Ferroportin disease or type 4 haemochromatosis is an autosomal dominant iron overload disorder caused by mutations in the iron exporter ferroportin.1,2 Numerous mutations in ferroportin (SLC40A1) have been identified (see review by Pietrangelo3). The A77D mutation of ferroportin has thus far only been reported in Italy.2 We report the first A77D mutation of ferroportin which resulted in hepatic iron overload in an Australian family. The study was approved by and performed in accordance with the ethical standards of the Queensland Institute of Medical Research Human Research Ethics Committee and the Helsinki Declaration of 1975, as revised in 1983. Informed and written consent was obtained from the patient and family members.
The subject, a 45 year old Caucasian male, presented with complaints of lethargy and malaise. He had no risk factors for viral hepatitis, consumed minimal alcohol (20 g/week), and was married with two children. Physical examination was normal, including a normal body mass index.
Initial investigations revealed a haemoglobin level of 12.2 g/dl, white blood count of 3.8×103, and platelet count of 135×103. Serum ferritin concentration was 3500 μg/l with a transferrin saturation (TS) of 29%. Molecular analysis did not reveal the presence of the C282Y, H63D, or S65C mutations of HFE.
The subject was referred for further evaluation after complaining of ongoing lethargy and fatigue, myalgias, and arthralgia. On further clinical investigation he was found to have a mild lymphopenia, an alanine aminotransferase level of 63 IU/l, a serum ferritin concentration of 3340 μg/l, and a TS of 29%. He was non-reactive for hepatitis B surface antigen and negative for anti-hepatitis C virus IgG. Random blood sugar level and lipid profile were normal. HFE analysis was repeated and again the absence of common mutations was confirmed.
Liver biopsy was performed and revealed significant Kupffer cell iron loading with minimal staining in hepatocytes, as detected by Perls’ Prussian blue staining. No fibrosis was detected. Hepatic iron concentration was 96 μmol/g dry weight (normal 5–35) with a hepatic iron index of 2.1 (normal <1.1). No other secondary cause for iron loading (for example, thalassemia, porphyria cutanea tarda, or chronic liver disease) was detected.
Liver histology and biochemistry were suggestive of ferroportin disease. The entire coding region and splice sites of the ferroportin gene from the proband were polymerase chain reaction amplified and sequenced, as previously described.4 Other family members were subsequently evaluated.
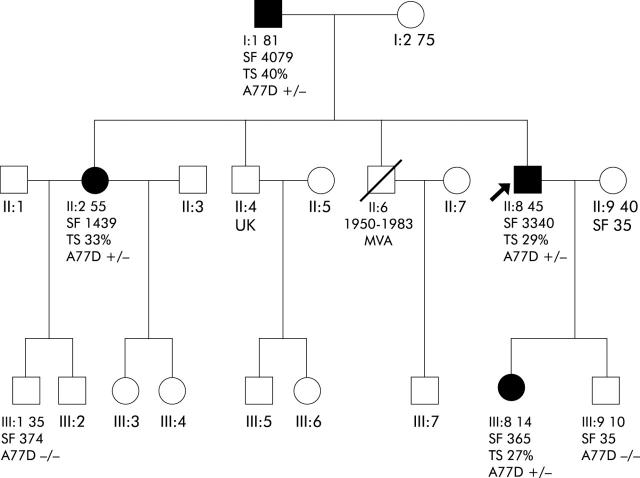
The presence of a cytosine to adenine change at nucleotide 230 of ferroportin, which results in mutation of an alanine to aspartic acid at amino acid 77 (A77D), was identified in the proband. Subsequently, this change was also identified in the proband’s father, sister, and daughter (fig 1 ▶). This is the same mutation which was identified in Italy by Montosi and colleagues.2 There is no known ancestral link between the family reported here and that in Italy. Thus it is likely that the A77D mutation has occurred in the two populations separately, as appears to be the case with the V162del mutation4–8 which has so far been reported in five geographic locations.
Figure 1.
Family pedigree. Family members and their age are shown. The proband is indicated by an arrow and affected family members by filled shapes. TS, transferrin saturation (%); SF, serum ferritin concentration (μg/l).
As knowledge about ferroportin disease is uncommon in the community, unlike HFE associated haemochromatosis, it is possible that some cases of this disorder are not recognised and thus remain undiagnosed. This particular case was not diagnosed until liver biopsy was performed. The raised serum ferritin level was initially attributed to viral illness. Because transferrin saturation and HFE genotype were normal, a diagnosis of iron overload was not initially considered.
In conclusion, we report the first identification of ferroportin disease caused by the A77D mutation in a region outside of Italy. This suggests that the A77D mutation may be more widespread than initially thought. This report also suggests that some cases of ferroportin disease may go undiagnosed. Ferroportin disease should thus be considered when a patient presents with a high serum ferritin, even when transferrin saturation and HFE genotype are normal.
Acknowledgments
The authors gratefully acknowledge the immense support and encouragement of the patient and his family. This work was supported by grants from the Haemochromatosis Society of Australia and the National Health and Medical Research Council of Australia (953219) to VNS.
Conflict of interest: None declared.
References
- 1.Njajou OT, Vaessen N, Joosse M, et al. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet 2001;28:213–14. [DOI] [PubMed] [Google Scholar]
- 2.Montosi G, Donovan A, Totaro A, et al. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J Clin Invest 2001;108:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis 2004;32:131–8. [DOI] [PubMed] [Google Scholar]
- 4.Wallace DF, Pedersen P, Dixon JL, et al. Novel mutation in ferroportin1 is associated with autosomal dominant hemochromatosis. Blood 2002;100:692–4. [DOI] [PubMed] [Google Scholar]
- 5.Devalia V, Carter K, Walker AP, et al. Autosomal dominant reticuloendothelial iron overload associated with a 3-base pair deletion in the ferroportin 1 gene (SLC11A3). Blood 2002;100:695–7. [DOI] [PubMed] [Google Scholar]
- 6.Roetto A, Merryweather-Clarke AT, Daraio F, et al. A valine deletion of ferroportin 1: a common mutation in hemochromastosis type 4. Blood 2002;100:733–4. [DOI] [PubMed] [Google Scholar]
- 7.Cazzola M, Cremonesi L, Papaioannou M, et al. Genetic hyperferritinaemia and reticuloendothelial iron overload associated with a three base pair deletion in the coding region of the ferroportin gene (SLC11A3). Br J Haematol 2002;119:539–46. [DOI] [PubMed] [Google Scholar]
- 8.Wallace DF, Browett P, Wong P, et al. Identification of ferroportin disease in the Indian subcontinent. Gut 2005;54:567–8. [DOI] [PMC free article] [PubMed] [Google Scholar]