2002; Zhang et al. 1999), the ipsilateral L4 or L5 DRG was placed in the recording chamber and mounted on the stage of an upright microscope (BX50-WI, Olympus). A U-shaped stainless steel rod with 3 pieces of fine nylon filaments crossed from one side to the other was used to gently hold the ganglion in place within the recording chamber. The DRG was continuously perfused with oxygenated ACSF at a rate of 2–5 ml/min. The temperature was maintained at 36 ± 1ºC by a temperature controller.
DRG cells were visualized under differential interference contrast (DIC). Images of the DRG neurons were clearly visible on a high resolution video monitor fed by a cooled CCD camera (CCD300T, Dage MTI, Michigan City, IN, USA). Intracellular, electrophysiological recordings were made from each cell with a microelectrode filled with 2M potassium acetate (pH=7.2). Satisfactory recordings were obtained with electrodes of 50–80 MΩ. Before electrode penetration, the DRG soma was visually classified by the diameter of its soma as small (<30 μm), medium (30–50 μm) or large (>50 μm). The electrophysiological data were collected with the use of single-electrode continuous current-clamp (AxoClamp-2B, Axon Instruments, Inc., Union City, CA, USA) and analyzed with pClamp 9 software (Axon Instruments, Inc).
In experiments to determine the incidence of spontaneous activity, individual DRG neurons were first impaled with a recording electrode. If spontaneous activity was absent during the first 60 sec of the impaling, incremental currents (up to 4 nA) were then injected to ensure that action potentials could be evoked indicating a healthy cell. If any spontaneous activity was present, then we would wait for 3 min to ensure that the activity was not caused by penetrating the somata with the sharp electrode. For experiments investigating co-localization of sympathetic fibers with identified cells, after the initial recording was completed and the recording electrode removed, a second, higher resistance, dye-filled electrode was used to impale the same neuron for intracellular injection of Lucifer Yellow, a technique similar to that described by Lawson and her colleagues (Djouhri and Lawson 1999; Djouhri et al. 2003; Fang et al. 2002; Lawson et al. 1997). A successful injection was verified under the same microscope by briefly exposing the neuron to UV light with proper emission/excitation filter set. The whole DRG was then fixed for TH staining (see below) to determine if the labeled neurons were surrounded by or co-localized with sympathetic fibers. In other experiments, some quiescent neurons were labeled using the same method.
In some experiments, in addition to determining spontaneous activity, we characterized the membrane properties and excitability more fully. Measured parameters were the threshold current (rheobase), action potential (AP) threshold, resting membrane potential (Vm), input resistance (Rin) and afterhyperpolarization (AHP) of the recorded DRG cell. The Vm was taken 3 min after a stable recording was first obtained. Depolarizing currents of 0.05 to 4 nA (100 ms duration) were delivered in increments of 0.05 nA until an action potential (AP) was evoked. The threshold current was defined as the minimum current required to evoke an action potential. The AP voltage threshold was defined as the first point on the rising phase of the spike at which the change in voltage exceeded 50 mV/ms. The duration of the AP was measured at the threshold voltage. The AP amplitude was measured between the peak and the AP threshold. The input resistance (Rin) for each cell was obtained from the slope of a steady-state I–V plot in response to a series of hyperpolarizing currents, 100 ms duration delivered in steps of 0.05 nA from 0.2 to −2 nA. The afterhyperpolarization (AHP) amplitude was measured between the maximum hyperpolarization and the final plateau voltage, and the AHP duration was measured at the voltage half way between these two points.
Extracellular fiber recording
For some experiments (Table 2), spontaneous activity was determined with extracellular fiber recording instead of intracellular recording. The spontaneous activity of the dorsal root fibers was extracellularly recorded using in vitro microfilament dissection technique (Zhang et al. 1997). Briefly, the L4 or L5 DRG with attached dorsal root and initial segment of sciatic nerve was removed and maintained in oxygenated ACSF at 37°C in a recording chamber. The Govrin-Lippmann and Devor method (Govrin-Lippmann and Devor 1978) was used to determine the incidence of ongoing discharge in those dorsal root fibers in which conduction velocity was able to be measured by electrical stimulation of the dorsal root of L4 and L5: briefly, for each fiber bundle of approximately equal diameter (40~50 μm), the sciatic nerve was stimulated with a gradually increasing intensity of current (0.1–0.5 ms square wave pulses, 1–2 Hz) up to 10 mA, resulting in a gradual recruitment of A-fibers then C-fibers until the number of fibers saturated. The total number of different spontaneous action potential waveforms was counted and summed for all strands and divided by the total number of activatable fibers (in all strands) recruited by electrical stimulation of the sciatic nerve to obtain the incidence of ectopic discharge.
Table 2.
Effect of K+ channel blockers on spontaneous activity
| Axotomy +ACSF (n=3 animals) | Axotomy +TEA (n=3 animals) | Axotomy +4-AP (n=3 animals) | |
|---|---|---|---|
| Incidence of Spontaneous activity | 21.1% ± 0.5 | 31.5% ± 1.0 | 31.8% ± 2.9 |
| P value vs. ACSF | - | 0.0001 | .0001 |
| Total number of fibers recorded | 503 | 555 | 576 |
Incidence of spontaneous activity determined on POD 7 after perfusion of the injured nerve with K+ blockers TEA (10 mM) or 4-AP (10 mM), or with vehicle (ACSF). The pump was still in place at the time spontaneous activity was measured. Standard errors are based on comparison between different animals. P values are based on Fisher’s exact tests on the combined data. There was no significant difference between the TEA and 4-AP groups.
TH immunostaining of sympathetic fibers in sectioned and whole mount DRGs
For measurement of sympathetic fiber density in sectioned DRGs (Figure 4), rats were anesthetized with pentobarbital sodium (40 mg/kg, i.p.) and fixed by perfusing 200–300 ml of Zamboni’s fixative through the left ventricle of the heart. The bilateral DRGs of L4 and L5 were removed, post-fixed in the fixative for 2 hr at 4 °C, and embedded in gelatin overnight. The ganglia were horizontally sectioned with a Vibratome at a thickness of 40 μm.
Figure 4.

Manipulation of spontaneous activity in injured nerve alters sympathetic fiber density in DRG. TH-positive fiber density was measured in 40 μm DRG sections as described. Sciatic nerve was perfused in all experimental groups. A: Sprouting, measured on POD 35–42 (i.e, 28 – 35 days after the end of the nerve blockade period), is significantly increased in transected vs. uninjured nerve (p< 0.001); perfusion of the transected nerve with TTX during the first 7 days after injury significantly (p<0.001) reduces this sprouting, back to levels statistically indistinguishable from that seen in normal uninjured nerve (one-way ANOVA with Tukey’s post test). B. Perfusing the transected nerve with potassium channel blockers TEA (10 mM) or 4-AP (10 mM) significantly (p<0.001) increased sympathetic fiber density compared to perfusion with vehicle (ACSF). The differences between the two channel blocker groups were not significant (one-way ANOVA with Tukey’s post test). In this experiment sprouting was measured at POD 14, 7 days after the end of channel blocker perfusion, hence the overall sprouting density in axotomized axons was lower than in Figure 4A. Sympathetic nerve sprouting is higher in L4 DRG than in L5 in this model, so data from these ganglia are presented and analyzed separately.
A whole mount DRG staining procedure was used to visualize the distribution of sympathetic fibers on the surface of the ganglion (Figure 3). The whole mount method was also used to visualize sympathetic fibers near individual neurons following intracellular recording and dye injection (Figures 5, 6), because only neurons on the ganglion surface are accessible to these procedures. For whole mount DRG staining, the unsectioned DRG was fixed in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) for 2 h. The capsule was always removed before whole mount staining or intracellular recording.
Figure 3.

Anti-TH immunostaining of sympathetic fibers in whole mount DRG. Sympathetic nerve fibers could be observed on the dorsal (top) and ventral (bottom) surfaces of both normal (A, B) and axotomized (C, D) DRGs, however, the axotomized DRGs had much higher fiber density on both sides of the ganglion. Scale bar=200μm.
Figure 5.
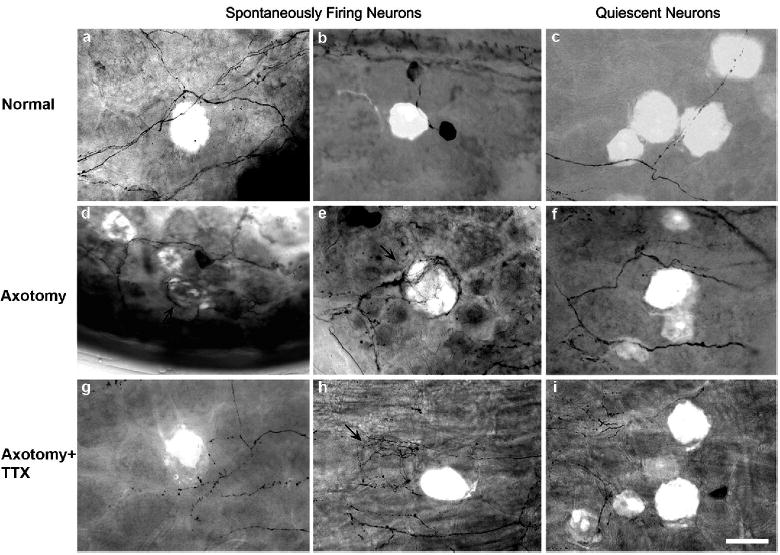
Examples of spontaneously active (left, center panels) or quiescent neurons (right panels) filled with fluorescent dye after recording followed by TH staining in whole mount DRGs. Compared with normal DRG (a & b), sympathetic fiber endings were more often co-localized with spontaneously active neurons after sciatic nerve transection. Basket structures (arrows) are formed around (d & e) or near (h) spontaneously active neurons. The quiescent neurons (c, f, i) were less specifically co-localized with sympathetic fiber endings. Scale=50 μm.
Figure 6.

The percentages of labeled spontaneously active and quiescent cells that had sympathetic fiber endings localized near their soma are indicated for each experimental group. In normal animals, the differences between spontaneously active and quiescent cells were not significant (p = 0.5); * - in all groups with sciatic nerve transection the differences were significant (Fishers exact test; p = 0.02 for the TTX group and p<0.001 for the axotomized and axotomized perfused with ACSF groups). Standard error bars are based on differences between experiments in different animals.
After blocking with 10% normal goat serum in PBS for 30 min, the tissue sections or whole DRGs were incubated in antibodies to TH (from Pel-Freeze, Rogers, AR, USA) at a dilution of 1:1,000 for 24 h at 4 °C, followed by the reaction with biotinylated secondary antibody and, finally, with Vector ABC reagent. The TH antibody is an affinity purified polyclonal rabbit antibody; the antigen is purified denatured rat TH isolated from pheochromocytoma cells. Specificity is demonstrated by ability to stain the noradrenergic and dopamine systems in rat brain with low background. Triton-X (0.3%) was used in all reaction solutions to enhance antibody penetration. Immunoreaction products were visualized by the diaminobenzidine method in the presence of H2O2 in 0.1 M phosphate buffer. Tissue sections were then mounted on gelatin-coated slides, air dried, dehydrated and coverslipped for light-microscopic observation. Whole mount DRGs were placed on slides coverslipped with anti-fade mounting medium for florescent light-microscopic observation.
Measurement of TH-IR fiber density in the DRG sections
Using ImagePro Plus software (Media Cybernetics, Inc., Silver Spring, MD, USA), images from all sections of each DRG were captured under a light microscope (20x) equipped with a SPOT Insight colored digital camera (Diagnostic Instruments, Inc., Burlingame, CA, USA). Using the ImagePro program, TH-IR fibers in each image were traced, and a new image containing all the traced fibers was generated from the original image. After applying a “thinning filter”, all traced fibers were converted to single-pixel lines. The total number of pixels within each image was then counted and converted to fiber length (in μm). The numerical density of the TH-IR fiber within each image was obtained by dividing the total fiber length by the size of the measured area (in mm2). The density of TH-IR fibers for each DRG was calculated after all images from all sections were measured and counted.
Determination of co-localization of TH-immunoreactive (IR) fibers and individual DRG neurons in whole mount ganglia
After individual neurons were identified by intracellular recording as spontaneously active (or quiescent, in separate experiments) and injected with Lucifer Yellow as described above, the whole DRG was fixed and stained for TH as described above. Whole mount preparations were observed with an upright microscope (Olympus BX50-WI). Dye-injected cells were scored as being co-localized with sympathetic sprouts if TH-positive fibers were observed to end anywhere on the cell surface. Fibers passing completely across the neuron but not ending there were not scored as co-localized (e.g. see Figure 5a). It was also noted whether cells had more extensive sprouting in the form of rings or basket formations.
Data analysis
All data are expressed as mean ± standard error of the mean (S.E.M). The proportions of spontaneously active fibers or neurons in different experimental groups, and of neurons with sympathetic fibers around them, were compared using Fisher’s exact test. Fiber density data were analyzed using ANOVA with Tukey’s posthoc test. Average values for electrophysiological parameters were compared using Students t-test.
Results
1. The development of hyperexcitability and spontaneous activity of DRG neurons after sciatic nerve transection depends on ectopic discharge from the injury site
1.1 Early nerve blockade reduced spontaneous activity in DRG neurons after sciatic axotomy
Between POD 35 and 49 days, the incidence of spontaneous activity was measured using whole-DRG intracellular recording in the following groups of DRG neurons: 2390 neurons from rats with axotomy but without any treatment (n=21), 915 axotomy with early nerve blockade (TTX perfusion of the nerve proximal to the injury site for the first 7 days POD, n=9), 404 axotomy with early ACSF perfusion (n=8), and 782 normal (n=10). The incidence of spontaneous activity in normal DRG was around 5% in both large- (21/415) and medium- (10/200) sized neurons. Sciatic nerve transection dramatically increased the incidence to 11.5 % (156/1352; p <0.001) in large- and 19.5% (119/609; p < 0.001) in medium-sized neurons, whereas, early nerve blockade significantly reversed this abnormal high incidence of spontaneous activity in DRG neurons, returning it to normal levels of 3.3 % (17/520; p = 0.18 vs. normal) in large-size neurons, and towards normal levels of 10.4% (26/249; p = 0.04 vs. normal) in medium-sized neurons. Perfusion with ACSF (vehicle) instead of TTX was indistinguishable from axotomy with no perfusion (p = 0.45, large cells; p = 0.30, medium cells). The effects of TTX perfusion were unlikely to be due to systemic effects, because perfusion of the (uninjured) contralateral nerve with the same concentration of TTX and at the same low pump rate (1 μl/h) as was used on the injured nerve was ineffective at reducing spontaneous activity, which remained at 19% in large diameter cells and 34% in medium diameter cells, not significantly lower than the axotomy perfused with ACSF group (large cells, p = 0.45; medium cells, p = 0.30). The incidence of spontaneous activity in C-cells after axotomy was already so low, (5/439, 1.1%; p = 0.60 vs. normal) that it was not possible to measure statistically significant decreases following early nerve blockade (Figure 1).
Figure 1.

Early nerve blockade decreased the incidence of spontaneous activity in DRG neurons following sciatic nerve transection. Sciatic nerve transection caused high incidence of spontaneous activity in both large and medium-sized DRG neurons. Early nerve blockade (perfusion with TTX) inhibited the abnormal change in spontaneous activity and almost normalized it in nerve injured DRG; perfusion with vehicle (ACSF), or perfusion of the uninjured contralateral nerve with TTX, did not inhibit spontaneous activity. *, significantly different from normal group; #, significantly different from axotomy group. Standard error bars are based on comparisons between different animals.
The particular patterns of spontaneous activity were also compared between neurons in the different experimental groups. As shown in Figure 2, most of the increase in spontaneous activity following axotomy was due to increased incidence of cells with either a bursting or irregular activity, and both these types of activity were reduced by nerve blockade. The percentage of tonically firing cells was much less sensitive to axotomy or TTX.
Figure 2.

The distribution of spontaneous activity patterns among spontaneous activity in large or medium-sized DRG neurons. Spontaneous activity was classified as bursting, irregular, or tonic, and the overall incidence of each subtype is indicated for each experimental group.
1.2 Early nerve blockade reduced excitability in DRG neurons after sciatic nerve transection
In some experiments, excitability parameters were measured in addition to determining incidence of spontaneous activity. In addition to a high incidence of spontaneous activity, sciatic nerve transection also caused abnormal depolarization of the resting membrane potential and reduction (hyperpolarization) of the AP threshold, decreased rheobase, decreased amplitude of AHP, and increased AP and AHP durations in both large- and medium-sized DRG neurons. These parameters are summarized in Table 1. Only depolarized resting Vm, hyperpolarized AP threshold and decreased rheobase were observed in small-sized neurons after sciatic nerve transection. More remarkably, instead of increasing, AP duration was significantly decreased in small-sized neurons.
Table 1.
Effect of early nerve blockade on electrical parameters
|
Large diameter cells (>50 μm) |
||||
|---|---|---|---|---|
| Normal (n=87 cells, 5 animals) | Axotomy (n=190 cells, 8 animals) | Axotomy +ACSF (n=170 cells, 7 animals) | Axotomy +TTX (n=137 cells, 6 animals) | |
| Vm (mV) | −60.06 ± 0.69 | −56.7 ±1.5*** | −56.7 ±0.5*** | −56.6 ±0.61** |
| Rheobase (nA) | 1.18± 0.07 | 0.61 ± 0.02*** | 0.71 ± 0.02*** | 0.93 ± 0.05* § |
| AP threshold (mV) | −38.5. ± 0.99 | −40.5 ± 0.57* | −40.7 ± 0.51* | −36.3 ± 0.73§ |
| AP duration (ms) | 0.83 ± 0.04 | 1.53 ± 0.05*** | 1.55 ± 0.05*** | 1.60 ± 0.07*** |
| AP amplitude (mV) | 39.2 ± 1.22 | 44.2 ± 0.61*** | 47 ± 0.58*** | 46 ± 0.80*** |
| AHP duration (ms) | 2.3 ± 0.11 | 5.0 ± 0.27*** | 4.5 ± 0.24*** | 4.4 ± 0.31*** |
| AHP amplitude (mV) | 13.1± 0.34 | 7.7 ± 0.27*** | 8.4 ± 0.26*** | 7.4 ± 0.30*** |
| Rin (MΩ) | 24.8 ± 0.97 | 33.5 ± 0.80 *** | 27.7 ± .80* | 31.4 ± 1.00 ** |
|
Medium diameter cells (30–50 μm) |
||||
|---|---|---|---|---|
| Normal (n=58 cells, 5 animals) | Axotomy (n=67 cells, 8 animals) | Axotomy +ACSF (n=81 cells, 7 animals) | Axotomy + TTX (n=73 cell, 6 animals) | |
| Vm (mV) | −57.6 ±0.90 | −54.3 ± 0.94* | −53.4 ± 0.92* | −53.8 ± 0.78* |
| Rheobase (nA) | 1.00 ± 0.08 | 0.57 ± 0.05*** | 0.65 ± 0.04*** | 0.81 ± 0.05* § |
| AP threshold (mV) | −34.4 ± 1.53 | −35.9 ± 0.94 | −36.6 ± 0.91 | −31.8 ± 1.10 § |
| AP duration (ms) | 1.16 ± 0.07 | 1.90 ± 0.09*** | 1.80± 0.09*** | 2.0 ± 0.09*** |
| AP amplitude (mV) | 44.1± 1.6 | 50 ± 1.54 | 49 ± 1.21* | 49.4 ± 1.20* |
| AHP duration (ms) | 2.61 ± 0.17 | 5.25 ± 0.42*** | 4.65 ± 0.29*** | 4.3 ± 0.33*** |
| AHP amplitude (mV) | 15.0 ± 0.70 | 10.2 ± 0.62** | 11.6 ± 0.56** | 9.8 ± 0.63*** |
| Rin (MΩ) | 41.7 ± 2.92 | 50.3 ± 2.3 | 41.5 ± 2.3 | 46.4 ± 3.10 |
|
Small diameter cells (<30 μm) |
||||
|---|---|---|---|---|
| Normal (n=42 cells, 5 animals) | Axotomy (n=35 cells, 8 animals) | Axotomy +ACSF (n=54 cells, 7 animals) | Axotomy +TTX (n=41 cells, 6animals) | |
| Vm (mV) | −58.1 ± 1.70 | −51.2 ± 1.83* | −52.4 ±0.98* | −52.02 ±1.23* |
| Rheobase (nA) | 1.06 ± 0.08 | 0.78 ± 0.06** | 0.73 ± 0.04** | 0.72 ± 0.04** |
| AP threshold (mV) | −17.62 ± 1.70 | −23.1 ±1.66* | −24.5 ±1.04** | −25.6 ±1.24** |
| AP duration (ms) | 3.0 ± 0.34 | 2.9 ± 0.25 | 2.80 ± 0.14 | 2.47 ± 0.11 |
| AP amplitude (mV) | 51.7 ± 1.18 | 48.3 ± 2.24 | 50.9 ± 1.12 | 50.0 ± 1.41 |
| AHP duration (ms) | 6.0 ± 0.55 | 5.3 ± 0.52 | 4.4 ± 0.27* | 3.45 ± 0.22* |
| AHP amplitude (mV) | 17.4 ± 0.93 | 16 ± 1.08 | 17.5 ± 0.50 | 17.06 ± 0.73 |
| Rin (MΩ) | 91.9 ± 7.56 | 79.4 ± 5.30 | 90 ± 4.7 | 76.5 ± 4.30 |
AP, action potential. AHP, afterhyperpolarization. Rin, input resistance.
Compared with normal value,
p<0.0001;
p<0.001;
p<0.05
Compared with Axotomy and Axotomy +ACSF group,
p<0.05
Cell diameters were obtained from microscopic observations made during intracellular recording.
Early nerve blockade inhibited the abnormal hyperpolarization of AP threshold in both large- and medium-sized neurons, and increased rheobase significantly in large- or medium-sized neurons. There was no clear effect observed on other abnormal parameters after early nerve blockade (Table 1). Also, the electrophysiological parameters of DRG neurons after sciatic nerve transection were not affected by ACSF perfusion (Table 1). These results suggest initial neuronal blockade at the time of nerve injury may prevent the subsequent development of spontaneous activity and hyperexcitability in medium- and large-sized DRG neurons after sciatic nerve transection, even when measured 4–5 weeks after the end of the nerve blockade period.
We always observed underlying membrane oscillations in spontaneously active neurons with bursting or irregular firing patterns, though this could not be observed in high frequency tonically firing cells. Similar observations have been reported by others [e.g. (Amir et al. 2005)]. Subthreshold membrane potential oscillations that were not sufficient to trigger action potentials were also observed in some neurons that were not spontaneously active. Like spontaneous activity, the incidence of such oscillations (observed 4 – 10 weeks after nerve injury) was increased after sciatic nerve transection and reduced by early nerve blockade. Of the non-spontaneously active neurons, subthreshold oscillations were observed in 3.5% (20/577) of cells from normal rats. This increased to 8.5% (107/1263) after axotomy or 11.0% (38/346) after axotomy with ACSF perfusion of the nerve (both significantly different from control; p<0.0001, but not different from one another, p = 0.17). Nerve blockade with TTX during the first week after injury reduced the percentage of oscillating cells to 5.7% (50/871), significantly lower than the axotomy group but not significantly different from the normal group. These oscillations were observed on 2 to 9 weeks after injury (longer times were not tested).
2. Relationship between sympathetic nerve sprouting and spontaneously active neurons
2.1. Distribution of TH-positive sympathetic fibers on the surface of DRGs before and after sciatic nerve transection
As shown in Figure 3, in normal rats (n=10), some TH-IR sympathetic fibers could be seen on the dorsal (Figure 3, top) and ventral (Figure 3, bottom) surface of the whole-mount DRG. But viewed in these orientations, the fibers were mainly located around the edge of the ganglion. None could be seen in the center region of the dorsal surface of the ganglion. Most fibers accompanied the vascular processes and some were from the small TH-IR dopaminergic neurons or “dark cells”, as previously described (Lawson 1979; Price and Mudge 1983; Tandrup et al. 2000). Fibers accompanying the vascular processes were from large bundles of TH-IR branches. The density of TH-IR fibers on the ventral side of the DRG was higher compared to the fiber density on the dorsal surface.
In DRG examined after nerve transection (n=21), TH-IR fibers sprouted vigorously and invaded the center portion of the dorsal surface of the DRG (Figure 3C and D). In some cases, the sprouted fibers formed ring or basket structures around large- and medium-sized DRG neurons. The overall density of the fibers increased significantly compared to normal DRG. The TH-IR dark cells disappeared completely in the axotomized DRG (Figure 3C and D). Similar changes occurred on both sides of the ganglion.
2.2. The density of sympathetic fibers in DRG sections after sciatic nerve transection was decreased by early nerve blockade and increased by potassium channel blockade
A great deal of evidence indicates that peripheral nerve injury increases the sprouting of sympathetic nerve in DRG after nerve transection. To determine whether ectopic firing in the DRG is related to the abnormal growth of sympathetic nerve fibers, we compared the density of sympathetic fiber sprouting between axotomized DRGs in which we experimentally increased or decreased the incidence of spontaneous activity reaching the DRG cell body. The density of sympathetic nerve fibers was counted in DRG sections by TH immunostaining. Early nerve blockade during the first 7-days post sciatic nerve transection (n=5) significantly (p<0.001, ANOVA, n=3) decreased the sprouting of sympathetic nerve in DRG as measured between POD 35 and 49, reducing it to a level just slightly higher than that seen in uninjured animals (Figure 4A;). Conversely, injured peripheral nerve fibers are sensitive to K+ channel blockers such as TEA and 4-AP, which can evoke robust increases in spontaneous firing at the nerve injured site. To further confirm the relationship between spontaneous activity and sympathetic nerve sprouting, TEA (n=3) or 4-AP (n=3) filled osmotic pumps were used to perfuse the injury site for 7 days in order to increase ectopic firing at the site of sciatic nerve transection. Perfusion with ACSF was used as a control (n=3). Consistent with previous studies (Devor 1983; Xie et al. 1993), dorsal root fiber recording on POD 7 indicated that spontaneous activity from DRG neurons were significantly increased following TEA or 4-AP perfusion by 1.5- fold for both treatments (Table 2). The two treatments did not differ from each other significantly (p = 0.21 for L4; 0.054 for L5). In addition, this increased ectopic discharge also resulted in a robustly higher density of sympathetic nerve fiber in the DRGs, which were collected and stained on POD 14 (Figure 4B). These results indicate that sympathetic nerve sprouting around DRG cell bodies following axotomy is directly affected by the spontaneous activity evoked by the injury.
2.3. Co-localization between sympathetic nerve sprouting and individual ectopic firing neurons
The data above clearly suggest that spontaneous activity in DRG directly correlates with the sprouting of sympathetic nerve after nerve transection. We further studied the relationship between ectopic firing and sympathetic nerve growth in DRG at the level of individual neurons. After sciatic nerve transection (n=21), we observed much higher density of sympathetic nerve fibers on the surface of DRG than on the normal ones (n=10) (Figure 3). During intracellular recording individual ectopically active or (in separate experiments) quiescent neurons were labeled with injection of the fluorescent dye Lucifer Yellow, followed by whole DRG TH immunohistochemical staining to detect sympathetic fibers. This allowed us to determine whether the sympathetic sprouting occurred preferentially around ectopically firing neurons. Two hundred and sixty one (261) ectopically firing neurons in 42 DRGs were successfully labeled, and sympathetic nerve fibers ended on 54% (142) of them (Figure 5). In 43 of these cells the sympathetic fiber endings took the form of a ring-like structure, and in 12 a basket structure. In the remaining cells the co-localization took the form of simple fiber endings on the surface of the cell. We observed that sympathetic nerve also formed basket-like structures on 19 unlabeled neurons, which were just adjacent to 19 of the labeled (spontaneously active) neurons. We noticed that spontaneously firing neurons, usually concentrated in several spots on the surface of DRG, suggesting that cross-talk may exist between spontaneously active DRG neurons. In separate experiments, we also randomly labeled 124 neurons without spontaneous activity in 17 DRGs after axotomy. Only 9 of them had sympathetic nerve fibers ending on the surface; none of these took the form of ring or basked structures. Hence growth of sympathetic fibers occurred preferentially around spontaneously active DRG cells (Figure 5 and 6).
In order to determine how general the co-localization phenomenon might be, we conducted a similar experiment in neurons from DRG that had been subjected to inflammation in the absence of nerve injury. DRG were inflamed by local deposition of a small drop of the immune activator zymosan (n=17). As shown previously (Xie et al. in press), this treatment causes sympathetic sprouting, spontaneous activity, and mechanical allodynia and hyperalgesia. In co-localization experiments such as those shown in Figure 5, conducted 15 – 35 days after localized inflammation of the DRG, 38% (35/93) of SA neurons were contacted by sympathetic fibers compared with only 11% (5/47) of quiescent neurons (p=0.0007).
2.4. Reducing sympathetic sprouting in DRG after sciatic nerve transection by early nerve blockade does not change the correlation between sprouting and individual spontaneously firing neurons
As we have described above, perfusing TTX onto the neuroma during the first 7 days after sciatic axotomy decreased but did not eliminate spontaneous activity in the DRG, and also decreased but did not eliminate sympathetic sprouting. To determine whether the preferential sprouting around spontaneously active neurons was preserved even when both phenomena were reduced by early nerve blockade, we repeated the above experiment in DRG in which the neuroma was perfused with TTX for the first 7 days after nerve transection (n=9). Recordings were made on POD days 14 – 45 (i.e., 7 to 37 days after the end of nerve blockade). Among 36 labeled ectopically firing neurons in 17 DRGs, 18 of them (50%) had adjacent sympathetic nerve sprouts. In 3 neurons the length of sympathetic nerve was longer than the half of the cell diameter. We also randomly labeled 76 neurons without spontaneous activity in 8 DRGs. Twenty of them (26%) had adjacent sympathetic nerve fibers ending on the cell surface (Figure 6, p = 0.02). This result indicated that the correlation between sympathetic nerve and ectopic firing neurons was not reduced by decreasing spontaneous activity, even though the overall incidence of both sprouting and activity was reduced. Using ACSF to replace TTX and perfuse transected sciatic nerve also did not change the relationship between sympathetic nerve and neurons with ectopic firing in the DRG (n=8). This result further indicates the specific correlation between ectopic firing neuron and sympathetic nerve sprouting. This relationship is independent of the absolute incidence of spontaneous activity or the absolute density of sympathetic nerve fibers.
Discussion
This study examined relationships between spontaneous activity and sympathetic sprouting around DRG cells in the complete sciatic nerve transection model of neuropathic pain. The primary new findings are that short term (1 week) blockade of the nerve proximal to the neuroma leads to reduction in spontaneous activity, hyperexcitability, and sympathetic sprouting in the DRG. These effects can be observed for at least 5 – 6 weeks after the end of the nerve blockade (longer times were not tested). In addition, we showed that experimentally increasing the spontaneous activity in the neuroma during the first week led to increased sympathetic sprouting, and that sympathetic sprouts were much more likely to terminate near spontaneously active rather than quiescent neurons.
The effects on sympathetic sprouting, hyperexcitability, and spontaneous activity of TTX perfusion near the nerve injury site are likely to be due to its local impulse blocking effects on the injured nerve because: 1) in our previous study, a similar effect on sprouting was observed using a very different class of drug, lidocaine, for local nerve blockade (Zhang et al. 2004) ; 2) TTX perfusion of the contralateral uninjured nerve did not reduce spontaneous activity in the ipsilateral DRG, arguing strongly against a systemic effect; and 3) perfusion of the injured nerve with chemically distinct drugs that increased spontaneous activity had opposing effects on the density of sympathetic sprouting.
Key role of early activity
It is well known that sciatic nerve transection induces abnormal spontaneous activity, originally arising from the neuroma. Later, spontaneous activity can originate in the DRG, and even cells not showing spontaneous activity may develop hyperexcitability (Amir 1999; Babbedge et al. 1996; Burchiel 1984a; b; DeSantis and Duckworth 1982; Govrin-Lippmann and Devor 1978; Kajander et al. 1992; Kirk 1970; Michaelis 2000; Wall and Devor 1983). Indeed, spontaneous activity is a key feature in many different neuropathic pain models (see Introduction). Such activity is prominent in medium diameter cells, many of which are nociceptors, as well as in large diameter cells, which are not normally nociceptors. Several mechanisms have been proposed to account for the apparent contribution of spontaneous activity in large diameter cells to pathological pain [e.g., see (Abdulla et al. 2003)]. Our data suggest that the initial, early period of ectopic discharges from the injury site plays a key role in setting up the later, more prolonged excitability changes, and in generation of spontaneous activity that occur in DRG cell bodies. Simply preventing this activity from reaching the DRG during the first week after injury results in a substantial reduction in spontaneous activity and hyperexcitability measured in the DRG cell bodies and dorsal roots 5 to 6 weeks after the nerve blockade has ended. Other studies have also suggested that blocking the initial period of spontaneous activity in various nerve injury models can have profound, long-lasting effects. Our previous work (Xie et al. 2005) with two different localized blockade methods, in two different partial injury neuropathic pain models, also provided evidence for the critical importance of spontaneous activity during the first week following nerve injury. Such blockade could lead to reduction in mechanical and thermal pain behaviors that long outlasted the duration of the blockade. Temporarily blocking spontaneous activity reduces or eliminates spontaneous pain, hyperalgesia, and allodynia in a variety of pain models, using methods to suppress spontaneous activity that vary widely in their specific targets (Boucher et al. 2000; Chaplan et al. 2003; Lai et al. 2002; Lyu et al. 2000; Seltzer et al. 1991; Xiao and Bennett 1995; Yoon et al. 1996), however, see (Suter et al. 2003).
In a previous study (Zhang et al. 2004), we showed that lidocaine, either systemic or locally applied to the injury site, could reduce sympathetic sprouting in the sciatic nerve transection model. The present study builds upon those findings in showing that experimentally increasing activity with local perfusion of K+ channel blockers can increase sprouting; that a different local blocker (e.g., TTX) has the same effect as lidocaine; and that individual spontaneously active neurons are preferentially targeted by sympathetic sprouts. A surprising finding in the Zhang et al. paper was that the reduction in sympathetic sprouting outlasted the duration of the lidocaine block by at least 7 days. In light of the results of the present study, it seems likely that this early blockade of spontaneous activity produced a decline in development of spontaneous activity in the DRG which lasted long after nerve blockade ended. If neuronal activity in fact causes sprouting, rather than simply correlating with it as demonstrated here (see below), this could in turn account for the observed long lasting decrease in sympathetic sprouting. Interestingly, prolonged effects of treatments of short-acting local anesthetics are also reported in clinical examples of sympathetically-maintained pain.
These findings, along with previous studies that emphasize the key role of the early period of spontaneous activity, support the notion of pre-emptive analgesia. Attempts to prevent development of chronic pain by providing analgesia early (e.g., immediately before or just after a surgery and before chronic pain has developed) have been conflicting and often disappointing (Kelly et al. 2001; Moiniche et al. 2002). However, many such clinical studies have used blockade periods that were much shorter than the 7 day blockade used in this study. In addition, this and previous studies suggest that analgesics that acted primarily at the level of the spinal cord would not be able to prevent the prolonged alterations in DRG properties that occur in animal models of pathological pain. Hence after the analgesic was removed, establishment of central sensitization by the abnormal DRG cells might still occur.
Relationship between spontaneous activity and sympathetic sprouting
The observation that sympathetic sprouting in this model occurs preferentially around large and medium diameter neurons, which are also most likely to develop high frequency spontaneous activity, provided the initial suggestion that sprouting might be related to spontaneous activity. This study demonstrated a correlation between sprouting and spontaneous activity at the level of individual neurons. Our study does not directly address mechanism and causality. The observation that spontaneous activity appears very early after injury, well before sprouting can be observed; as well as our ability to affect sprouting by manipulating spontaneous activity, suggest that if there is a causal relationship it is that activity somehow induces sprouting around individual neurons. However, our data do not address this issue directly, and we cannot eliminate an indirect or a bidirectional relationship between sprouting and activity.
Many mechanistic studies of sympathetic sprouting into the DRG have focused on possible roles of neurotrophic factors and/or cytokines. Some of these theories focus on the possible role of the satellite glia cells that surround DRG neurons, rather than on the neurons themselves [e.g., (Ramer et al. 1999; Walsh and Kawaja 1998; Zhou et al. 1999)], and it is worth noting that structural studies indicate that the sprouting fibers predominantly contact the satellite glia sheath rather than the DRG neurons(Shinder et al. 1999). The present findings are not inconsistent with a role of neurotrophic factors or cytokines in sprouting. There are a number of mechanisms by which abnormal activity in the DRG cell bodies might result in local elevation of trophic factors or cytokines. For example, activity might evoke release of substances from neurons that either enhance sympathetic sprouting directly, or stimulate nearby glial cells to produce substances such as NGF that then enhance sprouting. Many studies support the connection between neuronal activity and release of trophic factors (Castren et al. 1992; Ernfors et al. 1991; Gall et al. 1991a; Gall et al. 1991b; Gall and Isackson 1989; Isackson et al. 1991; Kim et al. 1994; Lu et al. 1991; Patterson et al. 1992; Thoenen 1991; Zafra et al. 1990; Zafra et al. 1992), as well as mechanisms by which neuronal activity is communicated to surrounding glial cells (Gunzel and Schlue 2000; Murphy et al. 1993; Schmidt et al. 1999). The latter include direct linking of neuronal and glial membrane potentials (Lohr and Deitmer 1999; Newman and Zahs 1998; Rouach et al. 2000), stimulation of calcium waves in glia by glutamate (Dani et al. 1992; Venance et al. 1995), and release of fractalkine by activated neurons (Chao et al. 1995; Kyrkanides et al. 1999; Watkins and Maier 2002; Winkelstein et al. 2001). Our observation that spontaneously active neurons tended to be found in clusters would be consistent with a role for highly localized factors. Highly localized effects of neuronal activity on sympathetic sprouting are also suggested by the finding that spontaneously active cells are more likely to have sympathetic fibers nearby even after early nerve blockade has greatly reduced the overall incidence of both activity and sprouting.
Acknowledgments
This work was supported in part by NIH grants NS39568 and NS45594 (J.Z.).
References
- Abdulla FA, Moran TD, Balasubramanyan S, Smith PA. Effects and consequences of nerve injury on the electrical properties of sensory neurons. 2003;81:663–682. doi: 10.1139/y03-064. [DOI] [PubMed] [Google Scholar]
- Amir R. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Kocsis JD, Devor M. Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons. J Neurosci. 2005;25:2576–2585. doi: 10.1523/JNEUROSCI.4118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbedge RC, Soper AJ, Gentry CT, Hood VC, Campbell EA, Urban L. In vitro characterization of a peripheral afferent pathway of the rat after chronic sciatic nerve section. J Neurophysiol. 1996;76:3169–3177. doi: 10.1152/jn.1996.76.5.3169. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Burchiel KJ. Effects of electrical and mechanical stimulation on two foci of spontaneous activity which develop in primary afferent neurons after peripheral axotomy. Pain. 1984a;18:249–265. doi: 10.1016/0304-3959(84)90820-0. [DOI] [PubMed] [Google Scholar]
- Burchiel KJ. Spontaneous impulse generation in normal and denervated dorsal root ganglia: sensitivity to alpha-adrenergic stimulation and hypoxia. Exp Neurol. 1984b;85:257–272. doi: 10.1016/0014-4886(84)90139-0. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Hu S, Peterson PK. Glia, cytokines, and neurotoxicity. Critical Reviews in Neurobiology. 1995;9:189–205. [PubMed] [Google Scholar]
- Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM, Dubin AE. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–1178. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien SQ, Li C, Li H, Xie W, Zhang J-M. Sympathetic fiber sprouting in chronically compressed dorsal root ganglia without peripheral axotomy. J Neuropathic Pain & Symptom Palliation. 2005;1:19–23. doi: 10.1300/J426v01n01_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglia of the injured peripheral nerve in a rat neuropathic pain model. J Comp Neurol. 1996;376:241–252. doi: 10.1002/(SICI)1096-9861(19961209)376:2<241::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- DeSantis M, Duckworth JW. Properties of primary afferent neurons from muscle which are spontaneously active after a lesion of their peripheral processes. Experimental Neurolgy. 1982;75:261–274. doi: 10.1016/0014-4886(82)90159-5. [DOI] [PubMed] [Google Scholar]
- Devor M. Potassium channels moderate ectopic excitability of nerve-end neuromas in rats. Neurosci Lett. 1983;40:181–186. doi: 10.1016/0304-3940(83)90299-9. [DOI] [PubMed] [Google Scholar]
- Devor M, Janig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Changes in somatic action potential shape in guinea-pig nociceptive primary afferent neurones during inflammation in vivo. J Physiol (Lond) 520 Pt. 1999;2:565–576. doi: 10.1111/j.1469-7793.1999.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Newton R, Levinson SR, Berry CM, Carruthers B, Lawson SN. Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Nav 1.7 (PN1) Na+ channel alpha subunit protein. J Physiol (Lond) 2003;546:565–576. doi: 10.1113/jphysiol.2002.026559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, Lawson SN. The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J Neurosci. 2002;22:7425–7433. doi: 10.1523/JNEUROSCI.22-17-07425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall C, Lauterborn J, Bundman M, Murray K, Isackson P. Seizures and the regulation of neurotrophic factor and neuropeptide gene expression in brain. Epilepsy Research - Supplement. 1991a;4:225–245. [PubMed] [Google Scholar]
- Gall C, Murray K, Isackson PJ. Kainic acid-induced seizures stimulate increased expression of nerve growth factor mRNA in rat hippocampus. Brain Res Mol Brain Res. 1991b;9:113–123. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Govrin-Lippmann R, Devor M. Ongoing activity in severed nerves: source and variation with time. Brain Res. 1978;159:406–410. doi: 10.1016/0006-8993(78)90548-6. [DOI] [PubMed] [Google Scholar]
- Grelik C, Bennett GJ, Ribeiro-da-Silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur J Neurosci. 2005;21:2475–2487. doi: 10.1111/j.1460-9568.2005.04089.x. [DOI] [PubMed] [Google Scholar]
- Gunzel D, Schlue WR. Mechanisms of Mg2+ influx, efflux and intracellular 'muffling' in leech neurones and glial cells. Magnesium Research. 2000;13:123–138. [PubMed] [Google Scholar]
- Habler H. Sympathetic-sensory coupling after L5 spinal nerve lesion in the rat and its relation to changes in dorsal root ganglion blood flow. Pain. 2000;87:335–345. doi: 10.1016/S0304-3959(00)00297-9. [DOI] [PubMed] [Google Scholar]
- Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Janig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res. 1996;113:161–184. doi: 10.1016/s0079-6123(08)61087-0. [DOI] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: physiological pathways and pharmacological modalities. 2001;48:1000–1010. doi: 10.1007/BF03016591. [DOI] [PubMed] [Google Scholar]
- Kim HG, Wang T, Olafsson P, Lu B. Neurotrophin 3 potentiates neuronal activity and inhibits gamma-aminobutyratergic synaptic transmission in cortical neurons. Proc Natl Acad Sci U S A. 1994;91:12341–12345. doi: 10.1073/pnas.91.25.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Na HS, Sung B, Nam HJ, Chung YJ, Hong SK. Is sympathetic sprouting in the dorsal root ganglia responsible for the production of neuropathic pain in a rat model? Neuroscience Letters. 1999;269:103–106. doi: 10.1016/s0304-3940(99)00435-8. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993;55:85–92. doi: 10.1016/0304-3959(93)90187-T. [DOI] [PubMed] [Google Scholar]
- Kinnman E, Levine JD. Sensory and sympathetic contributions to nerve injury-induced sensory abnormalities in the rat. Neuroscience. 1995;64:751–767. doi: 10.1016/0306-4522(94)00435-8. [DOI] [PubMed] [Google Scholar]
- Kirk EJ. Impulses in dorsal spinal nerve rootlets in cat and rabbit arising from dorsal root ganglia isolated from the periphery. J Comp Neurol. 1970;139:307–320. doi: 10.1002/cne.901550203. [DOI] [PubMed] [Google Scholar]
- Kummer W, Gibbins IL, Stefan P, Kapoor V. Catecholamines and catecholamine-synthesizing enzymes in guinea-pig sensory ganglia. Cell Tissue Res. 1990;261:595–606. doi: 10.1007/BF00313540. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, Olschowka JA, Williams JP, Hansen JT, O'Banion MK. TNF alpha and IL-1beta mediate intercellular adhesion molecule-1 induction via microglia-astrocyte interaction in CNS radiation injury. J Neuroimmunol. 1999;95:95–106. doi: 10.1016/s0165-5728(98)00270-7. [DOI] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, Na(v)1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Lawson SN. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol. 1979;8:275–294. doi: 10.1007/BF01236123. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig.[comment] J Physiol (Lond) 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp Brain Res. 1998;120:432–438. doi: 10.1007/s002210050416. [DOI] [PubMed] [Google Scholar]
- Liu B, Li HQ, Brull SJ, Zhang JM. Increased sensitivity of sensory neurons to tumor necrosis factor alpha in rats with chronic compression of the lumbar ganglia. J Neurophysiol. 2002;88:1393–1399. doi: 10.1152/jn.2002.88.3.1393. [DOI] [PubMed] [Google Scholar]
- Liu CN, Michaelis M, Amir R, Devor M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: relation to neuropathic pain. J Neurophysiol. 2000;84:205–215. doi: 10.1152/jn.2000.84.1.205. [DOI] [PubMed] [Google Scholar]
- Lohr C, Deitmer JW. Dendritic calcium transients in the leech giant glial cell in situ. Glia. 1999;26:109–118. [PubMed] [Google Scholar]
- Lu B, Yokoyama M, Dreyfus CF, Black IB. Depolarizing stimuli regulate nerve growth factor gene expression in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1991;88:6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu YS, Park SK, Chung K, Chung JM. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871:98–103. doi: 10.1016/s0006-8993(00)02451-3. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Jang W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- Michaelis M. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci. 2000;20:2742–2748. doi: 10.1523/JNEUROSCI.20-07-02742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology. 2002;96:725–741. doi: 10.1097/00000542-200203000-00032. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Wier WG, Baraban JM. Rapid communication between neurons and astrocytes in primary cortical cultures. J Neurosci. 1993;13:2672–2679. doi: 10.1523/JNEUROSCI.13-06-02672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Price J, Mudge AW. A subpopulation of rat dorsal root ganglion neurones is catecholaminergic. Nature. 1983;301:241–243. doi: 10.1038/301241a0. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bisby MA. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain. 1997;70:237–244. doi: 10.1016/s0304-3959(97)03331-9. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Thompson SW, McMahon SB. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain Suppl. 1999;6:S111–120. doi: 10.1016/S0304-3959(99)00144-X. [DOI] [PubMed] [Google Scholar]
- Roberts WJ. A hypothesis on the physiological basis for causalgia and related pains. Pain. 1986;24:297–311. doi: 10.1016/0304-3959(86)90116-8. [DOI] [PubMed] [Google Scholar]
- Rouach N, Glowinski J, Giaume C. Activity-dependent neuronal control of gap-junctional communication in astrocytes. Journal of Cell Biology. 2000;149:1513–1526. doi: 10.1083/jcb.149.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J, Prinz P, Deitmer JW. Glial hyperpolarization upon nerve root stimulation in the leech Hirudo medicinalis. Glia. 1999;27:32–38. doi: 10.1002/(sici)1098-1136(199907)27:1<32::aid-glia4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Beilin BZ, Ginzburg R, Paran Y, Shimko T. The role of injury discharge in the induction of neuropathic pain behavior in rats. Pain. 1991;46:327–336. doi: 10.1016/0304-3959(91)90115-E. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Shir Y. Sympathetically-maintained causalgiform disorders in a model for neuropathic pain: a review. J Basic Clin Physiol Pharmacol. 1991;2:17–61. doi: 10.1515/jbcpp.1991.2.1-2.17. [DOI] [PubMed] [Google Scholar]
- Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28:743–761. doi: 10.1023/a:1007090105840. [DOI] [PubMed] [Google Scholar]
- Song XJ, Hu SJ, Greenquist KW, Zhang J-M, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Suter MR, Papaloizos M, Berde CB, Woolf CJ, Gilliard N, Spahn DR, Decosterd I. Development of neuropathic pain in the rat spared nerve injury model is not prevented by a peripheral nerve block. Anesthesiology. 2003;99:1402–1408. doi: 10.1097/00000542-200312000-00025. [DOI] [PubMed] [Google Scholar]
- Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol. 2000;422:172–180. doi: 10.1002/(sici)1096-9861(20000626)422:2<172::aid-cne2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulse originate from dorsal root ganglia as well as from the periphery in normal and nerve injury rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Wall PD, Waxman S, Basbaum AI. Ongoing activity in peripheral nerve: injury discharge. Exp Neurol. 1974;45:576–589. doi: 10.1016/0014-4886(74)90163-0. [DOI] [PubMed] [Google Scholar]
- Walsh GS, Kawaja MD. Sympathetic axons surround nerve growth factor-immunoreactive trigeminal neurons: observations in mice overexpressing nerve growth factor. Journal of Neurobiology. 1998;34:347–360. doi: 10.1002/(sici)1097-4695(199803)34:4<347::aid-neu5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states [Review] Physiological Reviews. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–139. [PubMed] [Google Scholar]
- Xiao WH, Bennett GJ. Synthetic omega-conopeptides applied to the site of nerve injury suppress neuropathic pains in rats. J Pharmacol Exp Ther. 1995;274:666–672. [PubMed] [Google Scholar]
- Xie W, Deng H, Li H, Bowen TL, Strong JA, Zhang J-M. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. doi: 10.1016/j.neuroscience.2006.06.045. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: Early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhang J-M, Petersen M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995;73:1811–1820. doi: 10.1152/jn.1995.73.5.1811. [DOI] [PubMed] [Google Scholar]
- Xie YK, Xiao WH, Li HQ. The relationship between new ion channels and ectopic discharges from a region of nerve injury. Sci China B. 1993;36:68–74. [PubMed] [Google Scholar]
- Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J Comp Neurol. 2006;495:679–690. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- Yoon YW, Na HS, Chung JM. Contributions of injured and intact afferents to neuropathic pain in an experimental rat model. Pain. 1996;64:27–36. doi: 10.1016/0304-3959(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO Journal. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-M, Li H, Munir MA. Decreasing sprouting of noradrenergic sympathetic fibers in pathologic sensory ganglia: new mechanism and approach for treating neuropathic pain using local anesthetics. Pain. 2004;109:143–149. doi: 10.1016/j.pain.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Zhang J-M, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- Zhang J-M, Song XJ, LaMotte RH. An in vitro study of ectopic discharge generation and adrenergic sensitivity in the intact, nerve-injured rat dorsal root ganglion. Pain. 1997;72:51–57. doi: 10.1016/s0304-3959(97)00013-4. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Deng YS, Chie E, Xue Q, Zhong JH, McLachlan EM, Rush RA, Xian CJ. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11:1711–1722. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]


