Juvenile nasopharyngeal angiofibroma (JNA) is a rare locally invasive neoplasm composed of cavernous vascular channels set in an abundant myxoid stroma of fibroblasts and myofibroblasts.1,2 The histological similarity to erectile tissue, the almost exclusive occurrence in pubescent males, and expression of multiple steroid receptors suggest that JNA growth is stimulated by male sex hormones.1,3
The frequency of JNA is significantly increased in male familial adenomatous polyposis (FAP) patients, suggesting that it may arise through alterations of the adenomatous polyposis coli (APC)/β-catenin gene pathway.4 This was supported by the high frequency of recurrent β-catenin gene mutations detected in sporadic JNA, but no APC mutations have thus far been found.5–7
We analysed the sequence of the APC gene and the presence of recurrent β-catenin mutations in matched blood and tumour DNA from a 24 year old JNA affected FAP carrier who underwent restorative proctocolectomy and resection of an abdominal wall desmoid. The patient was the only JNA affected sibling of an FAP family. Matched DNA from blood and from frozen JNA tissue were analysed for APC mutations using the TNT Quick Coupled Transcription/Translation System (Promega, Madison, Wisconsin, USA) and heteroduplex analysis on agarose minigel,8 followed by sequencing. Using these techniques we detected a frameshift APC mutation, c.3927-3931delAAAGA, in both blood and JNA tissue. This mutation introduces a stop codon (pGlu1309fsX1312) in the APC gene region between the first and second 20 amino acid β-catenin binding repeats. Another frameshift APC mutation, consisting in a 5 bp deletion, c.3183-3187delACAAA, that introduces a stop codon (p.Lys1061fsX1062) in the region encoding the first 20 amino acid β-catenin binding repeat, was detected only in JNA DNA. Using restriction enzyme analysis,5 we ruled out the presence of the JNA associated activating mutations at codons 32 and 34 in exon 3 of the β-catenin gene. These results were confirmed in duplicate experiments. Due to lack of tumour sections, we were unable to perform laser capture microdissection to separate the vascular and stromal components of the tumour. However, the somatic mutation is expected to have been present in fibroblasts because of the clear stromal predominance in the JNA tissue analysed.
In the study by Abraham et al, activating β-catenin mutations were found in 12 of 16 sporadic JNAs analysed.5 The APC sequence corresponding to the mutation cluster region (MCR) of sporadic colorectal cancer9 was investigated in the four JNAs without β-catenin mutations but no mutations were detected.5 Guertl et al analysed 11 sporadic JNAs from nine patients for mutations in the MCR of the APC gene and for loss of heterozygosity (LOH) at the APC locus.6 No APC mutations were detected and none of the informative cases were LOH positive.6 Ferouz et al found no germline APC mutations in a series of nine JNA patients.7 Thus there was no direct evidence involving the APC gene in JNA, although this rare tumour is reported to occur 25 times more frequently in FAP affected adolescents than in an age matched population.4,7
This study documents for the first time the association between a somatic and a germline APC mutation in an FAP related JNA. Because of the stromal predominance in the tumour analysed (fig 1 ▶), the somatic mutation must have been present in the fibroblasts (that is, in the same cell type where nuclear accumulation of β-catenin, indicative of activation of the Wnt pathway, was previously demonstrated).5 We cannot exclude the presence or absence of the mutation in the vascular component. Our findings agree with the well known evidence of double hit APC inactivation in FAP associated fibroblastic tumours.10 Thus FAP associated JNA should be considered a sex dependent extraintestinal FAP manifestation.
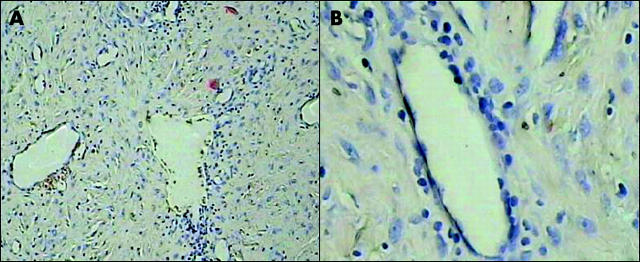
Figure 1.
Histopathological appearance of the nasopharyngeal angiofibroma described in this study. The tumour is composed of dilated vascular channels set in an abundant myxoid stroma containing fusiform fibroblasts and focal mononuclear cell infiltrates (A, ×125; B, ×400).
Acknowledgments
Supported by grants from “Associazione Italiana per la Ricerca sul Cancro” (AIRC) and the Italian Ministry of University and Research.
Conflict of interest: None declared.
References
- 1.Hicks JL, Nelson JF. Juvenile nasopharyngeal angiofibroma. Oral Surg Oral Med Oral Pathol 1973;35:807–17. [DOI] [PubMed] [Google Scholar]
- 2.Beham A, Kainz J, Stammberger H, et al. Immunohistochemical and electron microscopical characterization of stromal cells in nasopharyngeal angiofibromas. Eur Arch Otorhinolaryngol 1997;254:196–9. [DOI] [PubMed] [Google Scholar]
- 3.Hwang HC, Mills SE, Patterson K, et al. Expression of androgen receptors in nasopharyngeal angiofibroma: an immunohistochemical study of 24 cases. Modern Pathol 1998;11:1122–6. [PubMed] [Google Scholar]
- 4.Giardiello FM, Hamilton SR, Krush AJ, et al. Nasopharyngeal angiofibroma in patients with familial adenomatous polyposis. Gastroenterology 1993;105:1550–2. [DOI] [PubMed] [Google Scholar]
- 5.Abraham SC, Montgomery EA, Giardiello FM, et al. Frequent β-catenin mutations in juvenile nasopharyngeal angiofibromas. Am J Pathol 2001;158:1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guertl B, Beham A, Zechner R, et al. Nasopharyngeal angiofibroma: an APC-gene associated tumor? Hum Pathol 2000;31:1411–13. [PubMed] [Google Scholar]
- 7.Ferouz AS, Morh RM, Paul P. Juvenile nasopharyngeal angiofibroma and familial adenomatous polyposis: an association? Otolaryngol Head Neck Surg 1995;113:435–9. [DOI] [PubMed] [Google Scholar]
- 8.Cama A, Palmirotta R, Curia MC, et al. Multiplex PCR analysis and genotype-phenotype correlations of frequent APC mutations. Hum Mutat 1995;5:144–52. [DOI] [PubMed] [Google Scholar]
- 9.Miyoshi Y, Nagase H, Ando H, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Human Mol Genet 1992;1:559–63. [DOI] [PubMed] [Google Scholar]
- 10.Palmirotta R, Curia MC, Esposito DL, et al. Novel mutations and inactivation of both alleles of the APC gene in desmoid tumors. Hum Mol Genet 1995;4:1979–81. [DOI] [PubMed] [Google Scholar]