Several susceptible gene loci were identified as being involved in the aetiology of Crohn’s disease (CD).1 Recently, a non-synonymous single nucleotide polymorphism in the SLC22A4 gene encoding the organic cation transporter OCTN1 has been linked with CD in Caucasian populations (a 1672CT transversion, resulting in the amino acid substitution L503F).2,3 However, the functional consequences of this alteration are unclear as yet.
We have now discovered that L-ergothioneine (ET, 2-mercaptohistidine trimethylbetaine), a naturally occurring water soluble thiol compound of dietary origin, is the physiological substrate of OCTN1.4 Analysis of the concentration dependence of ET transport in OCTN1 transfected HEK293 fibroblasts by liquid chromatography tandem mass spectrometry revealed that the 503F variant was associated with a threefold higher substrate affinity (1/Km) and a twofold lower maximal transport velocity (Vmax), which resulted in a 50% higher initial transport capacity (Vmax/Km (503F) ≈ 1.5 × Vmax/Km (503L)) at low ET levels (⩽10 µmol/l) (fig 1A ▶). Analysis of the time course of ET transport showed a higher clearance for the 503F variant (CL (503F) ≈ 1.65 × CL (503L) at an ET concentration of 10 µmol/l) (fig 1B ▶). ET transport by 503L and 503F was sodium and pH dependent; only at unphysiologically low Na+ and pH values were the differences in transport activity between both variants lost (fig 1C ▶, D). Considering that maximal levels of ET found in tissues and in common foods are in the nanomolar to low micromolar range,5 our data suggest that carriers of the 503F allele accumulate higher ET concentrations in OCTN1 expressing cells compared with carriers of the wild-type 503L allele. Therefore, high tissue levels of ET may constitute a possible risk factor for CD.
Figure 1.
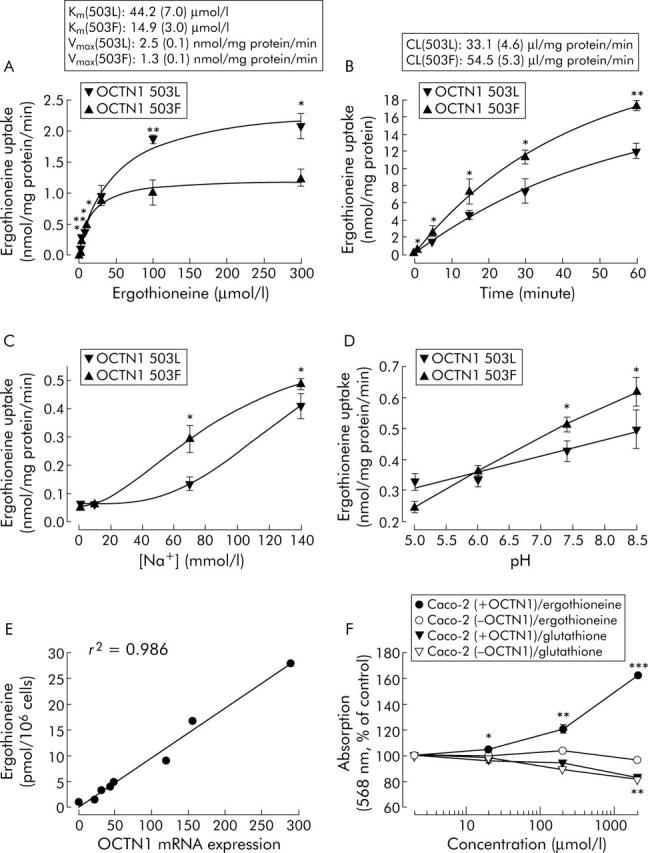
Ergothioneine and OCTN1. Concentration dependence, Km, and Vmax of specific ergothioneine (ET) uptake in HEK293 cells constitutively expressing the 503L variant or the 503F variant after one minute of loading (A); specific uptake and clearance (CL) over a time course after incubation with 10 µmol/l ET (B); effects of sodium (C) and pH (D) on specific uptake after one minute of loading with 10 µmol/l ET. In sodium reduced transport buffer, NaCl was isotonically replaced with choline chloride (which did not interfere with ET transport). An equal expression level of both OCTN1 mRNAs was controlled by quantitative real time polymerase chain reaction (TaqMan assay). Linear correlation of ET concentrations in CD14+ monocytes (fractionated from peripheral blood mononuclear cells by immunomagnetic beads) with OCTN1 mRNA expression (relative to the housekeeping gene GAPDH, lowest expression was set to 1) in eight healthy volunteers that were homozygous carriers of the 503L variant (E). MTT assay10 of the proliferation of Caco-2 colon tumour cells with and without OCTN1 mRNA expression after 24 hours of incubation with ET or glutathione. Resulting formazan formation was determined by absorbance at 568 nm (F). Data are means (SEM) of three (A–D) or 8–16 (F) independent experiments. *p<0.05, **p<0.01, ***p<0.001: significant differences between OCTN1 variants (A–D); significant differences compared with buffer controls (F), as determined by one way ANOVA with Holm-Sidak correction (α = 0.05).
The involvement of OCTN1 in the inflammatory process is further supported by observations that OCTN1 is strongly expressed in intestinal epithelial and immunological cells, particularly in CD14+ monocytes/macrophages playing a key role in the immunopathogenesis of CD,6 as well as by the finding that levels of SLC22A4 mRNA were upregulated by proinflammatory cytokines such as tumour necrosis factor α.7 Moreover, we found transcriptional regulation of SLC22A4 to determine essentially ET uptake: in CD14+ monocytes homozygous for the 503L variant, expression levels of SLC22A4 mRNA showed high interindividual heterogeneity and were directly proportional to cellular ET content (fig 1E ▶). Accordingly, in CD4+ and CD8+ lymphocytes lacking OCTN1 expression, we detected no ET (data not shown).
The physiological or pathophysiological functions of ET are as yet unknown. We tested the effect of ET on proliferation of the colon cancer epithelial cell line Caco-2 that was shown to be homozygous for the susceptible 503F allele and to express high levels of OCTN1 mRNA. Cell proliferation was enhanced in a dose dependent manner after exposure to ET concentrations above 20 µmol/l for 24 hours: at 200 µmol/l, proliferation was increased to 120 (3)% of the buffer control and intracellular ET concentration reached 6.7 (0.3) nmol/mg protein. In contrast, no stimulation of proliferation was seen when a Caco-2 variant without OCTN1 expression was employed; consequently, after treatment with 200 µmol/l ET, only diffusion controlled uptake to 0.67 (0.03) nmol/mg protein occurred. When incubated with glutathione, both Caco-2 cell lines exhibited an antioxidant typical inhibition of proliferation8 that was independent of OCTN1 expression (fig 1F ▶). Hence rather than antioxidant activities, stimulatory effects on cell proliferation appear to constitute the functional role of ET. ET may accelerate the inflammatory process by transcriptional activation of fibroblast repair proliferation, thereby also conferring susceptibility of CD patients to develop colorectal cancer.9
Collectively, our data suggest that the OCTN1 substrate ET is a proliferative factor in inflammatory diseases such as CD, and subjects carrying the 503F allele are at an increased risk due to a higher intracellular accumulation of ET.
Conflict of interest: None declared.
References
- 1.Newman B, Siminovitch KA. Recent advances in the genetics of inflammatory bowel disease. Curr Opin Gastroenterol 2005;21:401–7. [PubMed] [Google Scholar]
- 2.Peltekova VD, Wintle RF, Rubin LA, et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet 2004;36:471–5. [DOI] [PubMed] [Google Scholar]
- 3.Torok HP, Glas J, Tonenchi L, et al. Polymorphisms in the DLG5 and OCTN cation transporter genes in Crohn’s disease. Gut 2005;54:000–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A 2005;102:5256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melville DB. L-ergothioneine. Vitam Horm Leipzig 1958;17:155–204. [Google Scholar]
- 6.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis 2000;6:21–33. [DOI] [PubMed] [Google Scholar]
- 7.Tokuhiro S, Yamada R, Chang X, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet 2003;35:341–8. [DOI] [PubMed] [Google Scholar]
- 8.Eberhardt MV, Lee CY, Liu RH. Antioxidant activity of fresh apples. Nature 2000;405:903–4. [DOI] [PubMed] [Google Scholar]
- 9.Judge TA, Lewis JD, Lichtenstein GR. Colonic dysplasia and cancer in inflammatory bowel disease. Gastrointest Endosc Clin N Am 2002;12:495–523. [DOI] [PubMed] [Google Scholar]
- 10.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986;89:271–7. [DOI] [PubMed] [Google Scholar]


