Abstract
Background and aims: Melatonin, a sleep promoting agent, is involved in the regulation of gastrointestinal motility and sensation. We aimed to determine if melatonin was effective in improving bowel symptoms and sleep disturbances in irritable bowel syndrome (IBS) patients with sleep disturbance.
Methods: Forty IBS patients (aged 20–64 years; 24 female) with sleep disturbances were randomly assigned to receive either melatonin 3 mg (n = 20) or matching placebo (n = 20) at bedtime for two weeks. Immediately before and after the treatment, subjects completed bowel, sleep, and psychological questionnaires, and underwent rectal manometry and overnight polysomnography.
Results: Compared with placebo, melatonin taken for two weeks significantly decreased mean abdominal pain score (2.35 v 0.70; p<0.001) and increased mean rectal pain threshold (8.9 v −1.2 mm Hg; p<0.01). Bloating, stool type, stool frequency, and anxiety and depression scores did not significantly differ after treatment in both groups. Data from sleep questionnaires and polysomnography showed that the two week course of melatonin did not influence sleep parameters, including total sleep time, sleep latency, sleep efficiency, sleep onset latency, arousals, duration of stages 1–4, rapid eye movement (REM) sleep, and REM onset latency.
Conclusions: Administration of melatonin 3 mg at bedtime for two weeks significantly attenuated abdominal pain and reduced rectal pain sensitivity without improvements in sleep disturbance or psychological distress. The findings suggest that the beneficial effects of melatonin on abdominal pain in IBS patients with sleep disturbances are independent of its action on sleep disturbances or psychological profiles.
Keywords: melatonin, irritable bowel syndrome, sleep disturbance, polysomnography, pain
Sleep disturbance is commonly observed in patients with irritable bowel syndrome (IBS), occurring in 26–55%.1–3 Although the cause and effect association is not clear, there is some evidence supporting the “bad bowels cause bad dreams” hypothesis,4 including the finding that IBS patients have more frequent rapid eye movement (REM) sleep, a sleep phase that is characterised by arousal, than non-REM sleep.5 On the other hand, there is also evidence in support of the opposite association, that the bowel disturbance is a result of poor sleep rather than a cause of it. The severity of bowel symptoms has been documented to vary with the quality of the previous night’s sleep, in both male and female IBS populations.6,7
Melatonin (5-methoxy-N-acetyltryptamine), a close derivative of serotonin (5-HT), is a pineal gland neurohormone that is implicated in the control of the sleep-wake cycle. Its secretion is enhanced during darkness and suppressed during daylight. Melatonin has been documented to have a sleep promoting effect.8,9,10,11 It is documented that melatonin could reduce sleep latency, decrease wake up times after sleep onset, increase total sleep time and efficiency, and enhance sleep quality.11 However, many of the previous studies had small sample sizes and did not use objective measures of sleep parameters such as polysomnography (PSG).
Apart from the pineal gland, the gastrointestinal tract is another large source of endogenous melatonin. Many reports have shown that melatonin is involved in the regulation of gastrointestinal motility. It exerts both excitatory and inhibitory effects on the gut.12–14 The precise mechanism through which melatonin regulates gastrointestinal motility is not clear, although some studies suggest that this may be related to blockade of nicotinic channels by melatonin15 and/or the interaction between melatonin and Ca2+ activated K+ channels.16 Melatonin may also be involved in mediating gut visceral sensation because patients with functional abdominal pain are reported to have lower urinary excretion of 6-sulphatoxy melatonin and to exhibit a circadian rhythm of lower amplitude compared with healthy controls.17
In view of the high prevalence of sleep disturbance in IBS patients, and the possible double effects of melatonin in regulating sleep pattern and bowel function, we hypothesised that melatonin may be useful in the treatment of IBS, and its therapeutic effects might be most evident if it was used in IBS patients who suffer from concomitant sleep disturbance. The present study, therefore, aimed to assess the efficacy of exogenous melatonin given at bedtime in relieving IBS bowel symptoms, and in improving rectal sensitivity. It also aimed to explore the effectiveness of melatonin in treating IBS related sleep disturbance using objective measures.
SUBJECTS AND METHODS
Subjects
Forty two unselected IBS patients with sleep disturbances, aged 20–64 years, were recruited from the gastroenterology clinic or via an advertisement posted in the university campus. Patients had to satisfy the inclusion criteria of a diagnosis of IBS made by an experienced gastroenterologist (HKY) based on the Rome II criteria18 and suffered sleep difficulty defined as experiencing difficulty getting to sleep, awakening during the night, and/or early morning awakening. Subjects also had global a Pittsburgh sleep quality index (PSQI)19 score of greater than 5 and their sleep disturbance occurred at least two nights per week in the preceding 12 weeks to ensure that the disease was in an active phase. Patients were asked not to take any medications known to alter gastrointestinal function and sleep conditions within a month before and during the study, and their medications history was recorded. Subjects were excluded if they were pregnant or breast-feeding females; if they had organic gastrointestinal, anal, hepatic, or other systemic disorders; if they had previous gastrointestinal surgery history except appendectomy; or if they gave a history of cerebral disease or surgery.
Verbal and written consent was obtained from each subject. The National University Hospital Institutional Review Board (Singapore) approved the study.
Design
This was a randomised, double blind, placebo controlled study. Patients who met the inclusion and exclusion criteria were recruited and randomised into two groups to receive either melatonin 3 mg or an identically appearing placebo at bedtime for two weeks. Just prior to administration of the medication and after completing the two week course of the medication, all subjects were reviewed at the clinic and asked to complete several questionnaires, including the IBS symptoms evaluation score questionnaire (IBSSESQ), hospital anxiety and depression scale,20 PSQI, and the Epworth sleepiness scale (ESS)21 for assessment of their bowel symptoms, psychological status, sleep disturbance, and level of daytime sleepiness, respectively. The IBSSESQ was derived from the validated questionnaire provided by Francis and colleagues22 but modified by the addition of the validated “Bristol stool form scale”23 to facilitate easy recognition of stool consistency by subjects, adoption of the 11 point response format (0–10), and adoption of the total IBS symptom score. The latter was calculated from responses to the questionnaire and was used to estimate global symptom severity. Total IBS symptom score was considered to be high (that is, suggesting the presence of significant bowel dysfunction) when the value exceeded 20.0, this figure being the upper reference level (95th percentile) obtained from an earlier study designed to validate the total IBS symptom score in our local population (personal communication). All subjects were also asked to undergo two tests, namely the rectal manometry and overnight PSG on the day of the two clinic visits. During the treatment period, subjects were asked to complete the bowel and sleep diary daily and record any adverse events.
Rectal manometry procedure
A computer driven polygraf recorder (Medtronic Functional Diagnostics, Shoreview, Minnesota, USA) was used to assess rectal sensory thresholds, resting pressure, push pressure, and maximal voluntary squeeze pressure. Following defecation, patients were positioned on their left side with their hips and knees flexed. A well lubricated manometric catheter incorporating a 6 cm long latex balloon (Mediplus, High Wycombe, Bucks, UK) was first tested for leakage by inflating with air under water. It was then inserted into the rectum with the distal end of the balloon 5 cm proximal to the anal verge. The pressure within the balloon was measured using a water filled non-perfused channel via a water filled transducer connected to the polygraf. The tubing was then taped to the subject’s leg. After a 15 minute acclimatisation period, the balloon was inflated with progressively increasing volumes of air in an intermittent stepwise manner until the discomfort threshold was reached. The volume and pressure in the balloon were continuously recorded. During inflation, patients were asked to report when they first experienced the sensation of the balloon, the sensation of defecation, the urge to defecate, and pain.
After assessment of rectal sensory thresholds, the balloon was fully deflated and then slowly inflated with air to reach the volume of the first sensation of the balloon that was obtained in the previous stage. This was to ensure correct contact between the balloon and the rectal wall. This volume was not changed throughout the following procedure where patients were asked to do each of the following three manoeuvres three times at random to measure rectal pressures at different conditions: (a) to squeeze as hard as possible as if to hold back stool (maximal voluntary squeeze pressure), (b) to push as if to try to defecate (push pressure), and (c) to rest quietly (resting pressure). Each manoeuvre lasted 10 seconds and there were 30 seconds between every two manoeuvres. The pressure within the balloon was continuously recorded by the polygraf.
Overnight PSG
Overnight PSG was performed using the home ambulatory monitoring system (Compumedics P and E Series, Melbourne Australia) just before and after the two week treatment period. On the day of the test, participants were asked to refrain from using caffeine for at least six hours before the test, consume their dinner before 18:00, and abstain from any food and drink other than water after this time. A board certified technician visited the subjects in their usual sleeping environment (that is, the sleep studies were not done in an inhouse sleep laboratory). Each sleep study consisted of the full 16 channel polysomnogram, which included electroencephalogram (EEG), electrooculogram, nasal airflow pressure transducer and oronasal thermistor, electromyogram (EMG), microphone for snoring detection, electrocardiogram, thoracic and abdominal pietzo belts for movement detection, and bilateral leg EMG. Subjects were instructed to maintain their normal bedtime routine. During the following morning patients were again visited in their home where upon the recording machine was removed and all polysomnographic data were collected, scored, and analysed.
Sleep analysis
Objective sleep analysis: PSG
An experienced physician (LPH) and a technologist analysed all of the polysomnographic studies, scored them according to the Rechtschaffen and Kales criteria,24 and tabulated the data using a computer software program (Profusion PSG; Compumedics, Melbourne Australia). The following parameters were noted: (a) time in bed (TIB), defined as the beginning of the study until termination when the subject woke up in the morning; (b) total sleep time (TST)—total duration of sleep time, as defined by EEG criteria; (c) sleep onset latency—period from lights out to the first three consecutive stage 1 epochs, or the first stage 2 epoch; (d) REM onset latency—time from the first epoch of sleep to onset of REM sleep; (e) Sleep efficiency—TST/TIB×100%; (f) wakefulness after sleep onset (WASO)—total time spent awake after sleep onset; and (g) EEG arousal index—number of arousals from sleep/total sleep time (hour).
Subjective sleep analysis: sleep questionnaires
The PSQI and ESS sleep questionnaires were used to evaluate subjective sleep disturbance and daytime sleepiness, respectively. The PSQI consisted of 19 self rated questions which evaluated a variety of factors that influenced sleep parameters, including sleep latency, duration, efficiency, and frequency and severity of specific sleep related problems. In the ESS, which included eight questions, a score of 8 or more was considered to indicate sleepiness.
Drugs
The pharmacist dispensing the trial medications, the investigators, and the patients were blinded to the nature of the medication. The code for treatment allocation was only broken once the trial was completed. Melatonin 3 mg tablets were supplied by 21st Century Healthcare Pte Ltd (Singapore). Placebo tablets were supplied by Malaysia Chemist Pte Ltd (Singapore). Melatonin and placebo were identical with respect to preparation and packaging and were distinguishable only by the batch number held by the hospital pharmacy laboratory.
Statistical analysis
All statistical analyses were performed using standard SPSS package (version 12.0 for Windows). Continuous variables are expressed as arithmetic mean (SEM), and categorical variables were expressed as frequencies and percentages. Variables that were not normally distributed were transformed, as appropriate, by either logarithmic or square root transformations to satisfy normality assumptions. To compare the differences between the melatonin and placebo groups, the χ2 test was used for categorical variables and the independent sample t test for continuous variables. The paired t test was used to compare differences in the melatonin group before and after treatment. A two tailed p value less than 0.05 was considered statistically significant.
RESULTS
Patients
Of the 42 IBS patients with sleep disturbance who were recruited, two withdrew before taking the medication because of overseas travel and pregnancy, respectively. Thus 40 IBS patients were randomly assigned to receive either melatonin 3 mg or placebo. There were no significant differences in patient characteristics or IBS subgroups (table 1 ▶), or in baseline IBS symptom scores, including quality of life (table 2 ▶), baseline sleep disturbance scores (tables 3 ▶, 4 ▶), and baseline psychological scores (table 5 ▶) between the two groups. On recruitment, all IBS patients had subjective sleep complaints and objective sleep abnormalities, as evidenced by the following baseline sleep abnormalities: global PSQI score >5, ESS score >8, total sleep time <420 minutes, sleep onset latency >30 minutes, REM (%) <20%, and arousal index >10/h (tables 3 ▶, 4 ▶). Furthermore, total anxiety scores for both groups were more than 7 before treatment (table 5 ▶), suggesting underlying psychological distress. All patients completed the two week study and reported no obvious side effects.
Table 1.
Characteristics of irritable bowel syndrome (IBS) patients receiving either melatonin or placebo
| Parameter | Melatonin | Placebo |
| n | 20 | 20 |
| Age (y) (mean (SEM)) | 27.15 (1.95) | 27.70 (2.45) |
| Sex (M/F) | 8/12 | 8/12 |
| IBS subtype (n) | ||
| Constipation predominant | 8 | 6 |
| Diarrhoea predominant | 9 | 9 |
| Alternating | 3 | 5 |
There were no significant differences between the two groups.
Table 2.
Irritable bowel syndrome (IBS) symptom scores before and after two weeks of treatment
| Parameter | Before melatonin treatment (absolute baseline score)* | After melatonin treatment (decrease in score) | p Value† | ||
| Melatonin | Placebo | Melatonin | Placebo | ||
| Abdominal pain | 4.05 (0.28) | 3.85 (0.32) | 2.35 (0.30) | 0.70 (0.25) | <0.001 |
| Abdominal distension | 4.00 (0.36) | 3.15 (0.38) | 1.05 (0.38) | 0.15 (0.29) | 0.069 |
| Frequency of defecation | 3.25 (0.32) | 3.15 (0.43) | 1.85 (0.39) | 0.85 (0.37) | 0.070 |
| Stool type | 6.15 (0.60) | 6.25 (0.54) | 0.65 (0.60) | 0.55 (0.37) | NS |
| Abnormal sensation of defecation | 7.10 (1.02) | 7.70 (0.95) | 1.30 (0.80) | 1.35 (0.65) | NS |
| Quality of life | 5.80 (0.84) | 5.45 (0.65) | 1.25 (0.20) | 0.95 (0.17) | NS |
| Total bowel symptom score‡ | 30.35 (1.84) | 29.55 (1.93) | 7.30 (1.37) | 3.95 (1.25) | 0.078 |
All data are geometric mean (SEM).
*No statistically significant differences between the melatonin and placebo groups for any of the absolute baseline scores.
†Comparisons between the melatonin and placebo groups after two weeks of treatment.
‡Total bowel symptom score >20.0 suggests the presence of significant bowel dysfunction.
Table 3.
Sleep parameters taken from the Pittsburgh sleep quality index (PSQI) and the Epworth sleepiness scale (ESS) before and after two weeks of treatment
| Parameter | Before melatonin treatment (absolute baseline score) | After melatonin treatment (decrease in score) | ||
| Melatonin | Placebo | Melatonin | Placebo | |
| Global PSQI score | 7.50 (0.78) | 6.45 (0.58) | 2.25 (0.45) | 2.15 (0.53) |
| Sleep quality | 1.25 (0.17) | 1.10 (0.18) | 0.35 (0.15) | 0.25 (0.12) |
| Sleep latency | 1.60 (0.28) | 1.35 (0.21) | 0.55 (0.18) | 0.70 (0.18) |
| Sleep duration | 1.60 (0.23) | 1.40 (0.67) | 0.65 (0.20) | 0.90 (0.07) |
| Habitual sleep efficiency | 0.25 (0.12) | 0.15 (0.05) | 0.20 (0.14) | 0.00 (0.07) |
| Sleep disturbance | 1.40 (0.13) | 1.25 (0.11) | 0.05 (0.17) | 0.30 (0.13) |
| Use of sleep altering medications | 0.20 (0.17) | 0.15 (0.11) | 0.20 (0.17) | 0.15 (0.11) |
| Daytime dysfunction | 1.20 (0.27) | 1.05 (0.20) | 0.15 (0.22) | 0.45 (0.26) |
| ESS score | 10.05 (0.72) | 9.75 (0.77) | 1.65 (0.92) | 1.80 (0.76) |
All data are geometric mean (SEM).
There were no significant differences between the melatonin and placebo groups for any of the absolute baseline scores or after two weeks of treatment.
Table 4.
Polysomnographic parameters before and after two weeks of treatment
| Parameter | Before melatonin treatment (absolute baseline value) | After melatonin treatment (changes in value) | ||
| Melatonin | Placebo | Melatonin | Placebo | |
| Time in bed (min) | 469.40 (17.11) | 463.88 (16.14) | −8.98 (11.75) | 3.00 (13.37) |
| Total sleep time (min) | 342.57 (24.30) | 357.61 (20.56) | 31.38 (20.13) | 29.22 (18.48) |
| Sleep onset latency (min) | 51.73 (11.36) | 34.00 (8.45) | −14.97 (7.10) | −1.35 (4.91) |
| NREM (%) | 82.70 (1.90) | 84.59 (1.61) | −1.48 (1.85) | −2.57 (1.70) |
| Slow wave sleep (%) | 17.78 (2.33) | 21.41 (1.67) | −0.59 (1.47) | −1.55 (1.82) |
| REM (%) | 17.29 (1.90) | 15.42 (1.61) | 1.49 (1.85) | 2.54 (1.70) |
| REM onset latency (min) | 111.10 (13.41) | 100.88 (12.70) | −20.55 (12.33) | −0.30 (13.29) |
| Sleep efficiency (%) | 76.97 (3.16) | 77.66 (4.02) | 5.44 (2.64) | 5.55 (3.53) |
| WASO (min) | 38.80 (9.34) | 30.08 (6.19) | −9.50 (7.76) | −2.93 (7.03) |
| Arousal index | 10.13 (1.31) | 10.22 (1.24) | 1.03 (1.01) | 0.85 (1.06) |
All data are geometric mean (SEM).
NREM, non-rapid eye movement; REM, rapid eye movement; WASO, wakefulness after sleep onset.
No significant differences between the melatonin and placebo groups for any of the absolute baseline scores or after two weeks of treatment.
Table 5.
Psychological parameters before and after two weeks of treatment
| Parameter | Before melatonin treatment (absolute baseline score)* | After melatonin treatment (decrease in score) | ||
| Melatonin | Placebo | Melatonin | Placebo | |
| Total anxiety score | 9.55 (0.74) | 7.75 (0.87) | 0.80 (0.74) | 1.35 (0.39) |
| Total depression score | 5.55 (0.98) | 3.90 (0.52) | 0.75 (0.91) | 1.05 (0.48) |
All data are geometric mean (SEM).
No statistically significant differences between the melatonin and placebo groups for any of the absolute baseline scores or after two weeks of treatment.
Bowel parameters
Bowel symptoms
IBS bowel symptom parameters (that is, abdominal pain, abdominal distension, frequency of defecation, stool type, abnormal sensation of defecation, quality of life, and total bowel symptom score), as obtained from the IBSSESQ, were evaluated. Differences in these parameters before and after two weeks of treatment for the melatonin and placebo treated groups were compared. As shown in table 2 ▶, compared with the placebo group (0.70 (0.25)), the abdominal pain score was significantly decreased in the melatonin treated group (2.35 (0.30)) after two weeks of treatment (p<0.001). There was a tendency towards a greater reduction in abdominal distension (p = 0.069), stool frequency (p = 0.070), and total bowel symptoms (p = 0.078) following treatment with melatonin than with placebo. There were no statistically significant differences in post-treatment changes in stool type, abnormal sensation of defecation, or quality of life between the melatonin and placebo groups.
Rectal sensory thresholds
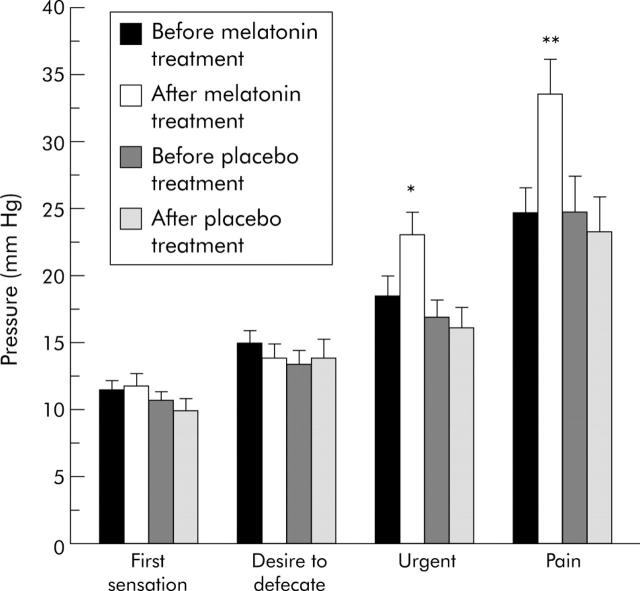
As shown in fig 1 ▶, among patients who were treated with melatonin for two weeks, rectal distension pressure thresholds that were required to induce the sensations of urgency and pain were significantly increased from 18.43 (1.52) mm Hg to 23.05 (1.75) mm Hg (p = 0.007), and from 24.81 (1.83) mm Hg to 33.66 (2.58) mm Hg (p = 0.003), respectively. In contrast, sensory thresholds for urgency and pain did not change at the end of the two week placebo treatment. Both melatonin and placebo given for two weeks did not change distension pressure thresholds for the first sensation of distension or the desire to defecate.
Figure 1.
Anorectal sensory pressure thresholds of irritable bowel syndrome patients before and after two weeks of treatment. Each bar represents mean (SEM) (n = 20). *p = 0.007 compared with urgency before melatonin treatment (paired t test); *p = 0.004 compared with urgency after placebo treatment (independent t test). **p = 0.003 compared with pain before melatonin treatment (paired t test); **p = 0.007 compared with pain after placebo treatment (independent t test).
When volume thresholds were measured, the same findings were observed (fig 2 ▶). Two weeks of melatonin treatment significantly elevated urgency and pain volume thresholds from a baseline of 116.20 (7.58) ml to 144.45 (9.92) ml (p = 0.011), and from a baseline of 157.6 (9.48) ml to 193.15 (12.21) ml (p = 0.014), respectively. There were no statistically significant differences in volume thresholds for urgency and pain before and after treatment for the placebo group. There were also no significant differences in volume thresholds for the first sensation of distension and the desire to defecate for both the melatonin and placebo treated groups, similar to pressure threshold measurements.
Figure 2.
Anorectal sensory volume thresholds of irritable bowel syndrome patients before and after two weeks of treatment. Each bar represents mean (SEM) (n = 20). *p = 0.011 compared with urgency before melatonin treatment (paired t test); *p = 0.01 compared with urgency after placebo treatment (independent t test). **p = 0.014 compared with pain before melatonin treatment (paired t test); **p = 0.02 compared with pain after placebo treatment (independent t test).
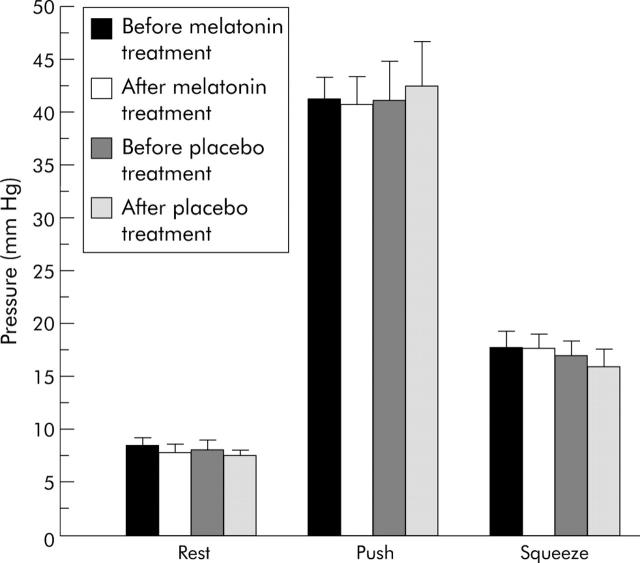
Rectal contraction or relaxation ability
As shown in fig 3 ▶, melatonin treatment did not result in any significant differences in rectal pressures during resting, pushing, or voluntary squeezing conditions.
Figure 3.
Anorectal manometry of irritable bowel syndrome patients before and after two weeks of treatment. Each bar represents mean (SEM) (n = 20). There were no significant differences in resting, pushing, or squeezing pressures before or after melatonin treatment.
Sleep parameters
Subjective sleep quantity and quality
As shown in table 3 ▶, changes in global PSQI score, sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep altering medications, and daytime dysfunction were not significantly different between the melatonin and placebo groups. No significant difference was found in ESS scores between the two groups after completion of the two treatments.
PSG results
Similar to findings from the subjective sleep measures, objective PSG measurements revealed no significant differences between the melatonin and placebo groups after two weeks of treatment (table 4 ▶). TIB, TST, sleep onset latency, REM onset latency, WASO, and arousal index were not significantly different between the two groups. There were no significant group differences in percentages of non-rapid eye movement (NREM), including slow wave sleep, REM sleep, and sleep efficiency (TST/TIB).
Psychological parameters
Changes in total anxiety and total depression scores after treatment in the melatonin group were similar to those in the placebo group (table 5 ▶).
DISCUSSION
To our knowledge this is the first study to explore the role of melatonin in treating IBS patients with sleep disturbance. The results showed that administration of oral melatonin 3 mg at bedtime for two weeks significantly alleviated abdominal pain in IBS patients. Therapeutic efficacy was associated with heightened pressure and volume thresholds for both urgency and pain sensations in melatonin treated patients. The results suggest that the beneficial effects of melatonin in IBS may be related to its action on gut visceral hypersensitivity. It could be argued that rectal distension procedures before melatonin treatment may have habituated the subjects, increased their vigilance, and influenced their responses to repeated procedures after the treatment. However, this possibility seems unlikely as changes in distension thresholds occurred only in the melatonin group and not in the placebo treated group, who would have been expected to experience the same potentially biased effect.
Interestingly, melatonin did not alter pressure or volume thresholds for the first sensation of distension or the desire to defecate. Although the mechanism responsible for the differential effect of melatonin in altering some but not all of the sensations to balloon distension cannot be clearly elucidated from the present study, we postulate that it may be related to the stress modulating effect of melatonin on the more unpleasant sensations. It is well known that noxious sensations of urgency and pain induced by balloon distension are often accompanied by acute stress reaction and negative emotions, both of which could enhance visceral sensitivity.25,26 It is thus possible that melatonin may have reduced these stress induced psychological and behavioural changes, and ameliorated rectal sensitivity to pain and urgency. Supporting evidence for this include animal and human studies. In one animal study, daily treatment of mice subjected to experimental stress with oral melatonin was shown to prevent chronic stress induced behavioural disturbances.27 Our previous study also showed that high dose melatonin alleviated stress induced defecation in rats (abstract reference). In a double blind placebo controlled study, oral melatonin was found to reduce stress associated with simulated travel in humans.28
In this study, the two week course of melatonin treatment did not significantly change the frequency of defecation or stool type. It also did not change rectal pressures during squeezing, pushing, or resting states. These results suggest that melatonin did not influence gut motility, and the target site for the effect of melatonin in IBS may not be gut smooth muscle. Despite improvement in abdominal pain, our subjects did not experience a better quality of life after melatonin treatment. This suggests that abdominal symptoms other than abdominal pain may exert a greater influence on quality of life than abdominal pain per se.
One study suggested that IBS patients tended to complain of poor sleep despite the absence of objective sleep abnormalities because of altered sleep perception.29 To avoid such a bias, we specifically selected IBS patients who had poor sleep and objectively proven sleep disturbance for inclusion into the study. We found that 3 mg melatonin given at bedtime for two weeks did not improve any of the subjective or objective sleep parameters. This is despite significant alleviation of abdominal pain in melatonin treated patients. The findings suggest that IBS daytime symptoms may worsen or improve, independent of sleep disturbance, and awakening during the night may not be attributed to occurrence of abdominal pain in IBS patients. Of note, although absolute differences in treatment response after melatonin compared with placebo for sleep onset latency (up to 15 minutes) and REM onset latency (up to 20 minutes) did not reach statistical significance, they were numerically quite large. Whether increasing the sample size and using a larger dose of melatonin for a longer treatment period could result in detectable changes in sleep parameters in IBS patients cannot be answered by this study.
We found no significant differences in anxiety or depression scores between the melatonin and placebo groups after two weeks of treatment. Although these results suggest the beneficial effects of melatonin on abdominal pain in IBS patients may be independent of its action on the patient’s psychological profile and emotional state, such conclusions need to be drawn with caution. Firstly, the psychological parameters used in this study were based on the hospital anxiety and depression scale, and this test may not be sufficiently sensitive to detect minor alterations in psychological state. Moreover, two weeks of melatonin 3 mg/day may not be sufficiently long or the dose may be too low to cause detectable psychological changes.
In conclusion, the results from the present study showed that administration of melatonin 3 mg at bedtime for two weeks significantly attenuated abdominal pain while enhancing rectal pain threshold. These changes occurred despite the absence of improvement in sleep disturbance or psychological distress. Our findings suggest that the beneficial effects of melatonin on abdominal pain in IBS patients with sleep disturbance are independent of its action on sleep disturbances or psychological profile. Future studies should focus on therapy with different doses of melatonin, prolonging the treatment period, and using a larger sample size to provide a clearer view of the role of melatonin in IBS and sleep disturbance.
Abbreviations
EEG, electroencephalogram
EMG, electromyogram
ESS, Epworth sleepiness scale
IBS, irritable bowel syndrome
IBSSESQ, IBS symptoms evaluation score questionnaire
NREM, non-rapid eye movement
PSG, polysomnography
PSQI, Pittsburgh sleep quality index
REM, rapid eye movement
TST, total sleep time
TIB, time in bed
WASO, wake after sleep onset
5-HT, serotonin
Conflict of interest: None declared.
These data were published in abstract form (Gut 2004;53(suppl VI):A69) and were presented orally at the 12th United European Gastroenterology Week, Prague, 2004.
Published online first 24 May 2005
REFERENCES
- 1.Fass R, Fullerton S, Tung S, et al. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol 2000;95:195–200. [DOI] [PubMed] [Google Scholar]
- 2.Corney RH, Stanton R. Physical symptom severity, psychological and social dysfunction in a series of outpatients with irritable bowel syndrome. J Psychosom Res 1990;34:483–91. [DOI] [PubMed] [Google Scholar]
- 3.Nyhlin H, Ford MJ, Eastwood J, et al. Non-alimentary aspects of the irritable bowel syndrome. J Psychosom Res 1993;37:155–62. [DOI] [PubMed] [Google Scholar]
- 4.Orr WC. Sleep and functional bowel disorders: can bad bowels cause bad dreams? Am J Gastroenterol 2000;95:1118–21. [DOI] [PubMed] [Google Scholar]
- 5.Kumar D, Thompson PD, Wingate DL, et al. Abnormal REM sleep in the irritable bowel syndrome. Gastroenterology 1992;103:12–17. [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith G, Levin JS. Effect of sleep quality on symptoms of irritable bowel syndrome. Dig Dis Sci 1993;38:1809–14. [DOI] [PubMed] [Google Scholar]
- 7.Jarrett M, Heitkemper M, Cain KC, et al. Sleep disturbance influences gastrointestinal symptoms in women with irritable bowel syndrome. Dig Dis Sci 2000;45:952–9. [DOI] [PubMed] [Google Scholar]
- 8.Kunz D, Mahlberg R, Muller C, et al. Melatonin in patients with reduced REM sleep duration: two randomized controlled trials. J Clin Endocrinol Metab 2004;89:128–34. [DOI] [PubMed] [Google Scholar]
- 9.Petrie K, Dawson AG, Thompson L, et al. A double-blind trial of melatonin as a treatment for jet lag in international cabin crew. Biol Psychiatry 1993;33:526–30. [DOI] [PubMed] [Google Scholar]
- 10.Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep 1997;20:124–31. [PubMed] [Google Scholar]
- 11.Bubenik GA, Blask DE, Brown GM, et al. Prospects of the clinical utilization of melatonin. Biol Signals Recept 1998;7:195–219. [DOI] [PubMed] [Google Scholar]
- 12.Storr M, Koppitz P, Sibaev A, et al. Melatonin reduces non-adrenergic, non-cholinergic relaxant neurotransmission by inhibition of nitric oxide synthase activity in the gastrointestinal tract of rodents in vitro. J Pineal Res 2002;33:101–8. [DOI] [PubMed] [Google Scholar]
- 13.Harlow HJ, Weekley BL. Effect of melatonin on the force of spontaneous contractions of in vitro rat small and large intestine. J Pineal Res 1986;3:277–84. [DOI] [PubMed] [Google Scholar]
- 14.Bubenik GA, Dhanvantari S. Influence of serotonin and melatonin on some parameters of gastrointestinal activity. J Pineal Res 1989;7:333–44. [DOI] [PubMed] [Google Scholar]
- 15.Barajas-Lopez C, Peres AL, Espinosa-Luna R, et al. Melatonin modulates cholinergic transmission by blocking nicotinic channels in the guinea-pig submucous plexus. Eur J Pharmacol 1996;312:319–25. [DOI] [PubMed] [Google Scholar]
- 16.Storr M, Schusdziarra V, Allescher HD. Inhibition of small conductance K+-channels attenuated melatonin-induced relaxation of serotonin-contracted rat gastric fundus. Can J Physiol Pharmacol 2000;78:799–806. [PubMed] [Google Scholar]
- 17.Roberts-Thomson IC, Knight RE, Kennaway DJ, et al. Circadian rhythms in patients with abdominal pain syndromes. Aust N Z J Med 1988;18:569–74. [DOI] [PubMed] [Google Scholar]
- 18.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut 1999;45 (suppl 2) :II43–7. [DOI] [PMC free article] [PubMed]
- 19.Buysse DJ, Reynolds CF 3rd, Monk TH, et al. he Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 22.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 23.Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Tech Coloproctol 2001;5:163–4. [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems for sleep stages of human subjects. Washington DC: US Government Printing Office, 1968.
- 25.Ford MJ, Camilleri M, Zinsmeister AR, et al. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology 1995;109:1772–80. [DOI] [PubMed] [Google Scholar]
- 26.Houghton LA, Calvert EL, Jackson NA, et al. Visceral sensation and emotion; a study using hypnosis. Gut 2002;51:701–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp C, Vogel E, Rettori MC, et al. The effects of melatonin on the behavioural disturbances induced by chronic mild stress in C3H/He mice. Behav Pharmacol 1999;10:73–83. [DOI] [PubMed] [Google Scholar]
- 28.Kirby AW, Clayton M, Rivera P, et al. Melatonin and the reduction or alleviation of stress. J Pineal Res 1999;27:78–85. [DOI] [PubMed] [Google Scholar]
- 29.Elsenbruch S, Harnish MJ, Orr WC. Subjective and objective sleep quality in irritable bowel syndrome. Am J Gastroenterol 1999;94:2447–52. [DOI] [PubMed] [Google Scholar]