Abstract
Background and aims: Increased pancreatitis associated protein (PAP) mRNA has been reported in active inflammatory bowel disease (IBD). The aims of the current study were to characterise PAP production in IBD and the effects of PAP on inflammation.
Patients and methods: Serum PAP levels were determined in healthy controls (n = 29), inflammatory controls (n = 14), and IBD patients (n = 171). Ex vivo PAP secretion in intestinal tissue was measured in 56 IBD patients and 13 healthy controls. Cellular origin of PAP was determined by immunohistochemistry. The effects of exogenous PAP on nuclear factor κB (NFκB) activation, proinflammatory cytokine production, and endothelial adhesion molecule expression were also analysed ex vivo.
Results: Patients with active IBD had increased serum PAP levels compared with controls, and these levels correlated with clinical and endoscopic disease severity. Ex vivo intestinal PAP synthesis was increased in active IBD and correlated with endoscopic and histological severity of inflammatory lesions. PAP localised to colonic Paneth cells. Incubation of mucosa from active Crohn’s disease with PAP dose dependently reduced proinflammatory cytokines secretion. PAP prevented TNF-α induced NFκB activation in monocytic, epithelial, and endothelial cells and reduced proinflammatory cytokine mRNA levels and adhesion molecule expression.
Conclusions: PAP is synthesised by Paneth cells and is overexpressed in colonic tissue of active IBD. PAP inhibits NFκB activation and downregulates cytokine production and adhesion molecule expression in inflamed tissue. It may represent an anti-inflammatory mechanism and new therapeutic strategy in IBD.
Keywords: pancreatitis associated protein, inflammatory bowel diseases, nuclear factor κB, inflammation, Paneth cells
Pancreatitis associated protein I (PAP I) is a member of the type III subclass of the REG gene family that was first identified in rat pancreatic juice after experimental pancreatitis.1 PAP I is also expressed in mice and humans, the amino acid sequences of these proteins showing a high degree of conservation.2 Human PAP is constitutively expressed in the pancreas and small intestine.3 Increased PAP mRNA has been documented in colonic mucosa from patients with active inflammatory bowel disease (IBD)4–6 as well as in experimental models of colitis.6,7
Although several functions have been proposed for PAP, the physiological relevance of PAP upregulation in inflammatory diseases remains unknown. Recent observations suggest that PAP may have a protective effect against inflammatory damage in pancreatic8,9 and extrapancreatic10 inflammatory conditions.
In the current study, we characterised PAP synthesis in IBD, examining its relationship with type of disease, ulcerative colitis (UC) or Crohn’s disease (CD), and its severity, based on clinical, endoscopic, and histological parameters. Given the protective action of PAP against inflammatory damage previously observed, we assessed whether PAP supplementation modulates signalling pathways in active IBD in ex vivo studies, particularly whether it alters proinflammatory cytokine synthesis in colonic inflamed mucosa from patients with CD and UC. After observing that this was in fact the case, and in order to gain a further mechanistic insight, we assessed whether PAP modulates activation of nuclear factor κB (NFκB) in three different cell lines as models of the cell types that orchestrate the chronic inflammation present in IBD—namely, monocytes, epithelial cells, and endothelial cells. Finally, we analysed if PAP could inhibit adhesion molecule expression in stimulated endothelial cells.
METHODS
The study was approved by the ethics committee of the Hospital Clínic de Barcelona, and all patients gave their written informed consent before enrolment.
Analysis of serum PAP in IBD
PAP levels in serum were analysed in active or inactive IBD patients (n = 171), in healthy control subjects (n = 29), and in a group of patients with documented acute infectious gastroenteritis (positive stool culture for Gram negative bacteria) as intestinal inflammatory controls (n = 14). Patients or controls with previously known intestinal or inflammatory diseases other than IBD, acute or chronic pancreatitis, previous intestinal resection, or chronic renal failure were excluded. In CD, clinical severity of the disease was estimated using the CD activity index,11 and in UC with the Lichtiger modified Truelove and Witts clinical activity index.12 Biological markers of IBD activity (C reactive protein (CRP) and erythrocyte sedimentation rate (ESR)) were also determined.
Colonic disease extension was determined by colonoscopy performed in all cases within six months before inclusion in the study. In CD patients, small intestinal involvement was assessed by a follow through performed within the same time period. Location of CD was categorised into three subgroups: ileal, colonic, and ileocolonic. Location of UC was categorised into two subgroups: distal, when lesions were confined to the rectosigmoid region, and extensive.
Serum PAP was measured using a commercially available ELISA kit (Dynabio SA, Marseille, France) and results are expressed as ng of PAP per ml of serum.
Intestinal PAP synthesis in IBD
Intestinal PAP synthesis was analysed using ex vivo culture of intestinal biopsy samples in 56 patients with IBD (41 with UC and 15 with CD) and 13 healthy controls. Colonic biopsy specimens were obtained from inflamed and/or non-inflamed mucosa of patients with active or inactive IBD. Patients receiving steroid treatment were excluded. Mucosal biopsies were taken from control patients free of intestinal inflammatory disease who underwent colonoscopy for cancer screening, in whom colonoscopy excluded the presence of lesions. Endoscopic severity of UC was assessed following a previously described scoring system.13 Evaluation of endoscopic severity in CD was referred to the area where biopsies were taken and categorised as: inactive: no lesions; mild: circumscribed aphthous lesions; moderate: superficial ulcers <1 cm in length; and severe: deep ulcerations or ulcers >1 cm in length. Biopsy specimens were examined blindly by a single gastrointestinal pathologist (RM). Assessment of histological severity of inflammation in biopsy samples was performed using previously described grading scores for UC14 or CD.15
For measurement of PAP secretion by colonic tissue, colonic biopsy samples weighing approximately 15–30 mg were cultured at 37°C with 5% CO2 for 24 hours. At the end of the culture period, supernatants were recovered and stored at −80°C for later analysis. Levels of PAP protein secreted were analysed measuring PAP concentration in the supernatant by ELISA (Dynabio SA). Results are expressed as ng PAP per mg of tissue.
Localisation of PAP in intestinal biopsies
Cellular origin of PAP was determined using immunohistochemistry. Colon or ileum paraffin sections from biopsy samples of healthy subjects, patients with UC, and patients with CD (n = 3 per group) were stained with haematoxylin-eosin, or immunostained with a rabbit polyclonal antibody against human PAP (1/50). This antihuman PAP antibody was generated by one of the investigators (JLI), as previously described.16 Localisation of PAP in Paneth cells was confirmed by immunostaining for lysozyme (1/400; EnVision System, Dako, Copenhagen, Denmark).
Effects of PAP on intestinal proinflammatory cytokine production
Sets of four endoscopic biopsies from controls and patients with active or inactive CD or UC were seeded on a well with medium alone or supplemented with various concentrations of PAP (25, 50, 500, or 2000 ng/ml). Biopsies were cultured at 37°C with 5% CO2 for 24 hours. Then, supernatants were recovered. Concentrations of interleukin 6 (IL-6), tumour necrosis factor α (TNF-α), interferon γ (IFN-γ), interleukin 12p70 (IL-12p70), interleukin 18 (IL-18), and interleukin 8 (IL-8) in tissue culture supernatants were measured using ELISA kits supplied by Diaclone (Besançon, France). PAP protein was obtained from human pancreatic juice collected by endoscopic retrograde pancreatography and purified using immunoaffinity chromatography, as previously described.10,16 Results are expressed as pg of cytokine per mg of tissue.
In order to determine whether PAP can penetrate into cultured tissue, colonic biopsy samples from CD patients (n = 3), incubated for 24 hours with medium alone or supplemented with 50 ng/ml PAP, were fixed with 4% formaldehyde, and immunohistochemistry against PAP was performed, as described previously.
Assessment of NFκB activation by immunofluorescence in HT29 colonic cells
Human HT29 epithelial colonic cell line was cultured at 37°C with 5% CO2. Cells were preincubated for one hour with various doses of PAP (50, 500, or 1000 ng/ml) and challenged with 10 ng/ml of TNF-α (Sigma, St Louis, Missouri, USA) for 30 minutes at 37°C. Thereafter, cells were incubated with a rabbit polyclonal anti-NFκB p65 (C-20) antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) for one hour, followed by incubation with a goat antirabbit FITC antibody (Santa Cruz Biotechnology) for one hour in the dark. After washing, slides were mounted for fluorescence microscopy.
Quantification of PAP effects on NFκB activation in different cell types
Human promonocytic THP-1 cells as model of immune cells, human HT29 epithelial colonic cells, and human umbilical vein endothelial cells (HUVECs) were used. All cell lines were maintained at 37°C with 5% CO2. Cells were preincubated for one hour with various doses of PAP (50, 100, 500, or 1000 ng/ml) and activated or not with 10 ng/ml TNF-α for 30 minutes at 37°C. Then, nuclear extracts were obtained using a nuclear extract kit from Active Motif (Rixensart, Belgium) and activation of NFκB was measured with a commercial ELISA kit (TransAM; Active Motif). Results are expressed as fold induction of NFκB after TNF-α stimulation.
Assessment of PAP effects on stimulated TNF-α expression in HT29 cells
HT29 cells maintained at 37°C with 5% CO2 were preincubated for one hour with various doses of PAP (50, 100, 500, or 1000 ng/ml) and stimulated or not with 10 ng/ml TNF-α for 30 minutes at 37°C. Then, total RNA was obtained to evaluate changes in TNF-α mRNA expression using quantitative real time reverse transcription-polymerase chain reaction (RT-PCR) analysis on a LightCycler detection system (Roche Applied Science, Basel, Switzerland). Expression levels of TBP gene (encoding the TATA binding protein) were used as an internal control. First strand cDNA was synthesised from 2 μg of total RNA using random hexamers and expanded by reverse transcriptase according to the manufacturer’s instructions (Roche Applied Science), subsequently diluted 1:10 with water, and stored at −20°C until use. TNF-α and TBP PCR products were detected using the Quantitect Probe PCR kit (Qiagen Operon, Cologne, Germany), following the manufacturer’s instructions, using the dual fluorescent Taqman probes (Qiagen Operon) 5′-FAM-TAG CCC ATG TTG TAG CAA ACC CTC AAG CT-TAMRA-3′ (position 435) and 5′-FAM-TCC CAA GCG GTT TGC TGC GGT A-TAMRA-3′ (position 811), respectively. The following primers were used: TBP forward (position 791) 5′-GCC CGA AAC GCC GAA TAT A-3′ and TBP reverse 5′-CGT GGC TCT CTT ATC CTC ATG A-3′ (position 855); TNF-α forward 5′- TCT TCT CGA ACC CCG AGT GA-3′ (position 407) and TNF-α reverse 5′- CCT CTG ATG GCA CCA CCA G-3′ (position 557). Quantitative real time RT-PCR was performed in a total volume of 20 µl containing 1× amplification buffer and 5 µl cDNA template. Samples were heated for 15 minutes at 95°C and amplified for 45 cycles (denaturation at 95°C for 10 seconds, annealing and elongation at 60°C for 60 seconds with a transition rate of 20°C/s). All samples were analysed in duplicate. Data evaluation was performed using the LightCycler data analysis software (version 3.5).
Effects of PAP on adhesion molecule expression in endothelial cells
HUVECs were incubated for 20 hours at 37°C with 5% CO2 and various concentrations of PAP (25, 50, 100, 250, or 1000 ng/ml) in the presence or absence of 10 ng/ml TNF-α, washed, and incubated for 45 minutes with an anti-E-selectin antibody (68-5H11), an anti-ICAM-1 antibody (HA58), or an anti-VCAM-1 antibody (51-10C9). Antibodies were purchased from BD Pharmingen (Heidelberg, Germany). After several washes, cells were incubated with a horseradish peroxidase conjugated secondary antimouse IgG antibody (Caltag, Burlingame, California, USA) for 30 minutes and a developing solution of OPD (Sigma). Absorbance was read at a wavelength of 450 nm. Results are expressed as per cent of OD450 nm reduction versus OD450 nm of TNF-α activated cells.
Statistical analysis
Data were analysed using ANOVA with Bonferroni post hoc test or the non-parametric Kruskal-Wallis test with Dunn’s post hoc test for multiple group comparisons, when appropriate. Repeated measures for the same patient were analysed using the Student’s paired t test. Correlation Z test was used to establish correlations between two quantitative variables. Values are expressed as mean (SEM). Statistical significance was set at p<0.05.
RESULTS
Study 1: analysis of serum PAP in IBD
The presence of chronic intestinal inflammation was associated with increased production of PAP, as estimated from serum levels of the protein. In comparison with healthy controls (n = 29), increased PAP levels in serum were already detected in patients with clinically inactive CD (n = 63) or UC (n = 34), and were further increased in those with active IBD (CD n = 45; UC n = 29), in association with severity of disease, as measured by the corresponding clinical activity indexes (fig 1A ▶). Serum PAP levels had a positive and significant correlation with clinical activity indexes in both CD (r = 0.70, p<0.0001) (fig 1B ▶) and UC (r = 0.44, p<0.001) (fig 1C ▶). PAP levels also correlated positively and significantly with serum CRP levels (CD r = 0.28, p<0.02; UC r = 0.45, p<0.001) and with ESR (CD r = 0.27, p<0.01; UC r = 0.45, p<0.001), although these correlations were weak. In contrast, serum levels of PAP in a group of patients with acute intestinal inflammation due to intestinal infection (n = 14) were identical to those of healthy control subjects (fig 1A ▶). CRP values of this group of inflammatory controls (4.26 (1.78)) were not different from those of IBD patients with active disease (3.89 (0.66)).
Figure 1.
(A) Serum pancreatitis associated protein (PAP) levels in inflammatory bowel disease (IBD) patients with disease of diverse severity. Inactive Crohn’s disease (CD, n = 63) or ulcerative colitis (UC, n = 34) patients had higher PAP levels than healthy controls (n = 29) or inflammatory controls (infectious diarrhoea, n = 14). In IBD patients, PAP levels increased in parallel with disease severity, categorised as: mild (CD n = 23, UC n = 14), moderate (CD n = 18, UC n = 9), or severe (CD n = 4, UC n = 6). *p<0.05 versus healthy controls and inflammatory controls; †p<0.05 versus inactive disease; ‡p<0.05 versus mild disease; §p<0.05 versus moderate disease; ¶p<0.05 versus severe UC. Correlation of serum PAP levels with CD activity index (CDAI) in CD patients (B) and with the Lichtiger modified Truelove and Witts clinical activity index in UC patients (C). Correlation of serum PAP levels with serum C reactive protein (CRP) levels in CD (D) and UC (E) patients. Correlation of serum PAP levels with erythrocyte sedimentation rate (ESR) in CD (F) and UC (G) patients. (H) Serum PAP levels in CD patients according to location of intestinal lesions: ileal (n = 26), ileocolonic (n = 24), or colonic (n = 20). (I) Serum PAP levels in UC patients according to disease extension: distal (n = 21) or extensive (n = 42). Results are expressed as ng PAP/ml serum.
In CD, serum PAP levels were not influenced by location of inflammatory lesions; similar values were observed when patients with ileal, ileocolonic, or colonic lesions of similar clinical severity were compared (fig 1H ▶). In UC, serum PAP levels were also not related to disease extension; similar levels were found in distal and extensive colitis of similar clinical severity (fig 1I ▶). When patients with CD and UC were stratified according to disease severity, PAP levels were similar in both diseases in mild and moderate cases but were significantly higher in severe CD compared with severe UC.
Study 2: characterisation of intestinal PAP production in IBD
Ex vivo analysis of PAP secretion in colonic tissue from IBD patients
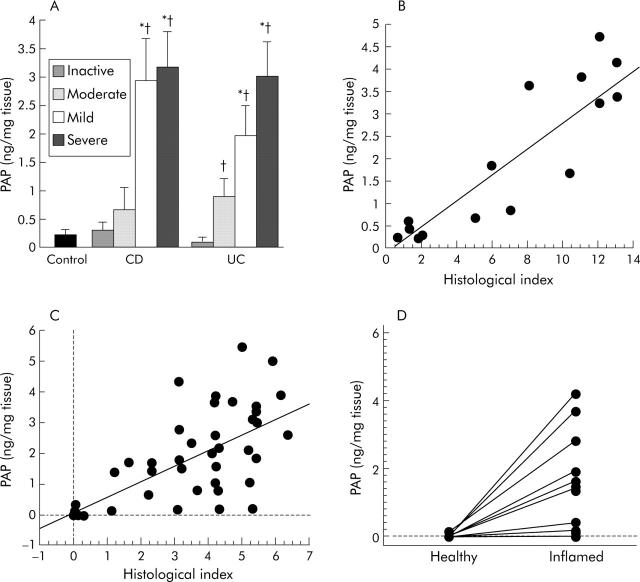
PAP secretion was significantly increased in the mucosa of patients with active CD (n = 10) and UC (n = 36) compared with mucosa from patients with inactive disease (CD n = 5; UC n = 5) or control patients (n = 13). In patients with IBD, ex vivo PAP secretion paralleled the severity of colonic inflammation assessed by endoscopy (fig 2A ▶). Levels of secreted PAP also correlated with histological severity of intestinal inflammation in both CD (r = 0.870; p = 0.0004) (fig 2B ▶) and UC (r = 0.616; p = 0.0001) (fig 2C ▶). When levels of secreted PAP of healthy and inflamed colonic mucosa from the same patient were compared (n = 10), low basal levels of PAP (0.01 (0.01) ng/mg tissue) secreted by the healthy tissue contrasted with the significantly higher levels observed in inflamed tissue (1.94 (0.21) ng/mg tissue; p<0.01) (fig 2D ▶), indicating that increased PAP secretion is restricted to areas involved by the chronic inflammatory process.
Figure 2.
Ex vivo pancreatitis associated protein (PAP) production in intestinal tissue of patients with inflammatory bowel disease (IBD) and controls. (A) PAP secretion paralleled the endoscopic severity of lesions in Crohn’s disease (CD, n = 15) and ulcerative colitis (UC, n = 41). *p<0.05 versus mild disease; †p<0.05 versus inactive disease. Correlation of PAP secretion levels with the histological index in CD (B) and UC (C). (D) Samples of healthy and inflamed intestinal mucosa from 10 IBD patients were assayed in parallel. Inflamed mucosa secreted significantly more PAP than healthy tissue (paired t test, p<0.01).
Paneth cells express PAP in active IBD
Immunostaining of human colonic mucosa from healthy controls did not reveal any positive signal for PAP. In contrast, positive staining for PAP protein in CD and UC colonic mucosa was clearly detected in epithelial cells located at the bottom of crypts (fig 3E ▶, F). Haematoxylin-eosin staining of colonic serial sections indicated that PAP expressing cells exhibited a characteristic morphology of Paneth cells, including the presence of acidophilic granules. Lysozyme staining confirmed localisation of PAP to Paneth cells in UC and also in CD (fig 3C ▶, D).
Figure 3.
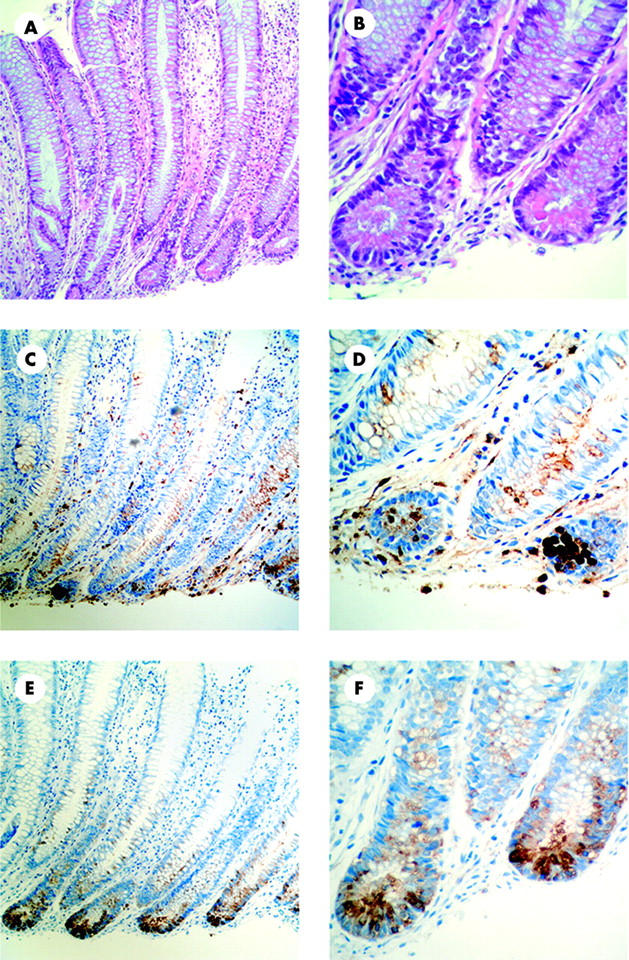
Pancreatitis associated protein (PAP) in the colon of Crohn’s disease (CD) and ulcerative colitis (UC) patients is synthesised in Paneth cells. Immunohistochemistry of consecutive colon paraffin sections are shown. Representative images from a UC patient have been chosen. Haematoxylin-eosin staining demonstrated the presence of metaplastic Paneth cells in the descending colon (A, 200×; B, 600×). Immunostaining for lysozyme precisely identified Paneth cells at the base of crypts (C, 200×; D, 600×). Immunostaining for PAP is seen in metaplastic Paneth cells (E, 200×; F, 600×). No staining was observed in sections incubated without primary antibody (data not shown). All sections were counterstained with haematoxylin.
Study 3: effects of PAP on intestinal inflammation
Proinflammatory cytokine secretion in CD and UC biopsies
Active CD is characterised by increased production of several proinflammatory cytokines such as TNF-α, IL-6, IL-8,17–19 IFN-γ,20 IL-1221 and IL-18.22 Secretion of all of these proinflammatory cytokines by the inflamed mucosa from patients with CD (n = 6) was significantly reduced on incubation with 50 ng/ml PAP to a variable extend (19–55%), with the most marked reductions observed with IFN-γ, TNF-α, and IL-6 (fig 4A ▶). Higher concentrations of PAP (500 or 2000 ng/ml) did not induce further inhibition of cytokine secretion, whereas incubation with 25 ng/ml had a significantly weaker inhibiting effect, as shown in fig 4B ▶. PAP had no significant effects on cytokine secretion by colonic mucosa from patients with inactive CD (n = 4) or from healthy controls (n = 4) in which baseline levels of cytokine secretion were low (data not shown). To rule out possible interference of PAP with the cytokine ELISA assays, PAP was added just before the cytokine assays to the culture medium of tissue samples incubated without PAP. Addition of PAP to the assay medium did not alter cytokine levels measured by ELISA.
Figure 4.

Reduction of cytokine secretion from mucosa samples incubated with pancreatitis associated protein (PAP). (A) Colonic biopsies from inflamed mucosa of patients with Crohn’s disease (CD, n = 6) were cultured for 24 hours in medium alone or with 50 ng/ml PAP, and concentrations of proinflammatory cytokines in culture supernatants were measured by ELISA. Secretion of the following cytokines was monitored: tumour necrosis factor α (TNF-α), interleukin (IL)-6, interferon γ (IFN-γ), IL-18, IL-12, and IL-8. (B) Dose-response relationship between PAP (25, 50, 500, 2000 ng/ml) supplemented medium and TNF-α secretion in the same biopsies as above. Results are expressed as per cent changes in cytokine secretion in tissue samples incubated with PAP relative to samples incubated with medium alone. *p<0.05 for each cytokine, paired t test.
In contrast with the uniform response observed in CD, incubation of inflamed colonic mucosa from patients with UC in the presence of PAP showed variable results. Globally, incubation of inflamed colonic mucosa with PAP 50 ng/ml did not significantly modify secretion of the proinflammatory cytokines relative to samples incubated in the absence of PAP: TNF-α (−17.3 (23.4); n = 8), IL-6 (+3.3 (22.8); n = 8), INF-γ (−-43.6 (19.7); n = 4), or IL-8 (−22.7 (19.3); n = 4). Nevertheless, 50% of patients studied responded with a decrease in the production of these cytokines after incubation with PAP. Higher concentrations of PAP (500 or 2000 ng/ml) did not induce further inhibition or increase the proportion of responses (data not shown). We did not analyse IL-18 or IL-12 secretion in UC biopsies because levels of these cytokines in tissue culture supernatants were not increased during inflammation, a finding in keeping with previous observations.21,22
To determine whether exogenous PAP can penetrate into cultured colonic tissue, biopsies incubated in the presence or absence of this protein were immunostained for PAP. As shown in fig 5 ▶, immunostaining was detected in the cytoplasm of epithelial cells of the samples incubated with this protein whereas in biopsies incubated with medium alone the signal was only present in Paneth cells, as mentioned above. This result indicates that epithelial cells are the main target for PAP and that these cells are able to internalise it.
Figure 5.

Presence of pancreatitis associated protein (PAP) in epithelial cells of colonic biopsies incubated with this protein. Immunohistochemistry for PAP in colonic sections from a patient with Crohn’s disease (CD) incubated for 24 hours with medium alone (A) or with 50 ng/ml PAP (B). (A) In the absence of PAP in the medium, immunostaining for PAP was only found in Paneth cells at the base of the crypts; no signal was found in other epithelial cells (400×). (B) In tissue samples incubated in the presence of exogenous PAP, the protein was diffusely identified in the cytoplasm of all epithelial cells (600×). All sections were counterstained with haematoxylin.
NFκB activation in different cell types
Expression of cytokines that are downregulated on incubation of inflamed CD tissue with PAP is in part regulated by NFκB.23–28 We hypothesised that inhibition of NFκB activation might be one of the mechanisms mediating the anti-inflammatory effect of PAP.
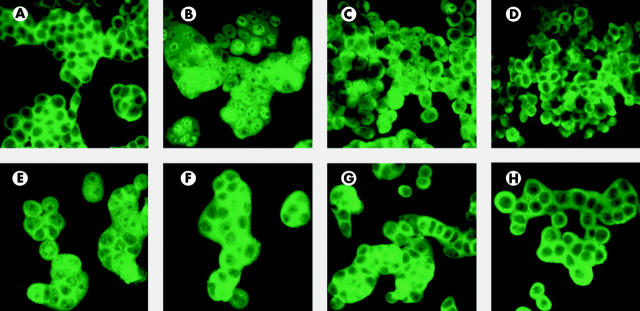
Initially, immunofluorescence studies were used to monitor the effects of PAP on NFκB activation in the colonic epithelial cell line HT29 challenged with TNF-α. In non-stimulated cells, NFκB p65 staining was detected only in the cytoplasm (fig 6A ▶). Stimulation of these cells with 10 ng/ml TNF-α induced translocation of NFκB p65 to the nucleus (fig 6B ▶). Incubation of stimulated cells with 50, 500, or 1000 ng/ml PAP inhibited translocation of NFκB p65 in most cells (fig 6C ▶, D, E), as staining remained predominantly cytoplasmic. Inhibition was stronger with 500 ng/ml PAP than with 50 ng/ml but did not further increase when a concentration of 1000 ng/ml was used. Incubation of non-stimulated cells with PAP did not result in activation of NFκB (fig 6F ▶, G, H).
Figure 6.
Effect of pancreatitis associated protein (PAP) on nuclear translocation of nuclear factor κB (NFκB) in colonic epithelial cells. The intracellular location of NFκB p65 was determined in HT29 cells by immunofluorescence using an anti-NFκB p65 antibody with FITC labelling. In non-stimulated cells incubated with medium alone, staining was cytoplasmic (A). (B) Cells stimulated with 10 ng/ml tumour necrosis factor α (TNF-α) showed diffuse immunostaining, indicating that NFκB had translocated from the cytoplasm to the nucleus. (C) Stimulated cells in the presence of 50 ng/ml PAP maintained predominantly cytoplasmic NFκB immunostaining, indicating inhibition of NFκB translocation. Incubation of stimulated cells with 500 ng/ml (D) or 1000 ng/ml (E) PAP resulted in nearly complete restriction of immunostaining to the cytoplasm, indicating stronger inhibition of NFκB translocation. In cells incubated with PAP 50 ng/ml (F), 500 ng/ml (G), and 1000 ng/ml (H) in the absence of TNF-α, immunostaining of NFκB was restricted to the cytoplasm. All preparations were blindly examined in a fluorescence microscope with 1000×.
To confirm the inhibitory ability of PAP on TNF-α induced NFκB activation, and to explore its differential effects in different cell types, we used a quantitative NFkB activation assay (TransAM). Monocytes, epithelial cells, and endothelial cells have been implicated in the pathogenesis of IBD,29 and these cell types show activation of NFκB in inflamed intestinal mucosa.30,31 To investigate which cell types may be affected by PAP, NFκB activation was measured in stimulated human THP-1 monocytic cells, HT29 epithelial cells, and HUVECs. As shown in fig 7 ▶, PAP inhibited TNF-α induced NFκB activation in all three cell types, in a dose dependent manner, with THP-1 cells showing the highest degree of inhibition. Addition of 100–500 ng/ml PAP to the culture medium in non-stimulated cells did not alter NFκB activation.
Figure 7.
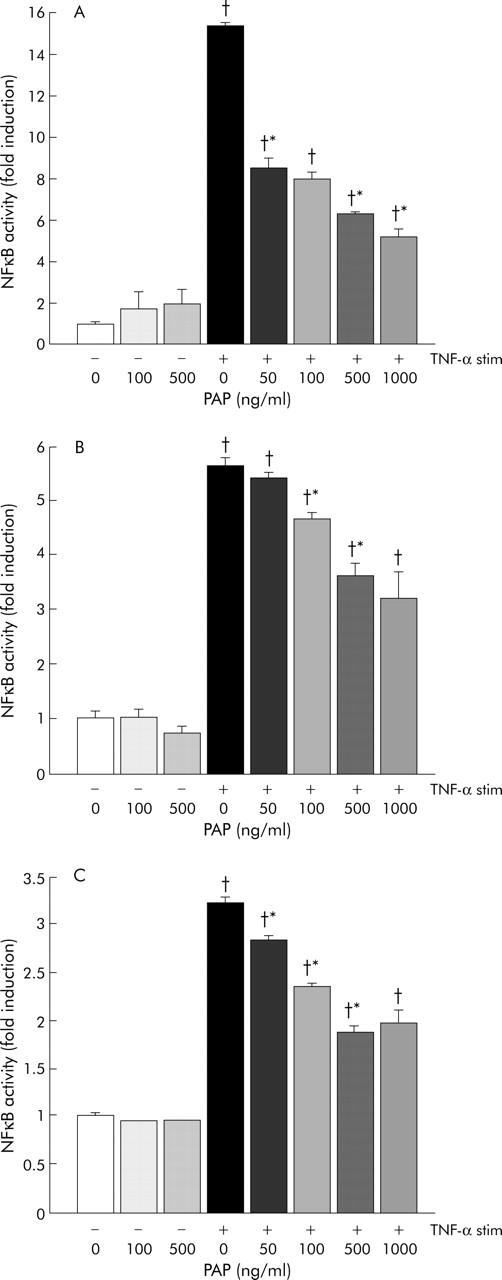
Effects of pancreatitis associated protein (PAP) on nuclear factor κB (NFκB) activation in THP-1 cells (A), HT29 cells (B), and human umbilical vein endothelial cells (C). Cells were preincubated for one hour with increasing PAP concentrations (0, 50, 100, 500, or 1000 ng/ml) and stimulated with 10 ng/ml tumour necrosis factor α (TNF-α) for 30 minutes. Nuclear extracts were obtained, and activated NFκB was measured using an oligonucleotide based specific ELISA (TransAM). NFκB activation in TNF-α stimulated cells was significantly reduced in a dose dependent manner by incubation with PAP. Results are expressed as NFκB-fold induction Experiments were performed in quadruplicate and all results are expressed as mean (SEM). *p<0.05 versus previous PAP dose; †p<0.05 versus TNF-α (−) PAP 0 ng/ml.
Effects of PAP on stimulated TNF-α transcription
To test the possible link between the effects of PAP on NFκB activation and cytokine production in cell lines, we monitored expression of TNF-α mRNA in stimulated HT29 cells after incubation with PAP by real time RT-PCR. As shown in fig 8 ▶, addition of PAP to the culture medium reduced TNF-α induced transcript levels in a dose dependent manner, confirming the inhibitory action of PAP on proinflammatory cytokine production through transcription regulation.
Figure 8.
Tumour necrosis factor α (TNF-α) mRNA expression normalised to endogenous control TBP gene. HT29 cells were preincubated for one hour with increasing pancreatitis associated protein (PAP) concentrations (0, 50, 100, 500, or 1000 ng/ml) and stimulated with 10 ng/ml TNF-α for 30 minutes. Total RNA was extracted and TNF-α mRNA expression was quantified by quantitative real time reverse transcription-polymerase chain reaction. TNF-α expression in stimulated cells was significantly reduced in a dose dependent manner by incubation with PAP. Results are shown as percentage relative to TNF-α expression in control cells (100%) given as mean (SEM) of four different experiments. *p<0.05 versus previous PAP dose; †p<0.05 versus control (TNF-α (−) PAP 0 ng/ml).
Endothelial adhesion molecule expression
Endothelial cells are activated in active IBD32,33 and this activation is accompanied by an increase in adhesion molecule expression. Endothelial expression of E-selectin, ICAM-1, and VCAM-1 is strongly induced by TNF-α and is dependent on NFκB activation.34 We explored whether PAP could also affect TNF-α induced adhesion molecule expression in HUVECs. Indeed, PAP significantly inhibited TNF-α induced adhesion molecule upregulation in a dose dependent manner. E-selectin upregulation was most sensitive to the effects of PAP whereas ICAM-1 and VCAM-1 upregulation were only affected by the highest doses of PAP (fig 9 ▶).
Figure 9.

Effect of pancreatitis associated protein (PAP) on expression of E-selectin (A), ICAM-1 (B), and VCAM-1 (C) in endothelial cells. Human umbilical vein endothelial cells were stimulated with 10 ng/ml tumour necrosis factor α (TNF-α) and incubated with increasing PAP concentrations (0, 25, 50, 100, 250, or 1000 ng/ml). TNF-α stimulation induced an increase in E-selectin, ICAM-1, and VCAM-1 expression and this expression was significantly reduced by co-incubation with PAP in all cases. Results are expressed as percentage of adhesion molecule expression relative to TNF-α stimulated cells. Experiments were performed in quadruplicate and all results are expressed as mean (SEM).
DISCUSSION
In this study, we have shown that serum PAP levels were increased in patients with IBD relative to healthy controls, and this increase seemed to be specific for chronic intestinal inflammation as serum PAP was not increased in patients with intestinal inflammation in the context of infectious diarrhoea. The concept that intestinal PAP production is elevated in active IBD had already been put forward in studies based on measurement of PAP mRNA in intestinal samples.4–6 In the current study, we explored whether disease type, location, or severity influenced PAP production. We provided evidence that in both CD and UC, serum PAP levels parallel disease severity. Nevertheless, patients with inactive disease had still higher serum PAP levels than those of control healthy subjects. The increase in PAP production in active disease bore no relationship to type or location of disease. This observation is at odds with a recent study involving CD patients which found increased PAP levels only in those with ileal disease.35 Our results, including a significant number of CD patients with disease limited to the colon and, even more convincingly, the observation of increased PAP levels in patients with UC, clearly indicates that production of this protein is also increased in the presence of inflammation limited to the colon. The notion that PAP production is increased in relation to the severity of intestinal inflammation was confirmed in the ex vivo studies measuring PAP production in inflamed intestinal tissue, in which highly significant correlations were found between histological and endoscopic severity of intestinal inflammation and PAP liberation to the culture medium.
Increased PAP production in both ileal and colonic disease is probably related to hyperplasia and metaplasia of Paneth cells. In the immunohistochemistry study performed in tissue samples obtained from patients with active IBD, we observed that PAP protein localised in Paneth cells in ileal and also in colonic tissue. This observation is in keeping with a recent study6 and may explain the marginal but significant increase in serum PAP in inactive IBD as hyperplasia and metaplasia of Paneth cells is maintained during periods of quiescent disease.
The role that increased PAP production may have on the course of intestinal inflammation is currently unknown. Here we provide evidence that exogenous PAP supplementation can penetrate into intestinal tissue, specifically into epithelial cells, and oppose the inflammatory process that takes place in IBD. We demonstrated that secretion of proinflammatory cytokines by colonic tissue of patients with active CD was inhibited by addition of PAP, and that downregulation of cytokine production occurred through a mechanism involving inhibition of NFκB activation. PAP inhibited TNF-α induced NFκB activation in three cell types that participate in the initiation and perpetuation of intestinal inflammation—that is, monocytes, epithelial cells, and endothelial cells.29
Increased expression of adhesion molecules is another important factor involved in the pathogenesis of IBD.36 We asked whether PAP could directly affect these determinants of leucocyte recruitment by monitoring expression of E-selectin, ICAM-1, and VCAM-1, as these molecules are upregulated through a NFκB dependent mechanism.34 TNF-α induced expression of these adhesion molecules was significantly reduced when cells were challenged in the presence of PAP. Hence PAP could also counteract the inflammatory response by inhibiting leucocyte recruitment into the intestine. Unfortunately, we could not directly assess the anti-inflammatory role of endogenous PAP due to the lack of blocking antibodies and absence of knockout animal models.
In the current study, we observed that supplementation with exogenous PAP did not have the same anti-inflammatory effect in the inflamed mucosa of CD and UC. We observed a significant and consistent reduction of cytokine production in all samples of inflamed tissue from CD patients, whereas only 50% of patients with UC responded with a reduction in cytokine production, and this decrease did not reach statistical significance when we examined the UC group as a whole. Such a difference could be attributable to the fact that immune cell activation patterns present in CD and UC are different. Activation of immune response in CD is of the Th1-type, with increased production of TNF-α, IL-12, IFN-γ, and IL-18, and a clinical response to immunoblockade of some of these cytokines such as TNF-α or IL-12,37 whereas the pattern of immune cell activation in UC is more complex. Discrepancies between the response of UC and CD to various forms of treatment, such as ciclosporin12,38 or methotrexate,39,40 has been previously documented in clinical controlled trials. Thus it is conceivable that if blockade of cytokines involved in the Th1-type response is a key element in reducing activation of the immune system by PAP in CD, this may not have the same effect in UC.
In conclusion, this study demonstrates that PAP synthesis is increased in IBD, to an extent that parallels the severity of intestinal inflammation. This increment is probably related to hyperplasia/metaplasia of Paneth cells, which is in keeping with the notion that these cells exert mostly a protective function in intestinal homeostasis. PAP secreted by these cells may dampen the inflammatory damage by affecting diverse components of the inflammatory response, including cytokine production, adhesion molecule expression, and activation of NFkB. Although the finding of increased levels of a potential anti-inflammatory factor paralleling disease severity may be puzzling, it may reflect activation of regulatory functions to limit tissue damage, as has been described for other molecules that downregulate various elements of the inflammatory cascade, such as IL-10 and IFN-γ.41,42 In that regard, increased PAP levels in inactive disease may also have a role in maintaining a balance between anti- and proinflammatory factors in an inflammation prone intestine. If PAP was eventually shown to be effective in the treatment of human CD, the use of a small human recombinant protein may have numerous advantages over antibody based therapies in terms of tolerance and immunogenicity.
Acknowledgments
This work was supported by grants SAF2002/02211 from Ministerio de Ciencia y Tecnología, FIS PI020286, 01/0099-01E, and C03/02 from the Instituto de Salud Carlos III. M Gironella is the recipient of a grant from Fondation Recherche Médicale.
We gratefully acknowledge Dr Jean Charles Dagorn for his help in the preparation of the manuscript and Elena Gonzalvo and Margarita Mainar for their technical assistance in immunohistochemistry.
Abbreviations
CD, Crohn’s disease
IBD, inflammatory bowel disease
UC, ulcerative colitis
PAP, pancreatitis associated protein
CRP, C reactive protein
ESR, erythrocyte sedimentation rate
HUVEC, human umbilical vein endothelial cells
IL, interleukin
IFN-γ, interferon γ
TNF-α, tumour necrosis factor α
NFκB, nuclear factor κB
RT-PCR, reverse transcription-polymerase chain reaction
Published online first 3 May 2005
Conflict of interest: None declared.
REFERENCES
- 1.Keim V, Rohr G, Stockert HG, et al. An additional secretory protein in the rat pancreas. Digestion 1984;29:242–9. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto H. The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells. J Hepatobiliary Pancreat Surg 1999;6:254–62. [DOI] [PubMed] [Google Scholar]
- 3.Christa L, Carnot F, Simon MT, et al. HIP/PAP is an adhesive protein expressed in hepatocarcinoma, normal Paneth, and pancreatic cells. Am J Physiol 1996;271:G993–1002. [DOI] [PubMed] [Google Scholar]
- 4.Dieckgraefe BK, Stenson WF, Korzenik JR, et al. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics 2000;4:1–11. [DOI] [PubMed] [Google Scholar]
- 5.Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet 2001;10:445–56. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa H, Fukushima K, Naito H, et al. Increased expression of HIP/PAP and regenerating gene III in human inflammatory bowel disease and a murine bacterial reconstitution model. Inflamm Bowel Dis 2003;9:162–70. [DOI] [PubMed] [Google Scholar]
- 7.Shytenberg A, Kandil E, Lin Y, et al. Up-regulation of pancreatitis-associated protein in a rat model of inflammatory bowel disease. J Surg Res 2003;114:292–3. [Google Scholar]
- 8.Fiedler F, Croissant N, Rehbein C, et al. Acute-phase response of the rat pancreas protects against further aggression with severe necrotizing pancreatitis. Crit Care Med 1998;26:887–94. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Kandil E, Lin YY, et al. Targeted inhibition of gene expression of pancreatitis-associated proteins exacerbates the severity of acute pancreatitis in rats. Scand J Gastroenterol 2004;39:870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller A, Fiedler F, Schmeck J, et al. Pancreatitis-associated protein protects the lung from leukocyte-induced injury. Anesthesiology 1999;91:1408–14. [DOI] [PubMed] [Google Scholar]
- 11.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s disease activity index (CDAI). Gastroenterology 1979;77:843–6. [PubMed] [Google Scholar]
- 12.Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med 1994;330:1841–5. [DOI] [PubMed] [Google Scholar]
- 13.Hanauer SB, Robinson M, Pruitt R, et al. Budesonide enema for the treatment of active, distal ulcerative colitis and proctitis: a dose-ranging study. U.S. Budesonide enema study group. Gastroenterology 1998;115:525–32. [DOI] [PubMed] [Google Scholar]
- 14.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 16.Keim V, Iovanna JL, Orelle B, et al. A novel exocrine protein associated with pancreas transplantation in humans. Gastroenterology 1992;103:248–54. [DOI] [PubMed] [Google Scholar]
- 17.Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol 1993;94:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsuyama K, Sasaki E, Toyonaga A, et al. Colonic mucosal interleukin-6 in inflammatory bowel disease. Digestion 1991;50:104–11. [DOI] [PubMed] [Google Scholar]
- 19.Banks C, Bateman A, Payne R, et al. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J Pathol 2003;199:28–35. [DOI] [PubMed] [Google Scholar]
- 20.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 1996;157:1261–70. [PubMed] [Google Scholar]
- 21.Pallone F, Monteleone G. Interleukin 12 and Th1 responses in inflammatory bowel disease. Gut 1998;43:735–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizarro TT, Michie MH, Bentz M, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol 1999;162:6829–35. [PubMed] [Google Scholar]
- 23.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol 1990;10:1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol 1994;14:4443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunsch C, Lang RK, Rosen CA, et al. Synergistic transcriptional activation of the IL-8 gene by NF-kappa B p65 (RelA) and NF-IL-6. J Immunol 1994;153:153–64. [PubMed] [Google Scholar]
- 26.Murphy TL, Cleveland MG, Kulesza P, et al. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol 1995;15:5258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suk K, Yeou KS, Kim H. Regulation of IL-18 production by IFN gamma and PGE2 in mouse microglial cells: involvement of NF-kB pathway in the regulatory processes. Immunol Lett 2001;77:79–85. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji-Takayama K, Aizawa Y, Okamoto I, et al. Interleukin-18 induces interferon-gamma production through NF-kappaB and NFAT activation in murine T helper type 1 cells. Cell Immunol 1999;196:41–50. [DOI] [PubMed] [Google Scholar]
- 29.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205. [DOI] [PubMed] [Google Scholar]
- 30.Rogler G, Brand K, Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998;115:357–69. [DOI] [PubMed] [Google Scholar]
- 31.Thiele K, Bierhaus A, Autschbach F, et al. Cell specific effects of glucocorticoid treatment on the NF-kappaBp65/IkappaBalpha system in patients with Crohn’s disease. Gut 1999;45:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binion DG, West GA, Ina K, et al. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 1997;112:1895–907. [DOI] [PubMed] [Google Scholar]
- 33.Pooley N, Ghosh L, Sharon P. Up-regulation of E-selectin and intercellular adhesion molecule-1 differs between Crohn’s disease and ulcerative colitis. Dig Dis Sci 1995;40:219–25. [DOI] [PubMed] [Google Scholar]
- 34.Collins T, Read MA, Neish AS, et al. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 1995;9:899–909. [PubMed] [Google Scholar]
- 35.Desjeux A, Barthet M, Barthellemy S, et al. Serum measurements of pancreatitis associated protein in active Crohn’s disease with ileal location. Gastroenterol Clin Biol 2002;26:23–8. [PubMed] [Google Scholar]
- 36.Panes J. Adhesion molecules: their role in physiopathology and treatment of inflammatory bowel disease. Gastroenterol Hepatol 1999;22:514–24. [PubMed] [Google Scholar]
- 37.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002;122:1592–608. [DOI] [PubMed] [Google Scholar]
- 38.Feagan BG, McDonald JW, Rochon J, et al. Low-dose cyclosporine for the treatment of Crohn’s disease. The Canadian Crohn’s Relapse Prevention Trial Investigators. N Engl J Med 1994;330:1846–51. [DOI] [PubMed] [Google Scholar]
- 39.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group Investigators. N Engl J Med 2000;342:1627–32. [DOI] [PubMed] [Google Scholar]
- 40.Oren R, Arber N, Odes S, et al. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology 1996;110:1416–21. [DOI] [PubMed] [Google Scholar]
- 41.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol 1995;101:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Autschbach F, Giese T, Gassler N, et al. Cytokine/chemokine messenger-RNA expression profiles in ulcerative colitis and Crohn’s disease. Virchows Arch 2002;441:500–13. [DOI] [PubMed] [Google Scholar]