Abstract
C3H10T1/2 cells differentiate along a chondrogenic pathway when plated onto the extracellular matrix (ECM) protein perlecan (Pln). To identify the region(s) within the large Pln molecule that provides a differentiation signal, recombinant Pln-sequence-based polypeptides representing distinct structural domains were assayed for their ability to promote chondrogenesis in C3H10T1/2 cells. Five distinct domains, along with structural variations, were tested. The N-terminal domain I was tested in two forms (IA and IB) that contain only heparan sulfate (HS) chains or both HS and chondroitin sulfate (CS) chains, respectively. A mutant form of domain I lacking attachment sites for both HS and CS (Pln Imut) was tested also. Other constructs consecutively designated Pln domains II, III(A-C), IV(A,B), and V(A,B) were used to complete the structure-function analysis. Cells plated onto Pln IA or Pln IB but no other domain rapidly assembled into cellular aggregates of 40-120 μm on average. Aggregate formation was dependent on the presence of glycosaminoglycan (GAG) chains, because Pln I-based polypeptides lacking GAG chains either by enzymatic removal or mutation of HS/CS attachment sites were inactive. Aggregates formed on GAG-bearing Pln IA stained with Alcian Blue and were recognized by antibodies to collagen type II and aggrecan but were not recognized by an antibody to collagen type X, a marker of chondrocyte hypertrophy. Collectively, these studies indicate that the GAG-bearing domain I of Pln provides a sufficient signal to trigger C3H10T1/2 cells to enter a chondrogenic differentiation pathway. Thus, this matrix proteoglycan (PG) found at sites of cartilage formation in vivo is likely to enhance early stage differentiation induced by soluble chondrogenic factors.
Keywords: perlecan, cartilage, chondrogenesis, proteoglycan
INTRODUCTION
Chondrogenesis occurs as a multistep process that is initiated by condensation of mesenchymal stem cells that subsequently undergo a specific program of differentiation. Studies from several laboratories clearly have established a role for specific soluble signals in this differentiation program that include bone morphogenetic proteins,(1) parathyroid hormone-related protein (PTHrP),(2) Indian hedgehog (Ihh)(3) and transforming and fibroblast growth factors (FGFs).(4,5) Of interest, several of these are known to interact with heparan sulfate proteoglycans (HSPG), a factor implicated in modulating their bioavailability.(6) In a previous report(7) our laboratory showed that a large HSPG found in the extracellular matrix (ECM) of developing cartilage perlecan (Pln; HSPG2) stimulated cells of a murine fibroblast line C3H10T1/2 to form aggregates in vitro similar to those found in condensing mesenchyme in vivo. These aggregates were shown to express the cartilage markers collagen type II and aggrecan, but not collagen type X.(7) In addition, Pln maintained the chondrogenic phenotype of adult chondrocytes in vitro.(8) Consistent with a fundamental role for Pln in endochondral bone formation, targeted disruption of the Pln gene in mice results in severe skeletal abnormalities at sites of cartilage growth and differentiation.(9,10) In the small subset of mouse embryos that survive to reach this stage, these abnormalities include a severe disorganization of the columnar structure of chondrocytes and defective endochondral ossification.(10) Interestingly, the phenotype of the Pln null mice is similar to that caused by activating mutations of FGF receptor 3 (FGFR3), interpreted to mean that these molecules modulate similar signaling pathways in developing cartilage.(10)
Pln is a multidomain protein consisting of five distinct regions, four of which display sequence similarity to other protein families.(11) The N-terminal domain I is unique to Pln. Within domain I are three glycosaminoglycan (GAG) attachment sites defined by the consensus amino acid triplet SGD. Although other potential sites for glycosylation exist in the protein core, the N-terminal sites are considered the major site for GAG attachment.(12) Domain II contains repeat sequences highly similar to the low-density lipoprotein (LDL) receptor, and domain III is comprised of three cysteine-rich globular repeats similar to domain IV of the laminin A chain. In mice, domain III contains an RGD sequence but in human Pln this sequence is missing.(13) Domain IV contains repeats similar to those found in the immunoglobulin G (IgG) superfamily member neural cell adhesion molecule (N-CAM). The C-terminal of domain V shows sequence similarity to the G domain of the laminin A chains. There also are epidermal growth factor (EGF)-like sequences spaced between the G-like repeats in Pln domain V. Given the potential for multiple functional interactions among these various structural domains, we aimed to determine which region(s) of Pln was responsible for the in vitro aggregation and chondrogenic activation of cultured C3H10T1/2 cells.
Each domain of Pln previously has been produced as a recombinant protein, and several of these also have been produced in various forms.(14-18) The N-terminal recombinant domain I (Pln I) was produced as two variants (Pln IA and IB) differing in GAG composition and also in GAG-deficient mutant form (Pln Imut). Several of the longer domains were expressed in consecutive pieces [Pln III(A-C), Pln IV(A,B), and Pln V(A,B)]. In this study, we cultured C3H10T1/2 cells on various combinations of the recombinant Pln domains. We found that only the GAG-bearing forms of domain I, but not other Pln domains, support formation of cellular aggregates. Like intact Pln, aggregates forming on domain I begin to express chondrocyte markers including staining with Alcian Blue and expression of collagen type II and aggrecan. These findings indicate that GAG-bearing domain I is sufficient to provide a permissive ECM signal to initiate chondrogenesis in vitro.
MATERIALS AND METHODS
Materials
Pln/HSPG2 was obtained from Becton-Dickinson Lab-ware (Bedford, MA, USA). The rabbit polyclonal antibody against rat aggrecan was provided by Dr. Kurt Doege (Shriners Hospital for Children, Portland Unit, Portland, OR, USA). The rabbit anti-mouse antibody against type X collagen (PXNC1-88) was provided by Dr. Greg Lunstrum (Shriners Children’s Hospital, Portland, OR, USA) The rabbit IgG antibody against mouse type II collagen was purchased from Biodesign International (catalog no. T40025R; Biodesign, Kennebunk, ME, USA). Species-specific TX-Red conjugated secondary antibodies were purchased from Amersham Corp. (Arlington Heights, IL, USA).
Immunofluorescent detection of ECM components
After culture on matrix for 6 days or 9 days, cell aggregates and monolayers were rinsed twice with Dulbecco’s phosphate-buffered saline (D-PBS) without calcium or magnesium. The specimens subsequently were fixed, washed three times (5 minutes at room temperature) with D-PBS, and incubated with the primary antibody for 1 h at 37°C in a humidified chamber. After three washes (5 minutes each at room temperature) in D-PBS, cell aggregates and monolayers were incubated with the secondary antibody for 45 minutes at 37°C in a humidified chamber and finally washed three times (5 minutes each) with D-PBS and mounted.
For aggrecan labeling, cell aggregates and monolayers were fixed in 100% methanol (10 minutes at room temperature); however, for type II and X collagen labeling, an ice-cold solution composed of 95% (vol/vol) ethanol plus 5% (vol/vol) acetic acid (30 minutes on ice) was used as fixative. To promote type II and X collagen antibody penetration, cell aggregates and monolayers were incubated with 0.02% type IV-S testicular hyaluronidase (H3884; Sigma, St. Louis, MO, USA) for 30 minutes at room temperature and rinsed three times with PBS before antibody incubation.
Culture of C3H10T1/2 cells on various matrix components
Cell culture was performed as previously described(7) with some modification. For confocal microscopic analysis of cell aggregates, Nalge-Nunc “permanox” chambered slides (4 well, catalogue no. 177437; Nalge-Nunc, Naperville, IL, USA) were used in place of the Nalge-Nunc 4-well plates used previously. Briefly, for coating wells, 5 μg of Pln or recombinant protein plus D-PBS was added to the well at a final volume of 200 μl and then incubated overnight at 37°C with lids askew. On the following day, the drywells were rinsed twice with D-PBS before addition of cells. C3H10T1/2 cells were added to wells at a density of 2 × 105 cells/well in CMRL-1066 medium. The CMRL-1066 media (Gibco Life Sciences, Rockville, MD, USA) was supplemented with 15% (vol/vol) heat-inactivated fetal calf serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin sulfate, ascorbic acid (50 μg/ml), citrate (50 μg/ml), and pyruvate (50 μg/ml) and changed daily throughout the experiment. Great care was taken not to disturb or remove aggregates. Formation of aggregates was assessed by visual inspection using light microscopy. Cells that had drawn together into dense, multilayered cellular aggregates reminiscent of condensing mesenchyme of developing cartilage, leaving areas of the well bare, were scored as positive as described previously.(7) For this investigation, chondrogenesis is defined as the process by which 10T1/2 cells condense to form aggregates that are positive for Alcian Blue and express collagen type II and aggrecan.
Confocal microscopic analysis of C3H10T1/2 cell aggregates
All data were acquired on a Zeiss inverted 100M Axioskop equipped with a Zeiss 510 LSM confocal microscope (Zeiss, Oberkochen, Germany) and a krypton argon laser (488 nm and 568 nm excitation lines). Simultaneous acquisition of nuclei stained with Syto 13 (505-550 band pass filter) and Texas Red conjugated secondary antibodies (590 long pass filter) used the fast-line switch feature for elimination of spectral bleed-through. Samples were imaged using a Zeiss 63X C-Apochromat water immersion lens (N.A. 1.2)
Enzymatic digestion of glycosaminoglycan chains
Digestion with both heparinase and chondroitinase ABC was performed as described previously.(7) Briefly, wells coated with Pln or recombinant domains of Pln were subjected to digestion with chondroitinase ABC or a mixture of heparinases I, II, and III (Sigma) for 4 h at 37°C. After digestion, the enzyme solution was removed and wells were rinsed once with D-PBS before the cells were plated.
Recombinant Pln fragments
Recombinant Pln fragments were prepared as described previously.(14-19) Briefly, complementary DNA (cDNA) encoding the specified Pln domain was inserted in frame behind the basement membrane protein BM-40. This construct was then inserted into the pRc/CMV vector and stably transfected into human embryonic kidney 293 cells. Positive clones were selected and media containing the various domains were collected. Recombinant protein was isolated by elution from a diethylaminoethyl (DEAE)-cellulose column using an NaCl gradient and purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining.
RESULTS
The response of C3H10T1/2 cells cultured on domain I of Pln mimics the response generated by native Pln
We reported previously that the murine embryonic cell line C3H10T1/2 aggregates and undergoes chondrogenic differentiation in response to culture on Pln-coated surfaces.(7) Our studies with native Pln also suggested that the N-terminal, GAG-bearing domain of Pln was at least one of the regions active in this assay. In this investigation we tested the hypothesis that a single domain of the Pln molecule is responsible for the in vitro aggregation and chondrogenic activation of C3H10T1/2 cells. To test this hypothesis, recombinant fragments of Pln were used as culture matrices for C3H10T1/2 cells (Table 1). When cultured on the recombinant N-terminal domain Pln IA, C3H10T1/2 cells responded as if cultured on native Pln (Fig. 1, compare B and C). Within 24 h, the majority of the cells condensed into multicellular masses of rounded cells, leaving the well bare with relatively few fibroblastic cells between the aggregates. By day 6 of culture, aggregates express markers of early chondrogenesis (Alcian Blue [not shown], type II collagen, and aggrecan; Fig. 2), but not type X collagen, a marker of chondrocyte maturation. Analysis of day 6 aggregates using confocal microscopy indicates the cells are tightly packed and in high density. The z-series (optical sectioning) analysis of aggregates labeled with the nucleic acid stain Syto 13 revealed that aggregates vary in thickness (42-120 μm). In addition, z-series analysis of aggregates labeled with antibodies specific to type II collagen and aggrecan illustrated that expression of these chondrogenic markers is localized to the periphery of the aggregate. Interestingly, expression of type II collagen appears to encompass the entire aggregate surface; however, aggrecan expression is distributed only at the top/dorsal surface of the aggregate (Figs. 2A and 2B).
TABLE 1.
AGGREGATE FORMATION ON PLN DOMAINSa
| Matrix component | Response |
|---|---|
| PBS | (-) |
| Pln | (+++) |
| Pln IA | (++/+++) |
| Pln IB | (+++) |
| Pln Imut | (-) |
| Pln II | (+/-) |
| Pln III(A-C) | (-) |
| Pln IV(A-C) | (-) |
| Pln V(A,B) | (-) |
–, No aggregates or Alcian Blue staining; +/-, inefficient aggregate formation with little or no Alcian Blue staining; ++, efficient aggregate formation with heterogeneous Alcian Blue staining; +++, efficient aggregate formation and homogeneous Alcian Blue staining.
C3H10T1/2 cells were cultured on the indicated Pln domain and aggregation and Alcian Blue staining was assessed after 3 days as described previously.7
FIG. 1.
Response of C3H10T1/2 cells to recombinant domains of Pln. C3H10T1/2 cells were either cultured on (A) plastic, (B) native Pln, (C) Pln IA, or (D) domain II thru V2. (A) On plastic, cells appeared fibroblastic while on (B) native Pln and (C) Pln IA alone, cells formed aggregates. However, when domains II thru V2 were used individually as matrices on which C3H10T1/2 cells were cultured, no aggregates were formed. (D) C3H10T1/2 cells cultured on domain II of Pln. The results obtained using Pln domains III thru V2 were similar. The cells form a dense monolayer of fibroblastic cells.
FIG. 2.
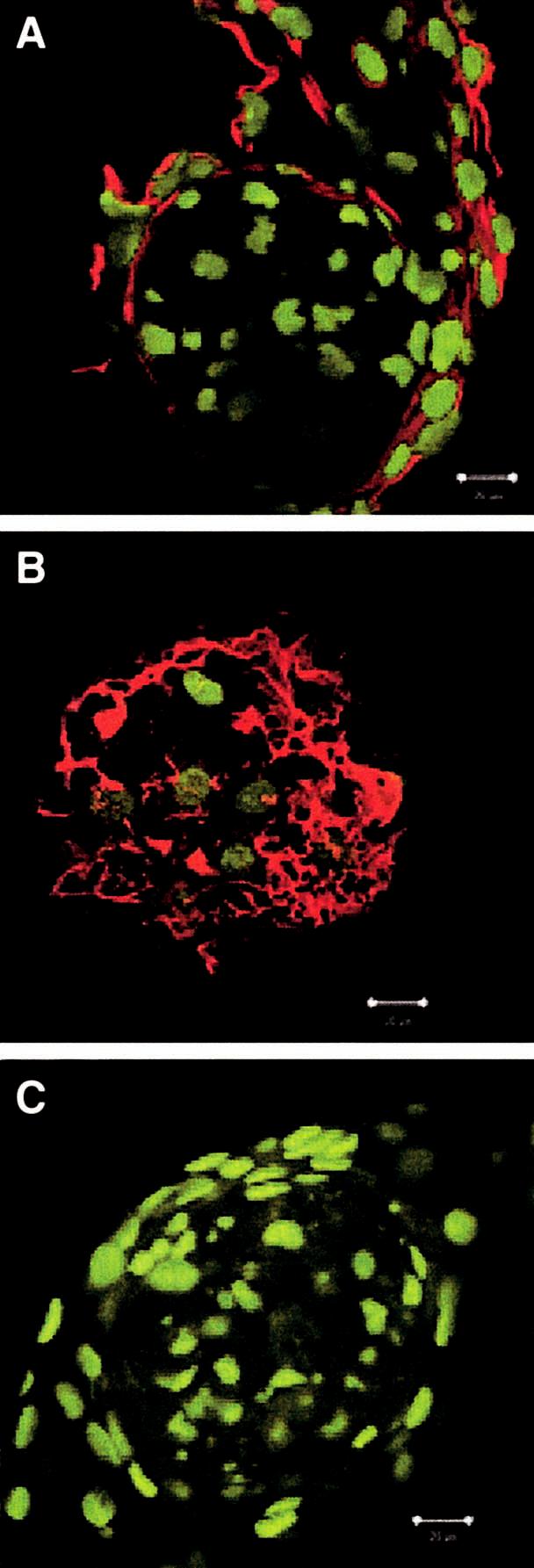
Pln domain IA induces differentiation to the same extent as native Pln. Cultures (day 6) from Pln IA-coated wells were probed with antibodies to (A) type II collagen (red signal), (B) aggrecan (red signal), (C) type X collagen (red signal), and optically sectioned with a confocal microscope (0.2 μm). The green signal is the nucleic acid stain Syto 13. Type II collagen is expressed on the exterior surface of Pln IA aggregates. In contrast, aggrecan expression is localized at the top/dorsal surface of the Pln IA aggregate. Day 6 Pln IA aggregates do not express type X collagen (magnification bar = 20 μm).
In contrast to Pln IA, C3H10T1/2 cells did not aggregate or differentiate when cultured on other recombinant Pln domains Pln II through Pln V (Fig. 1D). C3H10T1/2 cells cultured on recombinant Pln domains II-V responded like cells cultured on plastic, they attached, spread, and formed a dense monolayer. Thus, domain I of Pln is sufficient for the aggregation and differentiation of C3H10T1/2 cells into early chondrocytes in vitro.
The presence of GAG chains is required on the Pln IA core for activity
In our previous investigation, heparinase digestion of native Pln greatly reduced its aggregate-inducing activity in vitro.(7) Thus, in this investigation we tested the hypothesis that the presence of GAG chains on recombinant Pln domain I is necessary for the aggregation and differentiation of C3H10T1/2 cells in vitro. To test this hypothesis, recombinant Pln domain I variants of differing GAG chain composition were used as culture matrices for C3H10T1/2 cells. Of the three Pln domain I variants, the first comprised a protein core bearing HS chains only (Pln IA), the second bearing a mixture of HS and chondroitin sulfate (CS) chains (Pln IB(17)), and the third Pln Imut with mutations preventing GAG chain attachment.
When cultured on Pln IA and IB (data not shown) a robust recruitment of C3H10T1/2 cells into aggregates was observed (Fig. 3). This response is consistent with our previous observations of cells cultured on native Pln.(7,8) In contrast, C3H10T1/2 cells cultured on Pln Imut did not aggregate but attached and spread into a dense monolayer (Fig. 3). The full activity observed with Pln IB suggests that the presence of CS in conjunction with HS chains on the protein core does not interfere with activity. Moreover, our observations using Pln Imut suggest that the presence of GAG chains on the Pln protein core is necessary for C3H10T1/2 cell aggregation and differentiation activity.
FIG. 3.

Induction of morphological change by Pln IA variants. C3H10T1/2 cells were cultured on Pln IA and Pln Imut (lacking GAG chains) and photographed on day 6 of culture. C3H10T1/2 cells cultured on Pln Imut did not form aggregates.
Enzymatic digestion of GAG chains reveals chondroitin/dermatan sulfate chain participation
The redundancy in function between the Pln IA and Pln IB forms of Pln domain I suggested that both HS and chondroitin/dermatan sulfate chains might play a role in supporting differentiation. If a soluble factor secreted by the cells acted by binding HS, then digestion with heparinase should result in a reduction or loss of this activity. Thus, both Pln IA and Pln IB variants were digested with either heparinase or chondroitinase ABC and tested in the aggregation assay. As expected, heparinase but not chondroitinase digestion abolished the aggregate-promoting activity of native Pln (Figs. 4A, 4D, 4G, and 4J). Similarly, activity of the HS-bearing Pln IA variant was only lost when digested with heparinase but not with chondroitinase ABC (Figs. 4B, 4E, 4H, and 4K). Interestingly, neither digestion with heparinase nor chondroitinase ABC alone affected the activity of the Pln IB variant (Figs. 4C, 4F, and 4I). The maintenance of activity after heparinase digestion suggested that chondroitin/dermatan sulfate chains carried by the Pln IB variant also could promote aggregate formation. This was confirmed by the demonstration that digestion with a combination of enzymes destroyed aggregate-promoting activity (Fig. 4L). Thus, either HS or chondroitin/dermatan sulfate chains on Pln domain I is sufficient to trigger this response.
FIG. 4.
Activity of enzymatically modified PG IA and PG IB. Enzymatic digestion of Pln IA and Pln IB was performed to examine the role of GAG chains in differentiation of C3H10T1/2 cells. Cells on undigested (A) native Pln, (B) Pln IA, or (C) Pln IB formed numerous aggregates. When digested with heparinase, (D) Pln and (E) Pln IA failed to stimulate the C3H10T1/2 cells and cells cultured on (F) Pln IB again formed aggregates. Chondroitinase ABC digestion had little effect on (G) Pln, (H) Pln IA, or (I) Pln IB. The combination of the two enzymes resulted in loss of activity in all cases (J) Pln, (K) Pln IA, and (L) Pln IB (magnification bar in panel B = 100 μm).
DISCUSSION
Pln is an HSPG found in all basement membranes in developing mesenchyme and in cartilage.(7,19,20) Its functions include binding of growth factors and ECM proteins, modulation of cell and matrix adhesion, and protection of ECM from degradation.(9,10,19,21,22) In a previous study, we reported the ability of Pln to initiate aggregate formation and chondrocytic differentiation of pluripotent C3H10T1/2 cells.(7) This phenomenon also occurs when dishes are coated with the intact Pln molecule and also when soluble Pln is added at high concentrations (Gomes, Farach-Carson, and Carson, unpublished data, 2000). To determine more specifically which structural region of Pln is responsible for the chondrogenic activity, recombinant polypeptides representing distinct structural domains were tested using the same assay that we used previously with intact Pln. After culturing the cells on each domain individually, it was found that the N-terminal GAG-bearing domain I of Pln was sufficient to activate aggregate formation and expression of chondrogenic markers. No other domain had this aggregating ability, and C3H10T1/2 cells rapidly attached and spread on all other domains. From this, we conclude that Pln I with its attached GAG chains, like intact Pln, provides a sufficient signal to initiate the chondrogenic program in C3H10T1/2 cells. This finding is of interest in view of the previous report that Pln produced by bovine articular cartilage contains both chondroitin and HS GAG chains.(23)
The contribution of the core protein and GAG chains to the activity of domain I was explored further using Pln domain I variants of differing GAG composition or mutated to delete sites of GAG chain addition. We also tested the activity of preparations that had been digested with GAG lyases. The finding that Pln Imut displayed no aggregate-forming activity indicated a requirement for the presence of GAG chains and showed that the Pln I core protein alone provided an insufficient signal. This idea was supported further by the finding that heparinase digestion of Pln IA completely destroyed its activity. Because neither heparinase nor chondroitinase ABC digestion alone could destroy the aggregate-promoting activity of Pln IB having mixed GAG chains, we conclude that either HS or CS GAG chains attached to domain I can support aggregation and chondrocytic differentiation of C3H10T1/2 cells.
Previously, we found that the neoglycoprotein, heparin-bovine serum albumin (BSA) and GAG chains without core protein are incapable of supporting aggregation of C3H10T1/2 cells in vitro.(7) This suggested that the protein core to which the GAG chains are attached is important in generating an appropriate signal to initiate chondrogenesis. In this study, we were unable to block aggregate formation on Pln IA or Pln IB with polyclonal antibodies to the protein core (data not shown); however, we have no indication that these are function blocking antibodies. We also found that certain other PGs, including decorin and aggrecan (French, Höök, and Carson, unpublished studies, 2000), could support aggregation, but it has not been determined if they can support differentiation to an extent comparable with Pln. Aggregation alone does not indicate that chondrogenesis has occurred. We reported previously(7) that NIH 3T3 cells aggregate when cultured on Pln; however, these aggregates do not express markers of chondrogenesis (Alcian Blue reactivity, type II collagen, and aggrecan). In a previously reported study, SanAntonio et al.(24) proposed that GAG chains assist chondrogenic development through modifications of interactions between the cells and the ECM. These authors found that the addition of HS, heparin, or dermatan sulfate increased the formation of chondrogenic cell aggregates along with increasing sulfate incorporation, another index of chondrogenesis. High concentrations of exogenously added heparin inhibited aggregation. CS did not stimulate chondrogenesis. A role for GAGs, specifically HS, in expression of the chondrocyte phenotype or growth of cartilage nodules was proposed. Combined with the functional role for Pln domain I reported here, it seems likely that the GAG-bearing N-terminal region of Pln plays a pivotal role in early chondrogenesis. This idea is supported further by its appearance in developing cartilage in vivo.(7,23) Thus, a function of the Pln core protein might be to present the GAG chains to the target mesenchymal cells in an active form, a property that might be simulated to some extent by other HSPGs, but does not appear to be a property of heparin-BSA. Interestingly, the pattern of expression of chondrogenic markers within aggregates forming in vitro suggests that the aggregate itself is not homogenous. For example, whereas collagen II is expressed uniformly at the periphery of the aggregates, aggrecan appears to be expressed primarily at the top of the aggregate where the most differentiated cells are expected to be. The pattern of aggregate expression resembles that previously reported for N-CAM(25) where it was suggested that within aggregates a “sorting out” between differentiating and non-differentiating cells occurs. This marker localization is seen in aggregates on Pln domain I, providing further evidence that this region produces a sufficient signal for early chondrogenesis.
Mechanistically, there are several alternatives to explain the actions of Pln in stimulating early chondrogenesis. One possibility is that GAGs could interact directly with cell surface receptors and activate intracellular signaling pathways leading to cartilage differentiation. This type of interaction has been proposed.(26) Another more likely possibility is that GAG-bearing PGs could serve to disrupt the interactions between cell surfaces and ECM. This activity has been proposed for both HS-bearing and CS-bearing PGs,(24) and has led to the idea that PGs can serve as cell adhesion inhibitors. In the case of C3H10T1/2 cells, intact Pln or domain I could prevent the interaction of cells with the culture substratum and support the initial rounding and aggregation of the mesenchymal cells. Other domains of Pln, or Pln I lacking GAG chains, are devoid of this activity and permit cells to form focal adhesions and continue to proliferate. This idea of promoting chondrocyte differentiation by inhibiting ECM adhesion is consistent with our data and with that of others using micromass culture conditions to study chondrogenesis in vitro using nonadherent substrata.(27) It also is consistent with the ideas proposed by Solursh,(28) who demonstrated that prevention of cell spreading promotes a rounded morphology necessary for chondrocytic differentiation. In view of the findings reported here, a specific role for GAG-bearing Pln, specifically its N-terminal domain, in chondrocyte differentiation and cartilage development can now be considered.
ACKNOWLEDGMENTS
The authors are indebted to Dr. R. Liu, Dr. T. Thanh-Van, and Dr. C. Kirn-Safran as well as J. Julian, D. Baraniak, and J. Fongemie for their many helpful discussions and critical reading of this article. We thank Ginger Moore and Sharron Kingston for their excellent secretarial assistance. These studies were supported by the National Institutes of Health (NIH) grants HD25235 (to D.D.C.), R01DE13031 (to M.C.F.-C.), and a fellowship award from the Delaware Biotechnology Institute (to R.R.G.).
Footnotes
Address reprint requests to: Daniel D. Carson, Ph.D. 117 Wolf Hall University of Delaware Newark, DE 19716, USA
REFERENCES
- 1.Reddi AH. Cartilage morphogenesis: Role of bone and cartilage morphogenetic proteins, homeobox genes and extracellular matrix. Matrix Biol. 1995;14:599–606. doi: 10.1016/s0945-053x(05)80024-1. [DOI] [PubMed] [Google Scholar]
- 2.Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104:399–407. doi: 10.1172/JCI6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwamoto M, Enomoto-Iwamoto M, Kurisu K. Actions of hedgehog proteins on skeletal cells. Crit Rev Oral Biol Med. 1999;10:477–486. doi: 10.1177/10454411990100040401. [DOI] [PubMed] [Google Scholar]
- 4.Matsunaga S, Yamamoto T, Fukumura K. Temporal and spatial expressions of transforming growth factor-betas and their receptors in epiphyseal growth plate. Int J Oncol. 1999;14:1063–1067. doi: 10.3892/ijo.14.6.1063. [DOI] [PubMed] [Google Scholar]
- 5.Chintala SK, Miller RR, McDevitt CA. Basic fibroblast growth factor binds to heparan sulfate in the extracellular matrix of rat growth plate chondrocytes. Arch Biochem Biophys. 1994;310:180–186. doi: 10.1006/abbi.1994.1155. [DOI] [PubMed] [Google Scholar]
- 6.Ornitz DA. FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. BioEssays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.French MM, Smith SE, Akanbi K, Sanford T, Hecht J, Farach-Carson MC, Carson DD. Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J Cell Biol. 1999;145:1103–1115. doi: 10.1083/jcb.145.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard MA, Hogue DA, Cole WG, Sanford T, Snuggs MB, Montufar-Solis D, Duke PJ, Carson DD, Scott A, Van Winkle WB, Hecht JT. Cytoskeletal abnormalities in chondrocytes with EXT1 and EXT2 mutations. J Bone Miner Res. 2000;15:442–450. doi: 10.1359/jbmr.2000.15.3.442. [DOI] [PubMed] [Google Scholar]
- 9.Mercedes C, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 11.Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, Yamada Y, Hassell JR. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266:22939–22947. [PubMed] [Google Scholar]
- 12.Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozzo RV. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem. 1992;267:8544–8557. [PubMed] [Google Scholar]
- 13.Cohen IR, Grassel S, Murdoch AD, Iozzo RV. Structural characterization of the complete human perlecan gene and its promoter. Proc Natl Acad Sci USA. 1993;90:10404–10408. doi: 10.1073/pnas.90.21.10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costell M, Sasaki T, Mann K, Yamada Y, Timpl R. Structural characterization of recombinant domain II of the basement membrane proteoglycan perlecan. FEBS Lett. 1996;396:127–131. doi: 10.1016/0014-5793(96)01082-4. [DOI] [PubMed] [Google Scholar]
- 15.Schulze B, Sasaki T, Costell M, Mann K, Timpl R. Structural and cell-adhesive properties of three recombinant fragments derived from perlecan domain III. Matrix Biol. 1996;15:349–357. doi: 10.1016/s0945-053x(96)90138-9. [DOI] [PubMed] [Google Scholar]
- 16.Brown JC, Sasaki T, Gohring W, Yamada Y, Timpl R. The C-terminal domain V of perlecan promotes beta1 integrin-medicated cell adhesion, binds heparin, nidogen and fibulin-2 and can be modified by glycosaminoglycans. Eur J Biochem. 1997;250:39–46. doi: 10.1111/j.1432-1033.1997.t01-1-00039.x. [DOI] [PubMed] [Google Scholar]
- 17.Costell M, Mann K, Yamada Y, Timpl R. Characterization of recombinant perlecan domain I and its substitution by glycosaminoglycans and oligosaccharides. Eur J Biochem. 1997;243:115–121. doi: 10.1111/j.1432-1033.1997.t01-1-00115.x. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T, Costell M, Mann K, Timpl R. Inhibition of glycosaminoglycan modification of perlecan domain I by site-directed mutagenesis changes protease sensitivity and laminin-1 binding activity. FEBS Lett. 1998;435:169–172. doi: 10.1016/s0014-5793(98)01063-1. [DOI] [PubMed] [Google Scholar]
- 19.Hopf M, Göhring W, Kohfeldt E, Yamada Y, Timpl R. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem. 1999;259:917–925. doi: 10.1046/j.1432-1327.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 20.Timpl R. Proteoglycans of basement membranes. Experientia. 1993;49:417–428. doi: 10.1007/BF01923586. [DOI] [PubMed] [Google Scholar]
- 21.Mongiat M, Taylor K, Otto J, Aho S, Uitto J, Whitelock JM, Iozzo RV. The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J Biol Chem. 2000;275:7095–7100. doi: 10.1074/jbc.275.10.7095. [DOI] [PubMed] [Google Scholar]
- 22.Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, Underwood PA. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;19:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 23.SundarRaj N, Fite D, Ledbetter S, Chakravarti S, Hassell JR. Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J Cell Sci. 1995;108:2663–2672. doi: 10.1242/jcs.108.7.2663. [DOI] [PubMed] [Google Scholar]
- 24.San Antonio JD, Winston BM, Tuan RS. Regulation of chondrogenesis by heparan sulfate and structurally related glycosaminoglycans. Dev Biol. 1987;123:17–24. doi: 10.1016/0012-1606(87)90422-2. [DOI] [PubMed] [Google Scholar]
- 25.Tavella S, Raffo P, Tacchetti C, Cancedda R, Castagnola P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res. 1994;215:354–362. doi: 10.1006/excr.1994.1352. [DOI] [PubMed] [Google Scholar]
- 26.Perrimon N, Bernfield M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature. 2000;404:725–728. doi: 10.1038/35008000. [DOI] [PubMed] [Google Scholar]
- 27.Mello MA, Tuan RS. High density micromass cultures of embryonic limb bud mesenchymal cells: An in vitro model of endochondral skeletal development. In Vitro Cell Dev Biol Anim. 1999;35:262–269. doi: 10.1007/s11626-999-0070-0. [DOI] [PubMed] [Google Scholar]
- 28.Solursh M. Formation of cartilage tissue in vitro. J Cell Biochem. 1991;45:258–260. doi: 10.1002/jcb.240450306. [DOI] [PubMed] [Google Scholar]




