Abstract
Background and aims: Although regenerating gene (REG) Iα protein may be involved in the inflammation and carcinogenesis in the gastrointestinal tract, its pathophysiological role in ulcerative colitis (UC) and the resulting colitic cancer remains unclear. We investigated expression of the REG Iα gene and its protein in UC and colitic cancer tissues. We examined whether cytokines are responsible for REG Iα gene expression and whether REG Iα protein has a trophic and/or an antiapoptotic effect on colon cancer cells.
Methods: Expression of REG Iα mRNA and its gene product in UC tissues was analysed by real time reverse transcription-polymerase chain reaction and immunohistochemistry, respectively. The effects of cytokines on REG Iα promoter activity were examined in LoVo cells by luciferase reporter assay. The effects of REG Iα protein on growth and H2O2 induced apoptosis were examined in LoVo cells by MTT and TUNEL assays, respectively.
Results: REG Iα protein was strongly expressed in inflamed epithelium and in dysplasias and cancerous lesions in UC tissues. The level of REG Iα mRNA expression in UC tissues correlated significantly with severity of inflammation and disease duration. REG Iα promoter activity was enhanced by stimulation with interferon γ or interleukin 6. REG Iα protein promoted cell growth and conferred resistance to H2O2 induced apoptosis in LoVo cells. REG Iα protein promoted Akt phosphorylation and enhanced Bcl-xL and Bcl-2 expression in LoVo cells.
Conclusions: The REG Iα gene is inducible by cytokines and its gene product may function as a mitogenic and/or an antiapoptotic factor in the UC-colitic cancer sequence.
Keywords: REG, antiapoptosis, cytokine, proliferation, ulcerative colitis
The regenerating gene (Reg) was originally isolated from a complementary DNA (cDNA) library derived from regenerating rat pancreatic islets, and its human homologue was named REG Iα.1 REG Iα protein is predominantly expressed in the normal pancreas, and at lower levels in the stomach and colon, implying physiological roles for REG Iα in these organs.2,3 Indeed, recent studies have reported that REG Iα is overexpressed in pancreatitis,4,5Helicobacter pylori induced gastritis,6 and in gastric ulcer lesions,7,8 suggesting that has an important role in the pathogenesis of gastrointestinal inflammatory diseases. Moreover, it is interesting that REG Iα gene was identified as a distinctly overexpressed gene in inflammatory bowel disease by microarray analyses.9,10 However, it is not clear how REG Iα gene expression is enhanced in such inflammatory conditions.
Although we and others have previously suggested that Reg protein has a trophic effect on mammalian epithelial cells,11–13 its biological functions are still unclear. Recently, REG Iα was suggested to be involved not only in inflammatory diseases but also in carcinogenesis in various gastroenterological tissues, such as the stomach,14,15 colon,16 bile duct,17 and pancreas3; however, its involvement in ulcerative colitis (UC) associated colorectal cancer (colitic cancer) is not known. As the REG Iα gene is probably overexpressed in UC,9,10 REG Iα may play an important role as a trophic or other factor in the development of colitic cancers. In the present study, in order to elucidate the role of REG Iα in the UC-colitic cancer sequence, we investigated the relationship between REG Iα expression and clinicopathological factors in patients with UC and colitic cancer. Furthermore, in in vitro studies, we examined whether cytokines enhance REG Iα gene expression and whether REG Iα has a trophic and/or an antiapoptotic effect on colon cancer cells.
MATERIALS AND METHODS
Tissue specimens and histological examination
Colon biopsy specimens were obtained by endoscopy from 24 patients with UC (15 men and nine women; mean age 45.6 years (range 19–79); mean disease duration 6.4 years (range 0–19)), four patients with Crohn’s disease (two men and two women; mean age 36.0 years (range 26–50); mean disease duration 5.5 years (range 0–15)), eight patients with proctitis (six men and two women; age range 40–79 years), 10 patients with sporadic colon adenoma (seven men and three women; age range 55–85 years), and five normal controls (five men; age range 33–38 years) at Kyoto University Graduate School of Medicine. Tissue specimens were used for real time reverse transcription-polymerase chain reaction (RT-PCR) and histological analyses. This work was done with the approval of the Review Board of Kyoto University Hospital, and informed consent was obtained from all patients.
A total of seven colitic cancer lesions (location: five rectum, one sigmoid, one descending; histology: four well differentiated adenocarcinomas, three mucinous adenocarcinomas) were obtained from surgically resected specimens from four UC patients (two men and two women; age range 44–58 years; disease duration 11–25 years) and eight sporadic colon cancer lesions (location: three rectum, two sigmoid, one descending, one ascending, one caecum; two well differentiated adenocarcinomas, six moderately differentiated adenocarcinomas) were obtained from eight non-UC patients (five men and three women; age range 65–79 years) at Dokkyo University School of Medicine. The colitic cancer tissue specimens were fixed in 10% formalin solution, embedded in paraffin, and subjected to histological analyses. Sporadic colon cancer tissue specimens were used for real time RT-PCR and histological analyses. This work was done with the approval of the Dokkyo University Surgical Pathology Committee, and informed consent was obtained from all patients.
The diagnosis of UC was based on established endoscopic and histological criteria,18 and the degree of inflammation was evaluated according to Matts’ grade18 throughout the experiments.
Immunohistochemical staining
Immunohistochemical stainings for proliferating cell nuclear antigen (PCNA) and REG Iα protein were performed, as described previously,6,14 using antihuman PCNA antibody (PC10; Dako Japan, Kyoto, Japan; dilution 1:1000) and antihuman REG Iα antibody (dilution 1:2000).3 A cancerous specimen was considered positive for REG Iα when more than 20% of the tumour cells were positively stained.14
Real time RT-PCR
Total RNA was isolated from colonic biopsy samples and seven human colon cancer cell lines (Caco-2, COLO 205, DLD-1, HT-29, LoVo, SW403, and WiDr) with Trizol reagent (Gibco BRL, Rockville, Maryland, USA). Total RNA (5 μg) was reverse transcribed using oligo-dT primer (Applied Biosystems, Branchburg, New Jersey, USA) and RT product was amplified by PCR, as previously described.6,14 TaqMan quantitative real time PCR was performed with the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, California, USA). The following set of primers and probe for human REG Iα was prepared: human REG Iα 5′-CTA GAG GCA ACT GGA AAA TAC ATG TCT-3′ (sense), 5′-GTT GGA GAG ATG GTC CGG TTT-3′ (antisense), and 5′-FAM-AAC GGA GTC AAA AAT T (probe). In addition, a set of primers and probe for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was synthesised by Applied Biosystems.
Each amplification consisted of a 50 μl reaction mixture with 50 ng of cDNA, 250 nM of REG Iα probe (or 100 nM of GAPDH probe), 900 nM of REG Iα primer (or 200 nM of GAPDH primer), and 1×TaqMan universal PCR master mixture (Applied Biosystems). PCR cycling conditions were 50°C for two minutes, 95°C for 10 minutes, followed by 45 cycles at 95°C for 15 seconds and 60°C for 60 seconds. A template free negative control was included in all amplifications, and each assay was performed in duplicate. The intensity of the fluorescent dye was determined, and expression levels of REG Iα mRNA were normalised to GAPDH mRNA expression levels.
Cell culture and treatment
Colon cancer cell lines LoVo and SW403 were routinely maintained in RPMI1640 medium (Invitrogen, Grand Island, New York, USA) with 10% fetal bovine serum (Invitrogen) in a humidified incubator at 37°C with an atmosphere of 5% CO2. These cell lines were used for assessment of REG Iα promoter activity by interferon γ (IFN-γ; Roche, Mannheim, Germany), tumour necrosis factor α (TNF-α; Roche), interleukin (IL)-1β (Roche), IL-5 (Pepro Tech Inc, Rocky Hill, New Jersey, USA), IL-6 (Roche), IL-8 (Roche), and IL-13 (Pepro Tech Inc). In addition, LoVo cells were used for assays of cell growth and apoptosis.
Luciferase activity assay and transfection
The human REG Iα promoter from −1195 to +78 was generated from human stomach DNA by PCR using the following set of primers containing Mlu I and Bgl II restriction site, respectively: 5′-CTT ACG CGT GAA TTC CTG GGC TCA AGT GA-3′ and 5′’-CCC GAA GAT TTT AGA TCT ACA GTG C-3′’. The cloned nucleotides of the promoter were inserted into the position between Mlu I and Bgl II restriction site, upstream of the luciferase gene in the pGL3-Basic vector (Promega, Madison, Wisconsin, USA), and the construct was named hREG Iα-Luc.
LoVo and SW403 cells (2×104) were seeded 24 hours before transfection in 12 well plates (Iwaki, Funabashi, Japan). Cells were cotransfected with 700 ng of a hREG Iα-Luc construct and 7 ng of Renilla luciferase plasmid pRL-TK (as a control of transfection efficiency) in the Optimen medium (Gibco, Grand Island, New York, USA) using FuGENE 6 transfection reagent (Roche, Indianapolis, Indiana, USA) according to the manufacturer’s protocol. Forty eight hours later, cells were stimulated by IFN-γ, TNF-α, IL-1β, IL-5, IL-6, IL-8, and IL-13 for 3, 6, 12, and 24 hours.
Luciferase assays were performed using the Dual Luciferase Reporter Assay system (Promega) according to manufacturer’s protocol. Firefly luciferase and Renilla luciferase activities were assayed by luminometer (Lumat LB 9506; Berthold, Germany). Results obtained were normalised for Renilla luciferase activity and expressed as fold of activity of the untreated cell group at the 0 hour time point.
Transfection and expression of the human REG Iα cDNA
The full length of human REG Iα cDNA was inserted into the pIRES2-EGFP vector containing cytomegalovirus promoter driving the enhanced green fluorescent protein gene (EGFP) (Clontech, Palo Alto, California, USA). After cloning and verifying the nucleotides of the human REG Iα cDNA by sequencing, the construct was named pIRES2-hREG Iα, and pIRES2-EGFP vector without insert was used as a control.
Plasmids were stably transfected into LoVo cells using FuGENE 6 transfection reagent, as described above. To select cells with stable expression of the pIRES2-hREG Iα and pIRES2-EGFP, cells were cultured for over 3–4 weeks in medium containing G418 (Gibco; 400 μg/ml) and surviving colonies were pooled.
Preparation of conditioned medium
To prepare conditioned medium, we cultured human embryonic kidney (HEK) 293T cells in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. According to the manufacturer’s protocol, cells were transfected with 10 μg of pIRES2-hREG Iα or control plasmid using FuGENE 6 transfection reagent. The medium was replaced by serum free RPMI1640 medium after a 48 hour incubation period. The conditioned medium was then collected and stored frozen as a source of recombinant REG Iα protein.
Cell growth assay
Cell growth was assessed by Cell Counting Kit-8 (Dojindo, Kumamoto, Japan). LoVo cells (5×103), stably transfected with pIRES2-hREG Iα (LoVo-REG Iα cells) or pIRES2-EGFP (LoVo-EGFP) vector, were plated in 96 well microplates. Cells were incubated in serum free RPMI1640 medium for 24, 48, and 72 hours. After addition of 10 μl of the Cell Counting Kit-8 reagent followed by a three hour incubation, the plates were read at 450 nm in a spectrophotometer (Molecular Devices Co., Sunnyvale, California, USA). To assess the effect of REG Iα in the medium, we added anti-REG Iα antibody (50 μg/ml) to serum free RPMI1640 medium,13,19 and cells incubated for 72 hours were also evaluated in the same procedure.
Cell growth assay was also performed to examine the trophic effect of REG Iα protein on colon cancer cells. Briefly, LoVo cells (5×103) were cultured in 96 well microplates for 24 hours. Then, the medium was changed to conditioned medium containing human recombinant REG Iα or control medium, and cells were incubated for 24, 48, and 72 hours. To assess the effect of REG Iα in the medium, we added anti-REG Iα antibody to control or REG Iα containing medium, and cells incubated for 72 hours were also evaluated in the same procedure.
TUNEL assay
LoVo-REG Iα cells (1×104) and control LoVo-EGFP cells were cultured in four well culture slides (Falcon, Bedford, Massachusetts, USA). Twenty four hours later, cells were incubated for two hours with different concentrations (0–10 mmol/l) of H2O2 in serum free medium. Thereafter, cells were incubated in routine medium for 24 hours. After wash with phosphate buffered saline, slides were fixed with 10% buffered formalin for 15 minutes and then stained by In Situ Cell Death Detection Kit (Roche) according to the supplied protocol. TdT mediated dUTP nick end labelling (TUNEL) index was calculated as the percentage of positive cells. To assess the effect of REG Iα in the medium, we added anti-REG Iα antibody (50 μg/ml) to routine medium immediately after treatment with H2O2 (5 mmol/l).
TUNEL assay was also performed to examine the antiapoptotic effect of REG Iα protein on colon cancer cells. LoVo cells (5×103) were cultured in four well culture slides for 24 hours, followed by a 24 hour incubation with conditioned medium containing human recombinant REG Iα or control medium. Then, cells were incubated for another two hours with different concentrations (0–10 mmol/l) of H2O2, and subjected to TUNEL assay 24 hours later. To assess the effect of REG Iα in the medium, we added anti-REG Iα antibody to control or REG Iα containing medium immediately after treatment with H2O2 (5 mmol/l).
Detection of phosphorylated, non-phosphorylated Akt, and Bcl family proteins
LoVo cells were cultured in 10 cm dishes for 24 hours. After washing with phosphate buffered saline, the medium was changed to conditioned medium containing human recombinant REG Iα or control medium, and cells were incubated for another 12 hours. Cells were then mixed with lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40 (NP-40), 50 mM NaF, and 1×proteinase inhibitor (Complete Mini; Roche). Protein extract (10 μg) was fractionated by sodium dodecyl sulphate polyacrylamide gel electrophoresis. After transfer to a polyvinylidene difluoride membrane, western blots were performed using anti-Akt, antiphospho specific Akt (Ser473) (New England Biolabs, Beverly, Massachusetts, USA), anti-Bcl-2, anti-Bcl-xL, anti-Mcl-1 (BD Sciences, San Jose, California, USA), and anti-β-actin antibodies (Sigma Chemical Co., St Louis, Missouri, USA), as previously reported.20
Statistical analysis
All values are expressed as mean (SEM). Statistical differences between the two groups were assessed by the unpaired two tailed t test or by the Mann-Whitney U test when data were not parametric. The relationship between REG Iα mRNA level and disease duration was assessed by linear regression analysis. A p value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Expression of REG Iα mRNA in normal colonic and ulcerative colitis mucosa
REG Iα mRNA expression was detectable by real time RT-PCR in all colonic mucosa from both control and UC patients. As shown in fig 1A ▶, the level of REG Iα mRNA expression was significantly greater in UC tissues than in normal colonic tissues. Furthermore, levels of REG Iα mRNA expression in Crohn’s colitis and proctitis tissues were also significantly greater than that in normal colonic tissues. We next analysed the relationship between REG Iα mRNA expression and several clinicopathological factors, and found that REG Iα mRNA expression was significantly enhanced as the severity of endoscopic or histological features increased (fig 1B, 1C ▶ ▶). Moreover, REG Iα mRNA expression in UC patients of long duration (⩾10 years) was significantly greater than those of short duration (<10 years) (fig 1D ▶). We further subdivided patients by both disease duration and histological findings and analysed REG Iα mRNA expression in each group. As shown in fig 1E ▶, in high-Matts score group REG Iα gene expression level in patients with long duration UC was significantly greater than that in patients with short duration UC. In addition, in low-Matts score group REG Iα gene expression level in patients with long duration was also greater than in patients with short duration although this was not statistically significant (p = 0.16). REG Iα mRNA expression in UC tissues was significantly correlated with disease duration (fig 1F ▶) but not with age or sex (data not shown).
Figure 1.
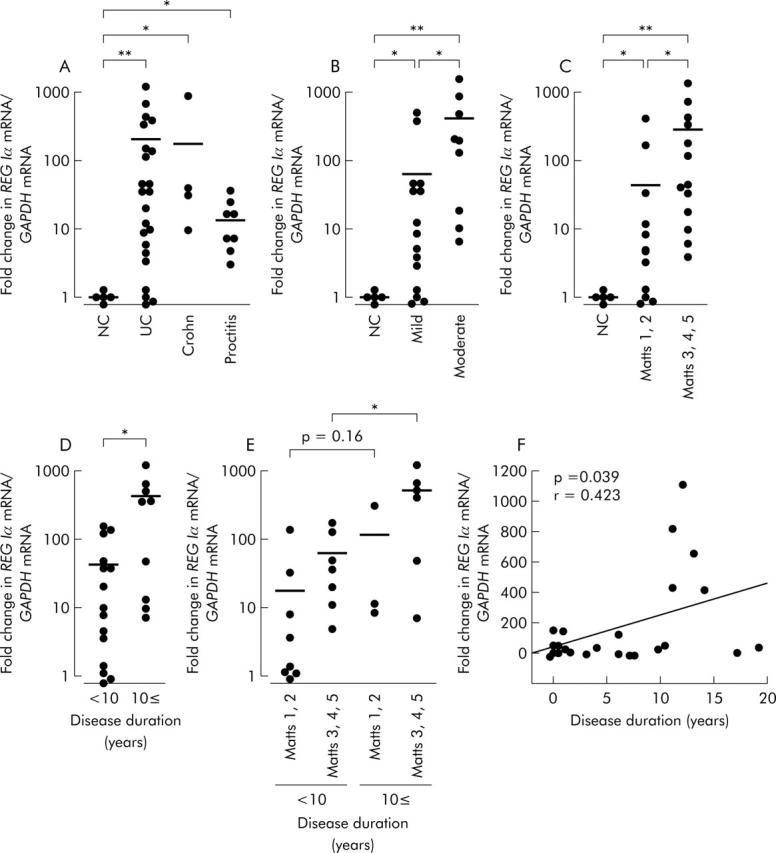
REG Iα mRNA expression in ulcerative colitis tissues. (A) REG Iα mRNA expression levels in normal colon (NC), ulcerative colitis (UC), Crohn’s colitis, and proctitis tissues. Comparison of REG Iα mRNA expression levels among groups subdivided by endoscopic appearance (B), histological findings (C), or disease duration (D, E). (F) Correlation between REG Iα expression levels and disease duration. All results are expressed as fold change in REG Iα mRNA/GAPDH mRNA ratio relative to the normal control group. Significant difference between two groups: *p<0.05, **p<0.01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Expression of REG Iα protein and PCNA in normal colonic and ulcerative colitis mucosa, and in dysplasia and colitic cancer
In normal colonic mucosa, REG Iα immunoreactivity was detected only in a few epithelial cells in the basal portion of crypts (fig 2A ▶). Colonic epithelial cells positive for PCNA were also observed in the basal portion of crypts (fig 2B ▶).
Figure 2.
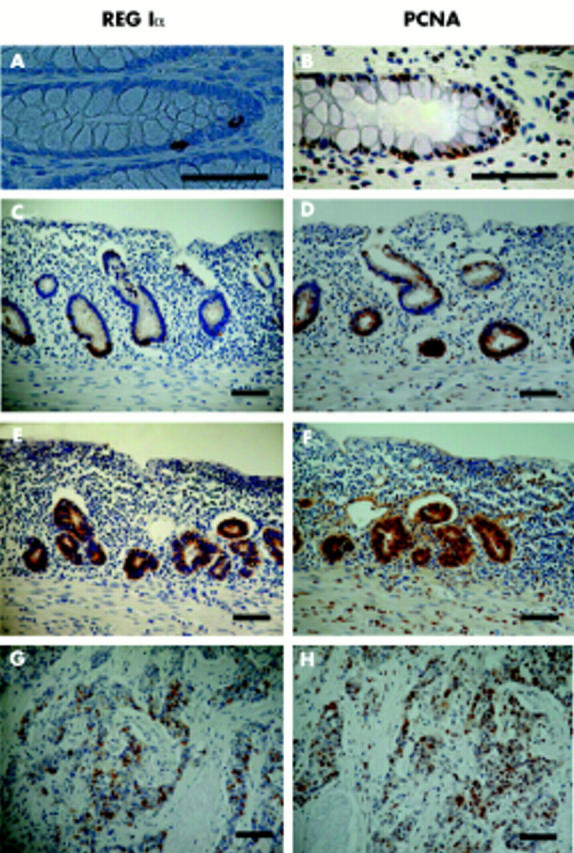
Immunostaining for REG Iα protein and proliferating cell nuclear antigen (PCNA) in normal colon (A, B), ulcerative colitis (C, D), dysplasia (E, F), and colitic cancer (G, H) tissues. REG Iα: A, C, E, G; PCNA: B, D, F, H. Bars = 100 μm.
In UC mucosa, both the number of REG Iα positive cells in crypts and the intensity of REG Iα immunoreactivity were increased. Strongly REG Iα positive cells were mainly observed in the lower part of the colonic mucosa (fig 2C ▶). The number of PCNA positive cells in crypts was also increased, and the distribution of such cells was similar to that of REG Iα positive cells (fig 2D ▶).
REG Iα immunoreactivity was detected in the cytoplasm of not only dysplastic but also colitic cancer cells (fig 2E, 2G ▶ ▶). REG Iα expression was detected in all seven of the colitic cancer lesions, originating from four patients examined. PCNA was also strongly expressed in both dysplastic and cancerous cells in all colitic cancer tissues examined (fig 2F, 2H ▶ ▶).
Expression of REG Iα mRNA in colon cancer cell lines, sporadic colon adenomas, and cancers
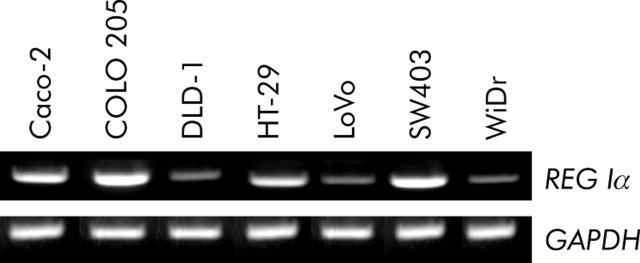
Expression of REGIα mRNA was detected in all seven colon cancer cell lines examined using the RT-PCR method (fig 3 ▶). Then, we examined REG Iα mRNA expression in colon adenomas, cancers, and their neighbouring normal colon mucosa by real time RT-PCR. REG Iα mRNA expression level in colon adenomas (n = 10; 159 (51)) was significantly higher than that in normal colon mucosa (n = 10; 1.0 (0.2)) (p<0.05). Moreover, the REG Iα mRNA expression level in colon cancers (n = 8; 1260 (710)) was significantly higher than that in normal colon mucosa (p<0.05).
Figure 3.
Detection of REG Iα mRNA in various human colon cancer cell lines by reverse transcription-polymerase chain reaction. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Effects of cytokines on REG Iα promoter activity in colon cancer cell lines
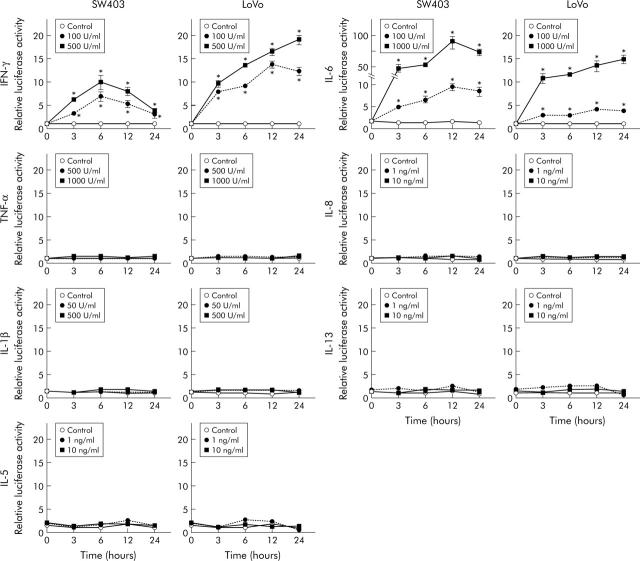
The effect of several cytokines on human REG Iα promoter activity was analysed by transient expression assays in two human colon cancer cell lines (fig 4 ▶). In both LoVo and SW403 cells transfected with the hREG Iα-Luc construct, luciferase activity was significantly elevated at three hours after stimulation with IFN-γ (100 and 500 U/ml) and this elevation was sustained for 24 hours. Similarly, human REG Iα promoter activity was enhanced in both cell lines at three hours after treatment with IL-6 (100 and 1000 IU/ml) and again the elevation was sustained for 24 hours. In contrast, treatment with TNF-α, IL-1β, IL-5, IL-8, or IL-13 did not affect luciferase activity in the two cell lines.
Figure 4.
REG Iα promoter activity in LoVo and SW403 cells in response to various cytokines. Cells were cotransfected with hREG Iα(−1195/+78)-Luc construct and Renilla luciferase plasmid pRL-TK (as a control for transfection efficiency). Forty eight hours later, cells were stimulated with interferon γ (IFN-γ), tumour necrosis factor α (TNF-α), interleukin (IL)-1β, IL-5, IL-6, IL-8, or IL-13. Luciferase activity was measured in extracts from transfected LoVo and SW403 cells and normalised to Renilla luciferase activity. All results are expressed as fold of the activity of the untreated cell group at the 0 hour time point. Vertical lines represent means (SEM) of four independent experiments. *p<0.05 versus control at the same time point.
Effect of REG Iα on cell growth in LoVo cells
LoVo cells transfected with pIRES2-hREG Iα (LoVo-REG Iα) showed significantly higher WST-8 cleavage levels than LoVo cells transfected with pIRES2-EGFP (LoVo-EGFP; control) at each time point (24–72 hours) of incubation (fig 5A ▶). Increased WST-8 cleavage in the LoVo-REG Iα cell group was suppressed almost to control levels by addition of anti-REG Iα antibody (fig 5B ▶), suggesting that enhanced cell growth in LoVo-REG Iα cells is caused by secreted REG Iα protein.
Figure 5.
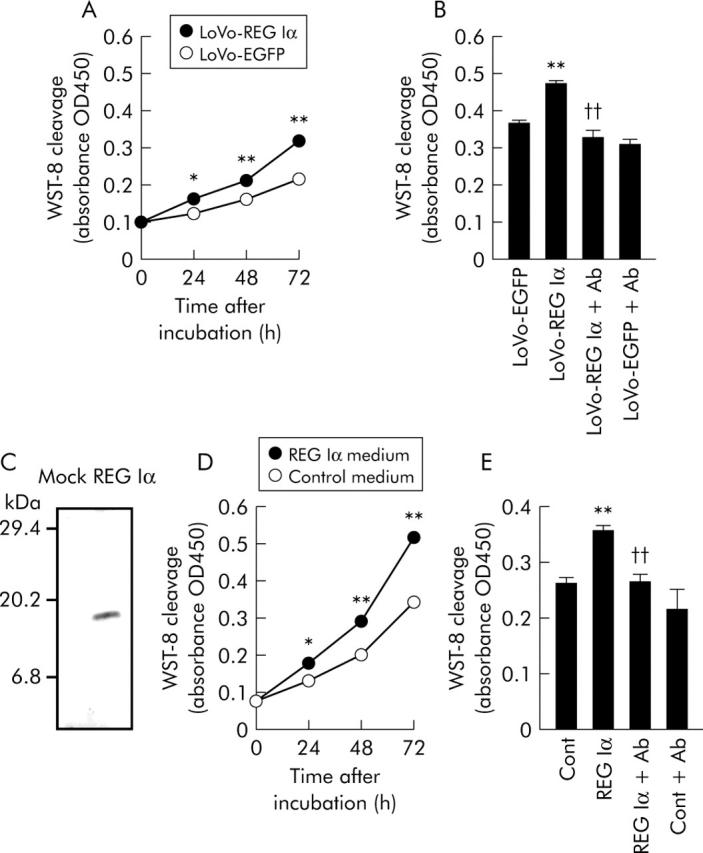
Effects of REG Iα gene induction or REG Iα conditioned medium on the growth of human colon cancer cells. (A) Effect of REG Iα gene induction on growth of human colon cancer cells. LoVo cells transfected with pIRES2-hREG Iα (LoVo-REG Iα) or pIRES2-EGFP (LoVo-EGFP; control) plasmids were cultured for 24, 48, and 72 hours. WST-8 cleavage in each group was measured by ELISA (absorbance OD450), as described in materials and methods. (B) Effect of anti-REG Iα antibody on cell growth of LoVo-REG Iα and LoVo-EGFP. LoVo-REG Iα and LoVo-EGFP cells were incubated with or without anti-REG Iα antibody (Ab, 50 μg/ml) for 72 hours. (C) Source of REG Iα protein. Human embryonic kidney HEK293T cells were transfected with a human REG Iα cDNA expression plasmid or a control plasmid, and the medium conditioned by these cells was collected. Release of REG Iα protein into the conditioned medium of human REG Iα cDNA transfected cells was confirmed by western blot analysis with an antihuman REG Iα monoclonal antibody. (D) Time course of the effect of REG Iα conditioned medium on growth of human colon cancer cells. LoVo cells were incubated with REG Iα or control medium for 24–72 hours. (E) Effect of anti-REG Iα antibody on growth of colon cancer cells promoted by REG Iα protein. Anti-REG Iα antibody (50 μg/ml) was added to control or REG Iα medium, followed by incubation for 72 hours. All results are expressed as the mean (SEM) of four samples. *p<0.05, **p<0.01 versus control (LoVo-EGFP cells (A, B) or control medium treated cells (D, E)) group at the same time point. ††Significantly lower than LoVo-REG Iα cells (B) or REG Iα medium treated cells (E) (p<0.01).
REG Iα conditioned medium significantly increased WST-8 cleavage in LoVo cells after 24–72 hours of incubation time (fig 5D ▶). As shown in fig 5E ▶, the increased WST-8 cleavage in REG Iα-treated LoVo cells was abolished by concomitant administration of anti-REG Iα antibody.
Antiapoptotic effect of REG Iα in LoVo cells
The LoVo-REG Iα cell group showed significantly lower TUNEL positivity than the LoVo-EGFP group when they were treated with H2O2 at concentrations of 2.5–10 mM, suggesting that REG Iα overexpressing cells are more resistant to apoptosis induced by H2O2 (fig 6A ▶). The decreased TUNEL positivity in the LoVo-REG Iα cell group was significantly reversed by treatment with anti-REG Iα antibody (fig 6B ▶), suggesting that secreted REG Iα protein confers an antiapoptotic effect on colon cancer cells.
Figure 6.
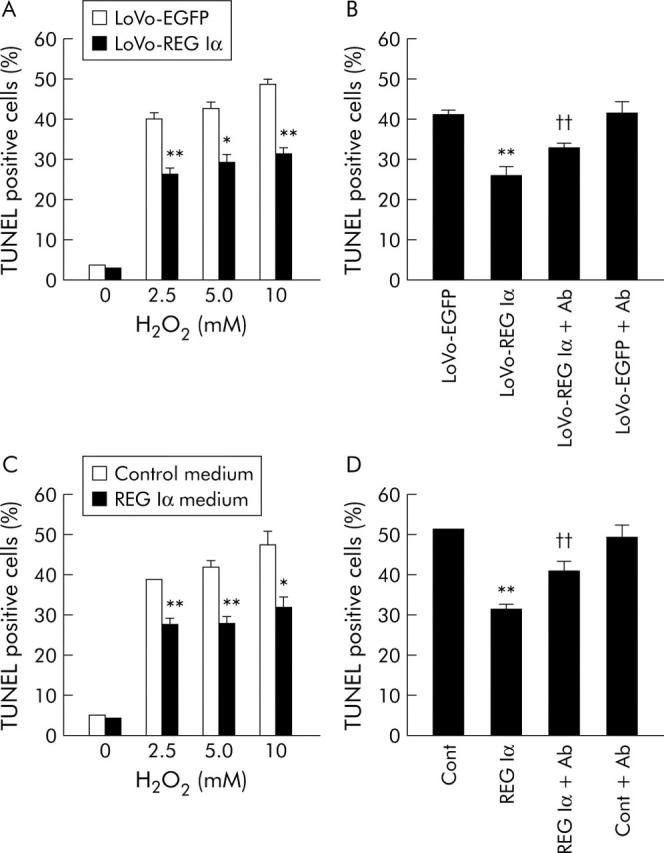
Effects of REG Iα gene induction or REG Iα conditioned medium on H2O2 induced apoptosis of human colon cancer cells. (A) Resistance to H2O2 induced apoptosis in human colon cancer cells transfected with REG Iα cDNA. LoVo cells transfected with pIRES2-hREG Iα (LoVo-REG Iα) or pIRES2-EGFP (LoVo-EGFP; control) plasmids were treated with H2O2 (0–10 mM) for two hours, followed by incubation in fresh culture medium for 24 hours. The percentage of TUNEL positive cells was evaluated, as described in materials and methods. (B) Effect of anti-REG Iα antibody (Ab, 50 μg/ml) on H2O2 (5 mM) induced apoptosis in LoVo-REG Iα and LoVo-EGFP cells. (C) Resistance to H2O2 induced apoptosis in human colon cancer cells treated with REG Iα conditioned medium. LoVo cells were incubated with conditioned medium containing recombinant human REG Iα or control medium for 24 hours, followed by incubation for two hours with medium containing H2O2 (0–10 mM). (D) Effect of anti-REG Iα antibody on H2O2 (5 mM) induced apoptosis in LoVo cells. Anti-REG Iα antibody (50 μg/ml) was added to control or REG Iα medium. All results are expressed as the mean (SEM) of four samples. *p<0.05, ** p<0.01 versus control (LoVo-EGFP cells (A, B)) or control medium treated cells (C, D)) at the same dose point. ††Significantly higher than LoVo-REG Iα cells (B) or REG Iα medium treated cells (D) (p<0.01).
LoVo cells treated with REG Iα conditioned medium showed a significantly lower TUNEL positivity than cells treated with control medium when they were exposed to H2O2 at concentrations of 2.5–10 mM (fig 6C ▶). The decreased TUNEL positivity in REG Iα treated LoVo cells was significantly reversed by concomitant administration of anti-REG Iα antibody (fig 6D ▶).
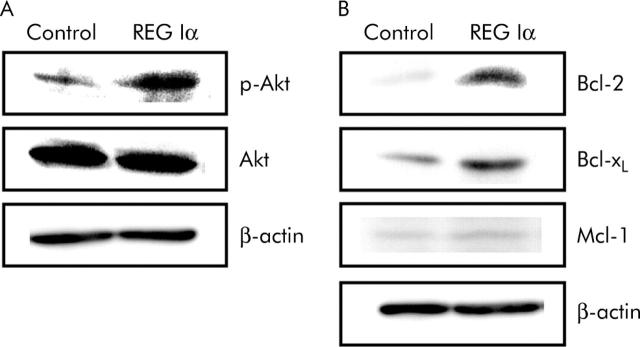
Treatment with REG Iα conditioned medium enhanced the phosphorylation of Akt in LoVo cells (fig 7A ▶). Moreover, REG Iα conditioned medium clearly increased Bcl-2 and Bcl-xL expression in LoVo cells whereas REG Iα did not affect Mcl-1 expression (fig 7B ▶).
Figure 7.
Effects of REG Iα conditioned medium on phosphorylation of Akt and expression of Bcl family proteins in colon cancer cells. Western blotting indicated that phosphorylation of Akt (A) and expression of Bcl-2 and Bcl-xL but not Mcl-1 (B) were enhanced in LoVo cells after 12 hours of incubation with REG Iα conditioned medium.
DISCUSSION
In the present study, we have shown that REG Iα is expressed in only a few small cells in the mid to basal portion of a colonic crypt where putative stem cells reside and continuous cell renewal is occurring.21,22 Interestingly, in the gastric epithelium, REG Iα is expressed not only in enterochromaffin-like and chief cells but also in immature small cells in the proliferating neck zone of gastric glands.14,23 As REG Iα protein is indeed mitogenic to gastrointestinal epithelial cells,11 REG Iα produced in the proliferative zone may play a role in the self renewal of gastrointestinal epithelium under certain physiological conditions.
It is noteworthy that several investigators have identified REG Iα as one of the most abundantly expressed genes in the colonic mucosa of patients with UC by gene chip analyses,9,10 even though only a few REG Iα positive cells are present in normal colonic mucosa. Confirming these data, we demonstrated in this study that levels of both REG Iα gene and its protein were significantly increased in UC mucosa. Furthermore, we also showed that enhancement of REG Iα gene expression in the colonic mucosa was correlated with severity of inflammation in UC, as assessed both endoscopically and histologically. These data strongly suggest that REG Iα is important in the inflammatory process of UC.
What factors could be responsible for the increase in REG Iα expression in UC mucosa? As various cytokines play important roles in the inflammation of UC, we examined in this study whether those cytokines enhance transcription of the REG Iα gene in vitro, and found that both IFN-γ and IL-6 significantly stimulated REG Iα promoter activity in colon cancer cells. In contrast, IL-8, IL-1β, TNF-α, IL-5, or IL-13 could not enhance REG Iα promoter activity in colon cancer cells. Previous studies have shown that, in addition to IFN-γ and IL-6, TNF-α and CINC-2β, a mouse homologue of IL-8, can enhance REG Iα gene expression in rat pancreatic acinar cells19,24 and in rat gastric mucosa in vivo.25 The discrepancies between these results and our present data may be due to the use of different species, cell types, or experimental conditions. It is well known that expression of both IL-6 and IFN-γ is prominently increased in UC mucosa.26–28 Thus although we did not measure levels of those cytokines in this study, it appears reasonable to suppose that increased levels of IL-6 and IFN-γ in the mucosa of UC are at least in part responsible for the enhanced expression of the REG Iα gene and its product. It may be noted that although UC is known as a Th2 dominant disease,29,30 none of Th2 cytokines tested in this study had any effect on REG Iα promoter activity. These data may suggest that the enhanced REG Iα expression in UC mucosa is not a Th2 specific phenomenon but rather a reflection of a general inflammatory condition where IL-6 and IFN-γ expression are elevated.
It is important to clarify whether REG Iα is involved in the carcinogenesis of UC associated colorectal cancer. Interestingly, we found in this study that REG Iα was expressed not only in the epithelial cells of UC mucosa but also in epithelial cells of dysplastic lesions and in colitic cancer cells. Moreover, we have clearly shown that REG Iα expression is significantly increased in patients with longstanding colitis that presents a high risk of colitic cancer.31,32 These findings strongly suggest involvement of REG Iα in the UC-colitic cancer sequence. During UC, colonic epithelial cells are continuously injured by inflammation that may induce sustained regeneration of epithelial cells. In the present study, we have shown that REG Iα protein has not only growth promoting but also antiapoptotic actions on colonic cells, and moreover that REG Iα protein exerts its antiapoptotic effect at least in part by activating Akt signalling and enhancing Bcl-xL and Bcl-2 expression. Thus REG Iα protein induced by UC may play a role in protecting colonic epithelial cells from apoptosis. Conversely, however, as REG Iα expression is sustained at high levels in chronic UC, its antiapoptotic action as well as its growth promoting effects may contribute to the development of colitic cancer from UC tissues. Of note, we also found that the distribution of REG Iα positive cells was similar to that of PCNA positive cells in colitic cancer tissues as well as in dysplastic and colitis mucosa. Therefore, REG Iα produced in epithelial cells as well as in cancer cells may exert a direct action on epithelial cells in colitis, and also on cancer cells themselves. In support of this view, addition of anti-REG Iα antibody to the incubation medium abolished not only the growth promoting effect of REG Iα conditioned medium but also the enhanced growth of REG Iα transfected cells. Similarly, anti-REG Iα antibody blocked the antiapoptotic effect of REG Iα conditioned medium and abrogated the reduction in apoptosis in REG Iα transfected cells. These data strongly suggest that REG Iα secreted from epithelial cells or cancer cells exerts growth promoting and antiapoptotic actions in a paracrine or autocrine fashion. On the other hand, it may be noted in this study that colitic cancers develop in only a limited number of patients with UC, although REG Iα is ubiquitously expressed in the inflamed UC mucosa. However, it may be emphasised that carcinogenesis is a complicated event in which many factors are involved. Thus REG Iα may play a role in the UC-colitic cancer sequence as one of the growth promoting as well as antiapoptotic factors, and function in colitic cancer development not as a tumour initiator but as a tumour promoter.
In the present study, we have shown that REG Iα gene expression is increased not only in UC mucosa and colitic cancers but also in sporadic colon adenomas or cancers, using real time RT-PCR analysis, although its expression is not always detected in sporadic colon cancers by northern blot analysis.16,33 In addition, REG Iα gene expression was detected in all seven colon cancer cell lines examined. These findings may suggest that REG Iα is involved not only in the UC-colitic cancer sequence but also in the adenoma-carcinoma sequence, although the mechanism of carcinogenesis in UC associated colorectal cancer is believed to be distinct from that in sporadic colorectal cancer.34,35 The role of REG Iα in the adenoma-carcinoma sequence should also be clarified in future studies.
In summary, we have shown that REG Iα is expressed not only in epithelial cells of UC mucosa but also in precancerous dysplastic epithelial cells and colitic cancer cells. The promoter activity of the REG Iα gene is stimulated by IFN-γ and IL-6. Moreover, REG Iα protein has both mitogenic and antiapoptotic effects on human colon cancer cells in vitro. Together, these results suggest that cytokine induced REG Iα in the colonic mucosa plays an important role in the development of UC associated colorectal cancer.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
We are grateful to Dr Hiroshi Okamoto in Tohoku University Graduate School of Medicine, Sendai, Japan, for providing anti-REG Iα antibody.
Abbreviations
Reg, regenerating gene
IFN, interferon
TNF, tumour necrosis factor
IL, interleukin
CINC, cytokine induced neutrophil chemoattractant
UC, ulcerative colitis
TUNEL, TdT mediated dUTP nick end labelling
MTT, 3,-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
WST-8, 2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt
RT-PCR, reverse transcription-polymerase chain reaction
PCNA, proliferating cell nuclear antigen
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
EGFP, enhanced green fluorescent protein gene
Conflict of interest: None declared.
Published online first 24 May 2005
REFERENCES
- 1.Terazono K, Yamamoto H, Takasawa S, et al. A novel gene activated in regenerating islets. J Biol Chem 1988;263:2111–14. [PubMed] [Google Scholar]
- 2.Watanabe T, Yonekura H, Terazono K, et al. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. J Biol Chem 1990;265:7432–9. [PubMed] [Google Scholar]
- 3.Kimura N, Yonekura H, Okamoto H, et al. Expression of human regenerating gene mRNA and its product in normal and neoplastic human pancreas. Cancer 1992;70:1857–63. [DOI] [PubMed] [Google Scholar]
- 4.Satomura Y, Sawabu N, Mouri I, et al. Measurement of serum PSP/reg-protein concentration in various diseases with a newly developed enzyme-linked immunosorbent assay. J Gastroenterol 1995;30:643–50. [DOI] [PubMed] [Google Scholar]
- 5.Graf R, Schiesser M, Lüssi A, et al. Coordinate regulation of secretory stress proteins (PSP/reg, PAP I, PAP II, and PAP III) in the rat exocrine pancreas during experimental acute pancreatitis. J Surg Res 2002;105:136–44. [DOI] [PubMed] [Google Scholar]
- 6.Fukui H, Franceschi F, Penland RL, et al. Effects of Helicobacter pylori infection on the link between regenerating gene expression and serum gastrin levels in Mongolian gerbils. Lab Invest 2003;83:1777–86. [DOI] [PubMed] [Google Scholar]
- 7.Asahara M, Mushiake S, Shimada S, et al. Reg gene expression is increased in rat gastric enterochromaffin-like cells following water-immersion stress. Gastroenterology 1996;111:45–55. [DOI] [PubMed] [Google Scholar]
- 8.Kawanami C, Fukui H, Kinoshita Y, et al. Regenerating gene expression in normal gastric mucosa and indomethacin-induced mucosal lesions of the rat. J Gastroenterol 1997;32:12–18. [DOI] [PubMed] [Google Scholar]
- 9.Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet 2001;10:445–56. [DOI] [PubMed] [Google Scholar]
- 10.Dieckgraefe BK, Stenson WF, Korzenik JR, et al. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics 2000;4:1–11. [DOI] [PubMed] [Google Scholar]
- 11.Fukui H, Kinoshita Y, Maekawa T, et al. Regenerating gene protein may mediate gastric mucosal proliferation induced by hypergastrinemia in rats. Gastroenterology 1998;115:1483–93. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Yonemura Y, Yonekura H, et al. Pancreatic beta-cell replication and amelioration of surgical diabetes by Reg production. Proc Natl Acad Sci U S A 1994;91:3589–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadowaki Y, Ishihara S, Miyaoka Y, et al. Reg protein is overexpressed in gastric cancer cells, where it activates a signal transduction pathway that converges on ERK1/2 to stimulate growth. FEBS Lett 2002;530:59–64. [DOI] [PubMed] [Google Scholar]
- 14.Fukui H, Fujii S, Takeda J, et al. Expression of Reg Iα protein in human gastric cancers. Digestion 2004;69:177–84. [DOI] [PubMed] [Google Scholar]
- 15.Dahr DK, Udagawa J, Ishihara S, et al. Expression of regenerating gene I in gastric adenocarcinomas. Cancer 2004;100:1130–6. [DOI] [PubMed] [Google Scholar]
- 16.Rechreche H, Montalto G, Mallo GV, et al. pap, reg Iα and reg Iβ mRNAs are concomitantly up-regulated during human colorectal carcinogenesis. Int J Cancer 1999;81:688–94. [DOI] [PubMed] [Google Scholar]
- 17.Harada K, Zen Y, Kanemori Y, et al. Human REG I gene is up-regulated in intrahepatic cholangiocarcinoma and its precursor lesions. Hepatology 2001;33:1036–42. [DOI] [PubMed] [Google Scholar]
- 18.Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med 1961;120:393–407. [PubMed] [Google Scholar]
- 19.Akiyama T, Takasawa S, Nata K, et al. Activation of Reg gene, a gene for insulin-producing β-cell regeneration: Poly(ADP-ribose) polymerase binds Reg promoter and regulates the transcription by autopoly(ADP-ribosyl)lation. Proc Natl Acad Sci U S A 2001;98:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda N, Seno H, Konda Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene 2004;23:4921–9. [DOI] [PubMed] [Google Scholar]
- 21.Chang WWL, Nadler NJ. Renewal of the epithelium in the descending colon of the mouse. Cell population kinetics of vacuolated-columnar cells. Am J Anat 1975;144:39–58. [DOI] [PubMed] [Google Scholar]
- 22.Appleton DR, Sunter JP, deRodriguez MSB. Cell proliferation in the mouse large bowel, with detail of the analysis of experimental data. In: Appleton DR, ed. Cell proliferation in the gastrointestinal tract. London: Pitman, 1980:141–54.
- 23.Higham AD, Bishop LA, Dimaline R, et al. Mutations of Reg Iα are associated with enterochromaffin-like cells tumor development in patients with hypergastrinemia. Gastroenterology 1999;116:1310–81. [DOI] [PubMed] [Google Scholar]
- 24.Dusetti NJ, Mallo GV, Ortiz EM, et al. Induction of lithostathine/reg mRNA expression by serum from rats with acute pancreatitis and cytokines in pancreatic acinar AR-42J cells. Arch Biochem Biophys 1996;330:129–32. [DOI] [PubMed] [Google Scholar]
- 25.Kazumori H, Ishihara S, Hoshino E, et al. Neutrophil chemoattractant 2β regulates expression of the Reg gene in injured gastric mucosa in rats. Gastroenterology 2000;119:1610–22. [DOI] [PubMed] [Google Scholar]
- 26.Kusugami K, Fukatsu A, Tanimoto M, et al. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci 1995;40:949–59. [DOI] [PubMed] [Google Scholar]
- 27.Indaram AVK, Visvalingam V, Locke M, et al. Mucosal cytokine production in radiation-induced proctosigmoiditis compared with inflammatory bowel disease. Am J Gastroenterol 2000;95:1221–5. [DOI] [PubMed] [Google Scholar]
- 28.Autschbach F, Giese T, Gassler N, et al. Cytokine/chemokine messenger-RNA expression profiles in ulcerative colitis and Crohn’s disease. Virchows Arch 2002;441:500–13. [DOI] [PubMed] [Google Scholar]
- 29.Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest 2004;113:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato T, Kanai T, Watanabe M, et al. Hyperexpression of inducible costimulator and its contribution on lamina propria T cells in inflammatory bowel disease. Gastroenterology 2004;126:829–39. [DOI] [PubMed] [Google Scholar]
- 31.Gyde SN, Prior P, Allan RN, et al. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut 1988;29:206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macadam RCA, Sarela AI, Farmery SM, et al. Death from early colorectal cancer is predicted by the presence of transcripts of the REG gene family. Br J Cancer 2000;83:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brentnall TA, Crispin DA, Rabinovitch PS, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology 1994;107:369–78. [DOI] [PubMed] [Google Scholar]
- 35.Lashner BA, Shapiro BD, Husain A, et al. Evaluation of the usefulness of testing for p53 mutations in colorectal cancer surveillance for ulcerative colitis. Am J Gastroenterol 1999;94:456–62. [DOI] [PubMed] [Google Scholar]