SUMMARY
Psychological stress has long been reported anecdotally to increase disease activity in inflammatory bowel disease (IBD), and recent well designed studies have confirmed that adverse life events, chronic stress, and depression increase the likelihood of relapse in patients with quiescent IBD. This evidence is increasingly supported by studies of experimental stress in animal models of colitis. With the evolving concept of psychoneuroimmunology, the mechanisms by which the nervous system can affect immune function at both systemic and gut mucosal levels are gradually becoming apparent. Recent data suggest that stress induced alterations in gastrointestinal inflammation may be mediated through changes in hypothalamic-pituitary-adrenal (HPA) axis function and alterations in bacterial-mucosal interactions, and via mucosal mast cells and mediators such as corticotrophin releasing factor (CRF). To date, the therapeutic opportunities offered by stress reduction therapy remain largely unexplored, in part because of methodological difficulties of such studies. This paper reviews recent advances in our understanding of the pathogenic role of psychological stress in IBD and emphasises the need for controlled studies of the therapeutic potential of stress reduction.
INTRODUCTION
Both ulcerative colitis (UC) and Crohn’s disease are chronic, relapsing, and remitting diseases. There is marked temporal variation in mucosal inflammation, from near normal in remission to severe ulceration during relapse. The aetiology of both diseases involves a complex interaction between genes and environment.1,2 There has been substantial progress in identification of the genes responsible for predisposition to IBD3 but the environmental factors which trigger initial presentation and subsequent relapses, and the mechanisms by which they act, are less clearly understood. Psychological stress is one environmental factor which has long been anecdotally reported as having a relationship with activity in IBD,4 and there have been substantial recent advances in both proving this relationship and in elucidating the mechanisms by which it occurs.
This review first provides a definition of stress and an overview of the anatomy and physiology of the stress response. The modern concept of psychoneuroimmunology is reviewed before examining the evidence that both chronic stress, in the form of adverse life events, and acute experimental stress can affect systemic immune and inflammatory function, and increase disease activity in humans with IBD. We also review the evidence for experimental stress being able to both initiate and reactivate gastrointestinal inflammation in animal models of colitis. The possible roles of altered function of the HPA axis and increased intestinal permeability are also discussed. Finally, we review the potential therapeutic implications of the realisation that psychological stress can worsen disease activity in IBD.
STRESS AND THE STRESS RESPONSE
To maintain homeostasis, a living organism must constantly adapt at a molecular, cellular, physiological, and behavioural level to environmental alterations. Stress can be defined as any threat to an organism’s homeostasis.5 The function of the stress response is to maintain homeostasis and may involve both physiological and behavioural adaptations.
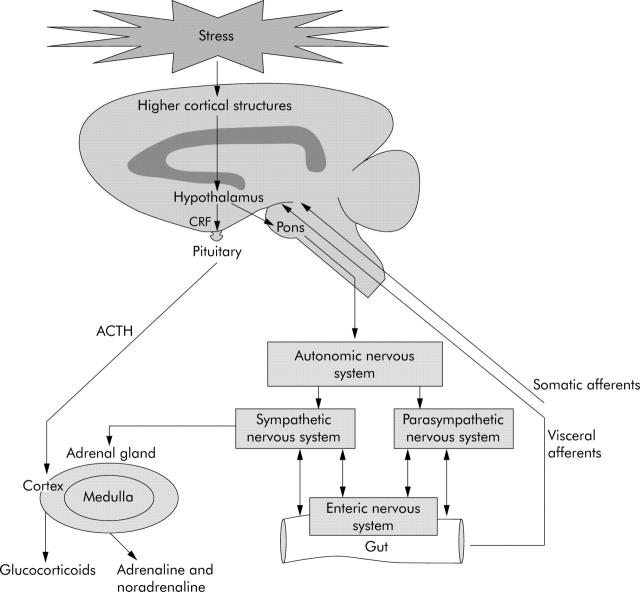
The stress response involves the complex integration of a series of interconnected regions within the brain, most notably the hypothalamus, amygdala, and hippocampus.6 This network receives input from both visceral and somatic afferents, and from higher cortical structures. In turn, it governs the neuroendocrine stress response via two interconnected effector pathways: the HPA axis and the autonomic nervous system (ANS) (fig 1 ▶).
Figure 1.
Pathways mediating the effects of stress on the gastrointestinal tract. (The effects of activation of the enteric nervous system on the gut in inflammatory bowel disease are shown in fig 2 ▶.) ACTH, adrenocorticotrophic hormone; CRF, corticotrophin releasing factor.
Stress stimulates the release of CRF from the hypothalamus causing the release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary gland. This in turn stimulates the secretion of cortisol, the principal glucocorticoid, from the adrenal cortex.
Stress activates direct descending neural pathways from the hypothalamus to the pontomedullary nuclei which control the autonomic response. Stimulation of the sympathetic nervous system in response to stress causes the release of adrenaline and noradrenaline from the adrenal medulla. Neurones of the sympathetic ANS also supply the entire gut directly while the vagus and sacral nerves provide parasympathetic input to the upper gut and to the distal colon and rectum, respectively.7 The efferent and afferent neurones of the sympathetic and parasympathetic ANS communicate with the gut’s own rich nerve supply, the enteric nervous system (ENS), and this network has been termed the brain-gut axis.7 The ENS contains 100 million neurones and regulates the motility, exocrine, and endocrine functions and microcirculation of the gastrointestinal tract.7
PSYCHONEUROIMMUNOLOGY
It is increasingly recognised that the HPA axis, ANS, and ENS can interact directly with the immune system. Psychoneuroimmunology is the study of the mechanisms by which behavioural factors and CNS function can influence inflammation and the immune system at both systemic and local tissue levels.8–10
Nerve fibres of the ANS form close effector junctions with lymphocytes and macrophages in lymph glands, bone marrow, thymus, spleen, and mucosa associated lymphoid tissue.8 A variety of neurotransmitters contained in the neurones of the ANS and ENS, including catecholamines, vasoactive intestinal peptide, angiotensin II, neurotensin, somatostatin, and substance (SP), are capable of affecting lymphocytes, macrophages, neutrophils, and other inflammatory cells at the neurone-immune cell junction and beyond.8–10 Lymphocytes and other inflammatory cells also carry receptors for the hormones and neuropeptides of the HPA axis such as growth hormone, ACTH, corticosteroids, and CRF.8,10
At high concentrations, glucocorticoids, released from the adrenal cortex in response to ACTH from the pituitary, have a mainly immunosuppressive action. They increase the synthesis of anti-inflammatory proteins such as lipocortin 1, interleukin (IL)-1 receptor antagonist, and IL-10.11 Transcription of several inflammatory cytokines and chemokines, such as IL-1, IL-6,12 and tumour necrosis factor α (TNF-α),13 is reduced by glucocorticoids via an inhibitory effect on the transcription factors AP-1 and nuclear factor κB.14,15 Glucocorticoids also promote apoptosis in inflammatory cell types which include T cells and eosinophils.16 However, at lower concentrations cortisol exhibits an immunostimulatory effect. For example, pretreatment with low dose cortisol increases the production of IL-6 and TNF-α by macrophages in response to lipopolysaccharide (LPS).17
Similarly, adrenaline and noradrenaline have mixed effects on immune and inflammatory function. Adrenaline infusion causes a rise in serum IL-6, and increases in LPS induced IL-8 and IL-10 synthesis by whole blood.18–20 It also increases the number of circulating cytotoxic T cells and natural killer (NK) cells, but decreases TNF-α production by monocytes.21,22
EFFECTS OF STRESS ON THE SYSTEMIC IMMUNE SYSTEM IN HUMANS
The effects of psychological stress on the systemic immune and inflammatory system are complex, and depend on both the duration and intensity of the stressor.23 Both chronic stress and acute stress are associated with alterations in systemic immune and inflammatory function which may have relevance to the pathogenesis of IBD.
Chronic psychological stress, adverse life events, and systemic immune and inflammatory function
Chronic sustained stress, such as that due to adverse life events, causes a prolonged increase in cortisol over several days which is characteristically associated with immunosupression.23 Bereavement, depression, and marital separation have all been shown to reduce the numbers of CD8+ lymphocytes, NK cells, and macrophages found in blood.24–28
However, in addition to immunosuppression, chronic psychological stress has also been shown to be associated with subclinical increases in inflammation. Patients with depression, and middle aged and elderly patients with reduced heart rate variability, a measure of increased sympathetic tone and chronic stress, have both been found to have a raised serum C reactive protein.29,30
Acute psychological stress, experimental stress, and systemic immune and inflammatory function
Examining the in vivo effects of psychological stress is difficult as stress is a subjective experience which is hard to define objectively and to simulate in a controlled experimental environment. Placing patients under sustained psychological stress would also be unethical. Despite these limitations, various experimental models have been designed which are capable of inducing mild acute psychological stress, as assessed by the ANS and HPA response.31,32
The dichotomous listening test, where different auditory inputs are played into the subject’s ears, has been one of the commonest techniques used to assess the effects of psychological stress on human gastrointestinal physiology.33 Difficult mental arithmetic tests (MAT), which include negative feedback and varying degrees of auditory distraction, are also used and are easily reproducible.34,35 The Trier social stress test (TSST) involves an oral presentation in front of a critical audience32,36,37 while the Stroop word-colour interference test involves identifying the colour of ink used to write a word which is different to the meaning of the word itself; for example the word blue written in red ink.38
Both real life acute stress and experimental stress tests cause acute stimulation of the sympathetic nervous system, with an almost immediate rise in adrenaline and noradrenaline.39 This is followed by a rise in cortisol but both changes are maintained over only a few hours. Stimulation of the stress axes in this way is associated with immune enhancement.23 There is an increase in serum levels and the production by whole blood of inflammatory cytokines known to be important in the pathogenesis of IBD. Whole blood from medical students taken the day before an examination produced more TNF-α, IL-6, and interferon γ (IFN-γ) when stimulated with LPS than when taken several weeks later.40 Similarly, blood stimulated with LPS for 24 hours produced more IL-6 and IFN-γ after an acute stressor than before,41–43 and serum levels of IL-6 and IL-2 receptor antagonist were increased two hours after a stressful behavioural task.44
Acute stress has also been shown to cause a leucocytosis in both healthy subjects and patients with quiescent UC, with a rapid redistribution of the lymphocyte population. There is a rise in the percentages of CD8+ cytotoxic T cells and NK cells, and a corresponding increase in their cytolytic activity, as assessed by chromium release assays.31,45–47
Platelet activation, as assessed by aggregation and production of inflammatory mediators and platelet dependent thrombin generation, has been shown to be increased by experimental stress in both healthy subjects and patients with quiescent UC.48–51 Platelets circulate in a higher activated state in patients with IBD52 and platelet activation may be important in pathogenesis by causing thrombosis formation and microinfarction secondary to microvascular ischaemia.53 The stress induced activation of platelets could be inhibited by beta blockers rather than aspirin, suggesting that sympathetic stimulation is key in the process.54 Platelet-leucocyte aggregate formation is also increased by acute experimental psychological stress51,55; this variable is raised in patients with IBD and may facilitate extravasation of leucocytes to localised areas of inflammation.56
Response to acute stress in the presence of chronic stress
The physiological and immune response to acute experimental stress is exaggerated by the presence of chronic psychological stress (table 1 ▶). Individuals with high chronic stress levels, such as those caring for a long term dependent or women with a strong family history of breast cancer, showed greater and more prolonged increases in sympathetic activation in response to acute stressors than controls.34,36 This was associated with a greater increase in NK cell numbers, albeit with an attenuated increase in their activity, in the chronically stressed subjects than in controls.34
Table 1.
Summary of the effects of adverse life events and acute experimental stress on systemic immune and inflammatory function in humans
| Stress | No of subjects | Effect | Reference |
| Stressful life events | |||
| Bereavement | 20 000 widows and widowers | Increased mortality | Lusyne58 |
| Bereavement | 37 widows | Decreased NK and cytotoxic T cell activity | Irwin24 |
| Major depression | 36 HV | Reduced NK cell number | Frank25 |
| Major depression | 46 depressed patients and 46 HV | Reduced NK cell activity | Chu26 |
| Geriatric depression | 166 depressed patients | Reduced CD4 and CD8 lymphocytes | Fortes27 |
| Adolescent depression | 36 depressed and 36 HV | Reduced NK cell number | Schleifer28 |
| Academic examinations | 64 adolescents | Reduced lymphocyte proliferation and neutrophil production of superoxides | Kang59 |
| Academic examinations | 38 HV | Increased TNF-α, IFN-γ, IL-6 production by LPS ;stimulated whole blood | Maes40 |
| Acute experimental stress | |||
| TSST | 32 HV | Increased IL-6 production by LPS stimulated ;whole blood | Goebel41 |
| TSST | 25 HV | Increased IL-1β and TNF-α production by LPS ;stimulated whole blood | Ackerman42 |
| TSST | 15 HV | Increased IFN-γ and IL-10 production by PBMC | Jacobs43 |
| Stroop | 13 HV | Increased serum IL-6 levels | Steptoe44 |
| TSST | 30 HV | Increased CD8 lymphocytes and NK cells | Marsland31 |
| TSST, MAT | 15 atopic dermatitis, 15 HV | Increased CD8 and NK cells | Schmid-Ott45 |
| TSST | 15 HV | Increased NK cells | Pawlak57 |
| MAT | 23 HV | Increased NK cell number and lytic activity | Pike34 |
| Dichotomous listening test | 20 quiescent UC | Increased leucocyte count and NK cell number | Mawdsley47 |
| Stroop | 11 HV under 35 | Increased thrombin induced platelet fibrinognen binding | Wallen49 |
| MAT | 12 HV | Increased platelet dependent thrombin generation | Kawano54 |
| Stroop | 40 HV | Increased platelet dependent ATP secretion | Malkoff50 |
| Stroop | 8 HV | Increased platelet dependent beta-thromboglobulin and serotonin secretion | Naesh48 |
| Stroop | 37 HV | Increased PLA formation | Steptoe55 |
| Dichotomous listening test | 20 quiescent UC | Increased platelet activation and PLA formation | Mawdsley 51 |
TSST, Trier social stress test; MAT, mental arithmetic test; Stroop, word-colour interference test; HV, healthy volunteers; NK, natural killer; LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumour necrosis factor α; IFN-γ, interferon γ; PLA, platelet-leucocyte aggregate; PBMC, peripheral blood mononuclear cell.
Response to acute experimental stress in the presence of chronic inflammatory disease
The immune response to acute experimental stress is also altered by the presence of chronic inflammatory disease (table 1 ▶). Patients with systemic lupus erythematosus (SLE) showed an increase in IL-4 producing peripheral blood mononuclear cells, as assessed by staining of intracellular cytokines, in response to the TSST, where as control subjects did not.43 The stress induced redistribution of the leucocyte subsets was also altered in patients with SLE, with an attenuated increase in the number of NK cells and their cytolytic activity in comparison with healthy controls.57
PSYCHOLOGICAL STRESS AND GASTROINTESTINAL IMMUNE AND INFLAMMATORY FUNCTION IN HUMANS
Chronic psychological stress and adverse life events in IBD
In the 1950s, IBD was classified as a psychosomatic disorder60,61 with many early studies finding an association between IBD and psychiatric diagnoses.62–64 However, a review in 1990 of 138 such studies found most to have serious flaws, while in the seven which did not, there was no association between psychiatric disease and UC.65 In contrast, in a study published in 2004, Mittermaier et al reported that patients with inactive IBD had a significantly increased chance of relapse over the next 18 months if their baseline score on the Beck’s depression inventory was raised.66
Placebo response rates in many therapeutic trials of IBD remain as high as 30–40%.67 This response rate relates not only to subjective measures such as patients’ feelings of well being, but also to objective measures such as the degree of mucosal inflammation seen at endoscopy, and provides further evidence to suggest that changes in psychological state can affect disease activity.68
Over the years there have been many case studies which have suggested that adverse life events can be a causal factor in relapse in IBD. However, although suggestive, these retrospective studies are frequently flawed by recall bias. Well designed prospective investigations of life events as causative factors for relapse in IBD are difficult to perform. They require a long study period to allow a sufficient number of relapses to occur to test for correlation, and a high degree of patient compliance for the collection of detailed diary records of life events and gastrointestinal symptoms. There are often confounding changes in medication during the study period. Lastly, the definition of what constitutes a stressful life event and what constitutes relapse is variable. In the latter context, many of the clinical scoring systems commonly used to assess disease activity in IBD such as the CDAI69 and SCCAI70 contain variables, like stool frequency, which are known to be affected adversely by stress but which do not necessarily reflect a worsening of inflammation.
The results of early studies of the association between adverse life events and disease activity in IBD were mixed (table 2 ▶).71–74,77–79 However, two more recent analyses, in which meticulous attempts were made to address these methodological problems, both found life events to be associated with a higher risk of subsequent relapse. Bitton et al found the number of stressful life events in the preceding month to be a risk factor for relapse in a one year prospective study of 60 patients with UC.75 Similarly, Mardini et al found depression and, to a lesser extent, life events to be predictors of relapse in a two year prospective study of 18 patients with Crohn’s disease.76
Table 2.
Summary of studies assessing association between stress and disease exacerbations in inflammatory bowel disease (IBD)
| Variable | Patients | Finding | Reference |
| Adverse life events | |||
| Retrospective analysis of life events preceding disease onset | 60 patients with UC | Positive association | Fava72 |
| Retrospective 2 year analysis of life events preceding disease relapse | 70 patients with CD, 44 patients with UC | Positive correlation with relapse in CD but not UC | Paar71 |
| Analysis of life events preceding relapse | 30 patients with UC | Positive association | Bach77 |
| Prospective 6 months of life events preceding exacerbation | 124 patients with IBD | Positive association with no lag time | Duffy78 |
| Prospective 2 year analysis of life events preceding disease relapse | 32 patients with IBD | No association | North73 |
| Prospective 1 year analysis of adverse life events and disease relapse | 108 patients with IBD | No association | Von Wietersheim74 |
| Retrospective analysis of incidence of adverse life events in preceding 12 months in patients with UC and healthy controls | 122 patients with UC | Greater incidence of life events in preceding 12 months in patients with UC | Tocchi79 |
| 1 year prospective analysis of life events and disease relapse | 60 patients with UC | Positive association of life events and relapse in the following month | Bitton75 |
| Retrospective 16 year analysis of prevalence of IBD in patients who lost a child and controls | 21 062 parents who lost a child | No increased prevalence of IBD | Li80 |
| Prospective 2 year analysis of depression and life events and disease relapse | 18 patients with CD | Positive association of depression and (less so) life events with disease relapse simultaneously and at 8–12 weeks | Mardini76 |
| Chronic perceived stress | |||
| Prospective 2 year analysis of PSQ score and disease exacerbation | 62 patients with UC | Increased risk of exacerbation with increased PSQ score | Levenstein81 |
| Analysis of mucosal endoscopic appearance and PSQ score | 46 patients with asymptomatic UC | Increased PSQ score in patients with mucosa abnormalities | Levenstein82 |
| Daily stress | |||
| Prospective 28 days of daily stress with self rated disease severity | 10 patients with CD | Positive association in some individuals | Garrett83 |
| Prospective 1 year analysis of stress with disease exacerbation | 11 patients with IBD | Positive association | Greene84 |
CD, Crohn’s disease; UC, ulcerative colitis; IBD, inflammatory bowel disease; PSQ, perceived stress questionnaire.
It is now also recognised that an individual’s stress response depends on their perception of the significance of the stressor, a factor which is not taken into account in a standard record of life events. The perceived stress questionnaire (PSQ) was developed to overcome this limitation. In a prospective cohort study of 62 patients with UC, a score in the upper tertile of the PSQ over the previous two years significantly increased the actuarial risk of an exacerbation81 and was also predictive of mucosal abnormalities in patients with UC who reported no symptoms.82 Ninety per cent of patients who scored in the upper tertile of the PSQ experienced a relapse during the study compared with 44% in the lower tertile.81 In these studies a chronic state of heightened stress appeared important in predicting relapse.
Acute psychological stress and gastrointestinal immune and inflammatory function
Both of the two small studies which have examined the role of acute daily stress in IBD suggest a positive association between disease relapse and exacerbation (table 2 ▶).83,84
There have been few examinations of the effects of experimental stress on gastrointestinal inflammation in humans. One study did find that physical stress, induced by immersion of the hand in iced water, increased the luminal jejunal concentration of the mast cell mediators tryptase and histamine in healthy volunteers and even more so in those with food allergies.85 We have found that acute psychological stress in the form of a dichotomous listening test increases the production of reactive oxygen metabolites by rectal mucosal biopsies in patients with quiescent UC (JE Mawdsley et al) (work in progress).
There is also limited anecdotal evidence that artificially induced alterations in neural function can affect gastrointestinal inflammation. One reported example is of a man whose previously refractory UC went into complete remission following a Brown-Sequard paralysis at the level of C5.86 Kemler described a patient who suffered recurrent flares of his UC in association with spinal cord stimulation given for post-traumatic pain in his arm.87 Neuromodulatory drugs such as lidocaine,88 which decreases neuronal release of SP, clonidine,89 and nicotine90 have been claimed for many years to have benefit in UC in clinical trials and remain potential therapeutic agents in IBD.91
Functioning of the stress axes in IBD
Several studies have suggested that the functioning of the HPA axis may be altered in patients with IBD and this may be important in relation to stress induced increases in disease activity. Usually, the release of CRF within the pituitary, and hence the serum concentration of cortisol, is increased by inflammatory cytokines, particularly IL-6. As the prevailing action of cortisol is anti-inflammatory in this context it provides a negative feedback which reduces inflammation. However, in a study of 64 patients with UC, serum concentrations of cortisol were found to bear no relation to serum IL-6 levels or to scores of disease activity.92
In a second study, Straub et al examined the correlation between sympathetic tone, as indicated by serum levels of neuropeptide Y, and HPA axis stimulation, as measured by serum cortisol, in healthy controls and patients with IBD.93 In healthy volunteers, the two variables were positively correlated. However, in patients with IBD, there was no such correlation, with a higher level of neuropeptide Y and a lower level of cortisol. Although in this study the concomitant use of oral steroids may have affected results, the authors suggest that in patients with IBD there may be uncoupling of the sympathetic nervous system and HPA axis. Chronically raised levels of inflammatory cytokines in the blood due to active IBD may thus blunt the response of the HPA axis to both inflammation and acute stress, as has been found in other chronic inflammatory diseases.
The function of the ANS may also be altered in patients with IBD as some authors have reported marked autonomic nervous hyperreflexia in patients with UC and CD.94
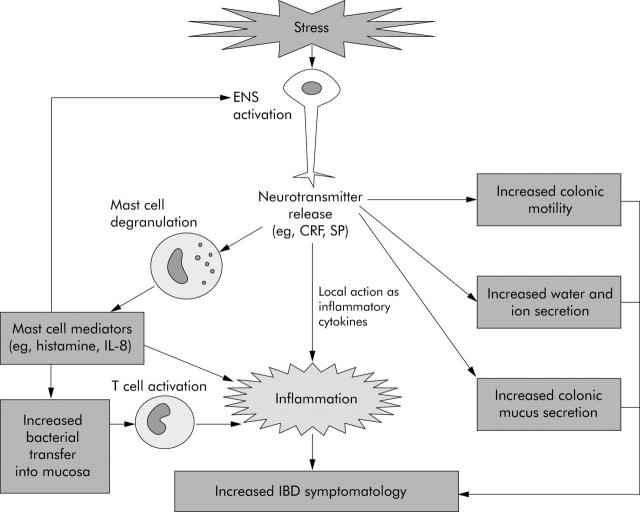
Acute psychological stress and gastrointestinal motility, and water and ion secretion (fig 2 ▶)
Figure 2.
Pathways by which the enteric nervous system (ENS) is likely to mediate stress induced increases in inflammatory bowel disease (IBD) symptomatolgy and disease activity. CRF, corticotrophin releasing factor; SP, substance P; IL, interleukin.
Acute psychological stress has effects on gastrointestinal motility, and water and ion secretion. Thus in healthy human volunteers, acute short term stress in the form of painful stimuli, dichotomous listening tests, and stressful interviews enhanced colonic motility.95 Dichotomous listening tests and cold pain stress have also been shown to increase jejunal water and sodium and chloride ion secretion.85,96,97 Although these are non-inflammatory changes, they could contribute to stress induced increases in symptomatology in patients with IBD.
Psychological stress and pain processing
Acute psychological stress has been shown to decrease thresholds for the perception of pain. Dichotomous listening tests decreased the threshold for the perception of pain in response to rectal distension in both patients with IBS and healthy volunteers.98,99 Although this experiment has not been repeated with patients with IBD, if stress does lower pain thresholds in these patients it may, in part, explain how acute stress can worsen IBD symptomatology.
The central release of SP from afferent neurones has been shown to be important in mediating stress induced gastrointestinal hyperalgesia. Central administration of an SP antagonist prevented restraint stress induced hypersensitivity to rectal distension in the guinea pig. However, the SP antagonist had no effect on rectal sensitivity in animals which had not been sensitised with restraint stress.100
The role of peripheral substance P (fig 2 ▶)
In addition to its central effects, peripheral release of SP from the ENS may have a role in stress induced increases in mucosal inflammation. Although there are no published data to show an increase in mucosal SP in response to stress, SP containing neurones are found in close association with mast cells, a cell type implicated as central in mediating stress induced permeability changes (see below). SP has been shown to increase histamine release from mucosal mast cells in patients with IBD.101 Lastly, SP can act not only as a neurotransmitter but also an inflammatory cytokine in its own right, enhancing cytokine production and stimulating chemotaxis of inflammatory cells.102 It also increases expression of leucocyte adhesion molecules on microvascular endothelium, and of CD11b on neutrophils, facilitating leucocyte adhesion at sites of inflammation.102
PSYCHOLOGICAL STRESS AND GASTROINTESTINAL IMMUNE AND INFLAMMATORY FUNCTION IN ANIMALS
Chronic psychological stress and animal models of IBD
Like humans, some animals may develop bowel inflammation when chronically stressed. Cotton top tamarins are at increased risk of a colitis closely resembling UC when subjected to the chronic stress of living in captivity, with remission being induced by return to natural living conditions.103 Similarly, Siamese gibbons can also develop a fatal colitis when held in captivity.104
Acute psychological stress and animal models of IBD
As in humans, experimental models of psychological stress have been developed to allow the study of the role of psychological stress in animal models of IBD. A period of restraint stress or wrap restraint stress, where an animal’s movements are restricted by gentle binding, is the commonest technique used in the rodent to simulate acute stress. This can be combined with either a cold environment or partial immersion in water. The water avoidance stress, where an animal is placed on a small platform surrounded by water, is another model used for acute stress.105 Prolonged maternal separation is used to simulate chronic stress and depression. A confounding factor, which requires use of appropriate control groups in all animal experiments, is that routine handling is inherently stressful.
Experimental psychological stress appears able to contribute to both the initiation and reactivation of gastrointestinal inflammation in animal models of colitis. Rats subjected to restraint stress for the four days prior to induction of colitis by 2,4,6-trinitrobenzenesulfonic acid (TNBS) developed an increased mucosal inflammatory response.106 Restraint stress in the absence of any other costimuli caused partial reactivation of mucosal inflammation in rats which had recovered from TNBS colitis six weeks previously; there was an increase in colonic myeloperoxidase, although there were no inflammatory changes detectable by light microscopy.107 A period of restraint stress also lowered the dose of TNBS required to reactivate colitis in mice which had recovered from TNBS colitis eight weeks previously.108 This susceptibility was transferable between mice by a population of CD4 rich lymphocytes taken from the spleen and mesenteric lymph nodes, suggesting that stress induced reactivation is dependent on the presence of key immune cells. Recipient mice did not develop colitis immediately but required a lower dose of TNBS in combination with restraint stress to cause mucosal ulceration than did controls.
Chronic stress appears to render an animal with colitis more vulnerable to the effects of acute stress. Adult rats which underwent previous prolonged maternal separation and which were then subjected to a series of inescapable foot shocks, experienced a greater severity of dextran sodium sulphate induced colitis than did controls.109
Functioning of the stress axes in animal models of IBD
There have been no studies to assess the functioning of the stress axes in animal models of IBD. However, it has been shown that reduced HPA axis function renders rodents susceptible to stress induced increases in gastrointestinal inflammation. If, as discussed above, HPA axis function is reduced in patients with IBD, this observation may be relevant to stress induced increases in disease activity. LEW/N rats have a reduced CRF content of the hypothalamus and paraventricular nucleus compared with controls. They have a markedly decreased plasma ACTH and corticosterone response to stressful stimuli, and have long been recognised as being more susceptible to inflammatory disorders such as the arthritis induced by intra-articular injections of streptococcal cell wall.110 LEW/N rats also show an increased susceptibility to TNBS induced colitis, an effect which was found to be dependent on coexisting stress.111 Thus although stress naive animals developed a similar degree of intestinal inflammation to control rats at seven days after TNBS administration, intestinal inflammation was greater in LEW/N rats than in appropriate control animals if they were restrained for six days prior to being given TNBS.
Psychological stress and intestinal barrier function and bacterial-host interactions (fig 2 ▶)
Experimental stress in animals increases intestinal mucosal permeability and also alters bacterial-host interactions. These changes are likely to contribute to mucosal inflammation, and fit with the hypothesis that such inflammation is driven by mucosal flora.112
Restraint stress in rats has been shown to increase jejunal and colonic permeability to inert marker molecules, such as mannitol and Cr-EDTA, and to antigenic proteins such as horse radish peroxidase (HRP).113 Under normal conditions, HRP passes through the epithelial cells via pinocytosis but, after chronic restraint stress, it also passed through the epithelial layer paracellularly. Although the gut mucosa appeared normal to examination under the light microscope, electron microscopy demonstrated increased HRP both within intracellular endosomes and in interepithelial cell tight junctions.114 Again, chronic stress appears to enhance the effect of acute stress, since prior maternal separation augmented restraint stress induced increases in permeability.115
The increase in permeability caused by stress appears to be dependent on cholinergic innervation, as it was blocked with atropine and was more marked in cholinesterase deficient Wistar-Kyoto rats.116 Mast cells are also important in mediating permeability changes, as mast cell deficient rats exposed to repeated restraint stress lost weight to a similar extent to wild-type rats but without showing changes in intestinal permeability.117 Restraint stress increased the histamine content of colonic mucosal mast cells in rats, an effect which appeared dependent on both central CRF and IL-1.118 Increased colonic permeability caused by stress was also mimicked by injection of peripheral CRF.114
In addition, it has recently been shown that bacterial-mucosal interactions are also affected by experimental stress in animal models. One hour sessions of water avoidance stress for 10 consecutive days increased the phagocytic uptake of killed Escherichia coli into follicle associated epithelium in mice.119 Follicle associated epithelium overlies Peyer’s patches and contains M cells which are in close association with dendritic cells, the principal antigen presenting cell of the gut. These changes were not found in villi associated epithelium and did not occur in mast cell deficient rats.119,120
Catecholamines also appear to have a role in altering bacterial adherence and uptake by gut mucosa. Chen et al reported that both dopamine and noradrenaline increased the adherence of E coli 0157 to murine caecal mucosa121 whilst Green et al found that pretreatment of isolated porcine jejunal Peyer’s patches with noradrenaline led to increased internalisation of Salmonella choleraesuis and E coli 0157 but not of non-pathogenic E coli.122
It is possible that mast cells, stimulated by neurotransmitters such as CRF and SP released by enteric neurones in response to stressful stimuli, increase bacterial adherence and uptake via the release of mast cell mediators. This in turn could lead to the sensitisation of T cells and the production of IFN-γ and TNF-α which may both initiate inflammation and cause a secondary increase in permeability. This theory is consistent with the recent finding that increases in intestinal permeability to the inert marker Cr-EDTA, in response to mixed restraint and acoustic stress, were dependent on the presence of T cells and IFN-γ.123 Also, it has recently been demonstrated that expression of mRNA for TNF-α in mucosa correlates with endosomal uptake of HRP in resected ileal mucosa mounted in Ussing chambers.124
Psychological stress and gastrointestinal motility, and water and ion secretion (fig 2 ▶)
As in humans, acute stress in the form of restraint stress, loud noise, inescapable foot shock, or water avoidance all increase colonic motility and defecation in the rodent.125 The mechanisms for these changes involve CRF and its receptors (see below).126
Similar alterations in ion and water transport to those seen in humans are also well described in animals in response to psychological stress. Increases in gastrointestinal water and chloride ion secretion occur in response to restraint stress in the rat.127 It is now recognised that, as with changes in intestinal permeability, this secretory response is related to both cholinergic nerves and mast cells as it was increased in cholinesterase deficient Wistar-Kyoto rats, blocked by pretreatment with atropine, and absent in mast cell deficient rats.116,117 Restraint stress also increased colonic mucus secretion in ex vivo colonic segments and in vivo, as measured by histological goblet cell depletion.128–130 This effect could also be reproduced by peripheral CRF administration128 and inhibited by mast cell stabilisers.
The role of CRF in mediating stress related gastrointestinal changes in animal models (fig 2 ▶)
CRF is a 41 amino acid neuropeptide which exerts its effects via two adenylate cyclase coupled receptors, CRF-R1 and CRF-R2.131 It has a pivotal role in mediating the effects of stress on the gastrointestinal tract in animal models, some of which may be relevant to stress induced increases in IBD activity in humans.
Central injection of CRF in the rat induces behaviour normally seen in response to stressful stimuli.132 It also reproduces the motility changes usually seen in response to stress, with decreased gastric emptying and increased colonic motility. The use of selective CRF receptor antagonists has proven that central CRF increases colonic motility via stimulation of CRF-R1 and delays gastric emptying via stimulation of CRF-R2.126
More recent evidence has also implicated a role for peripheral CRF in mediating stress induced motility changes. Peripheral administration of CRF antagonists has been shown to abolish restraint stress increases in colonic motility and decreases in gastric motility.133 As described above, both peripheral and central CRF are important in mediating stress induced increases in gastrointestinal permeability. Furthermore, CRF appears to have a secondary peripheral action as an inflammatory cytokine. It stimulates IL-1, IL-2, and IL-6 secretion and IL-2 receptor expression by peripheral blood mononuclear cells,134–136 T and B lymphocyte proliferation, and enhancement of NK cytotoxicity.137 It exerts a mixed effect on cytokine production by endothelial cells, with both a proinflammatory action via CRF-2 receptors and an anti-inflammatory action via CRF-1 receptors.138 More recent evidence for a functional role for CRF in animal models of gastrointestinal inflammation comes from the finding that pretreatment with a CRF antagonist reduced the inflammation caused by injection of C difficile toxin into rat terminal ileum.139
CRF levels are increased in caecal biopsies from rats with experimental colitis induced by intramural injection of peptidoglycan-polysaccharide polymers.140 In the chronically inflamed caecum, abundant immunoreactive CRF was found in inflammatory cells, mesenchymal cells, and myenteric plexi; in contrast, in non-inflamed caecum only minimal CRF containing cells were found. Whether CRF has a role in mediating stress related gastrointestinal changes in humans with IBD is unknown. However, there is some evidence of a functional role for CRF in the pathogenesis of IBD as CRF levels are increased in lamina propria mononuclear cells in colonic biopsies from patients with active UC.141,142
IMPLICATIONS FOR THERAPY OF IBD
If psychological stress is indeed a pathogenic factor in IBD, then stress reduction therapy may have therapeutic benefit. However, despite recent advances in our understanding of the relationship between psychological stress and IBD, most stress reduction therapy remains unformalised, and studies of its efficacy in patients with IBD are few. There are a wide variety of psychotherapeutic interventions which could be assessed making standardisation difficult. Due to the nature of the intervention, performing the trials in a blinded controlled manner is also difficult and, as already discussed, with placebo rates of up to 40%67,68 genuine therapeutic effects can be hard to detect.
Of those trials which have been reported, the results are conflicting. Schwarz and Blanchard found that combined complementary medical treatment, including cognitive behavioural therapy, muscle relaxation techniques, and patient education, was successful in reducing stress but did not improve IBD symptoms.143 Jantschek et al also failed to find that addition of psychosocial treatments to conventional therapy led to any reduction in the number, duration, or severity of relapses in a prospective two year study of 108 patients with Crohn’s disease.144 In contrast, Milne et al found that a stress management course in addition to conventional treatment significantly reduced Crohn’s disease activity index over the follow up period of one year in a randomised trial of 80 patients with Crohn’s disease.145 An uncontrolled study examining the benefits of hypnotherapy, a psychotherapeutic intervention used increasingly to treat refractory irritable bowel syndrome,146–148 found a non-significant trend to improvement in the IBDQ score of 12 patients with IBD after six weeks of treatment.149
In summary, further trials are required before a view can be taken as to whether any form of stress reduction therapy may have benefit for patients with IBD. However, given the considerable evidence both in humans with IBD and in animal models of IBD that stress can increase disease activity, perhaps such studies should now be attempted.
Points to remember: psychological stress in IBD.
Recent studies indicate that chronic stress, adverse life events, and depression can cause relapse in patients with IBD.
The effects of stress on inflammation in IBD are likely to be mediated through changes in hypothalamic-pituitary-adrenal function, alterations in bacterial-mucosal floral interactions, activation of mucosal mast cells, and peripheral release of corticotrophin releasing factor.
The symptoms of IBD may be exacerbated by the effects of stress on gut motility and fluid secretion.
There is a need for further controlled studies of the potential benefits of stress reduction therapy in IBD.
CONCLUSION
Ulcerative colitis and Crohn’s disease were initially considered examples of psychosomatic diseases in which psychological factors played a major role. However, as knowledge of the genetic, environmental, and molecular pathogenesis of IBD increased, the possible contribution to its aetiology of psychological stress was progressively neglected. Indeed, stress was often dismissed as a vague subjective concept, a view which some of the early and methodologically flawed studies of stress in relation to IBD did nothing to diminish.
In recent years, however, considerable evidence has accumulated that psychological stress does indeed contribute to the risk of relapse in IBD. Furthermore, laboratory research has indicated a variety of mechanisms by which stress can affect both the systemic and gastrointestinal immune and inflammatory responses. Translating these findings into therapeutic interventions based on stress reduction remains a challenge, the solution to which will not only benefit patients, but also shed further light on the pathogenesis of IBD.
Acknowledgments
We would like to acknowledge the Broad Medical Research Foundation for funding JEM’s research.
Conflict of interest: None declared.
REFERENCES
- 1.Ahmad T, Tamboli CP, Jewell D, et al. Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology 2004;126:1533–49. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology 2001;120:622–35. [DOI] [PubMed] [Google Scholar]
- 3.Hugot JP. Genetic origin of IBD. Inflamm Bowel Dis 2004;10 (suppl 1) :S11–15. [DOI] [PubMed] [Google Scholar]
- 4.Brown CH. Acute emotional crises and ulcerative colitis. Report of seven cases. Am J Dig Dis 1963;8:525–36. [DOI] [PubMed] [Google Scholar]
- 5.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 1992;267:1244–52. [PubMed] [Google Scholar]
- 6.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol 2003;463:235–72. [DOI] [PubMed] [Google Scholar]
- 7.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med 1996;334:1106–15. [DOI] [PubMed] [Google Scholar]
- 8.Ader R, Cohen N, Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet 1995;345:99–103. [DOI] [PubMed] [Google Scholar]
- 9.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut 2000;47:861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichlin S. Neuroendocrine-immune interactions. N Engl J Med 1993;329:1246–53. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PJ. Molecular mechanisms of corticosteroids in allergic diseases. Allergy 2001;56:928–36. [DOI] [PubMed] [Google Scholar]
- 12.Ray A, Sehgal PB. Cytokines and their receptors: molecular mechanism of interleukin-6 gene repression by glucocorticoids. J Am Soc Nephrol 1992;2 (suppl) :S214–21. [DOI] [PubMed] [Google Scholar]
- 13.Joyce DA, Gimblett G, Steer JH. Targets of glucocorticoid action on TNF-alpha release by macrophages. Inflamm Res 2001;50:337–40. [DOI] [PubMed] [Google Scholar]
- 14.Franchimont D, Kino T, Galon J, et al. Glucocorticoids and inflammation revisited: the state of the art. NIH Clinical Staff Conference. Neuroimmunomodulation 2003;10:247–60. [DOI] [PubMed] [Google Scholar]
- 15.Brattsand R, Linden M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment Pharmacol Ther 1996;10 (suppl 2) :81–90. [DOI] [PubMed] [Google Scholar]
- 16.Amsterdam A, Tajima K, Sasson R. Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem Pharmacol 2002;64:843–50. [DOI] [PubMed] [Google Scholar]
- 17.Renz H, Henke A, Hofmann P, et al. Sensitization of rat alveolar macrophages to enhanced TNF-alpha release by in vivo treatment with dexamethasone. Cell Immunol 1992;144:249–57. [DOI] [PubMed] [Google Scholar]
- 18.Sondergaard SR, Ostrowski K, Ullum H, et al. Changes in plasma concentrations of interleukin-6 and interleukin-1 receptor antagonists in response to adrenaline infusion in humans. Eur J Appl Physiol 2000;83:95–8. [DOI] [PubMed] [Google Scholar]
- 19.Van der PT, Lowry SF. Lipopolysaccharide-induced interleukin 8 production by human whole blood is enhanced by epinephrine and inhibited by hydrocortisone. Infect Immun 1997;65:2378–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegmund B, Eigler A, Hartmann G, et al. Adrenaline enhances LPS-induced IL-10 synthesis: evidence for protein kinase A-mediated pathway. Int J Immunopharmacol 1998;20:57–69. [DOI] [PubMed] [Google Scholar]
- 21.Kappel M, Poulsen TD, Galbo H, et al. Effects of elevated plasma noradrenaline concentration on the immune system in humans. Eur J Appl Physiol Occup Physiol 1998;79:93–8. [DOI] [PubMed] [Google Scholar]
- 22.Van der PT, Lowry SF. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor-alpha and IL-10. Am J Physiol 1997;273 (6 Pt 2) :R1885–90. [DOI] [PubMed] [Google Scholar]
- 23.Straub RH, Dhabhar FS, Bijlsma JW, et al. How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis. Arthritis Rheum 2005;52:16–26. [DOI] [PubMed] [Google Scholar]
- 24.Irwin M, Daniels M, Weiner H. Immune and neuroendocrine changes during bereavement. Psychiatr Clin North Am 1987;10:449–65. [PubMed] [Google Scholar]
- 25.Frank MG, Wieseler Frank JL, Hendricks SE, et al. Age at onset of major depressive disorder predicts reductions in NK cell number and activity. J Affect Disord 2002;71:159–67. [DOI] [PubMed] [Google Scholar]
- 26.Chu L, Tian S, Chen H et a l. Study on natural killer cell subset and activity in patients with depression. Zhonghua Yi Xue Za Zhi 2002;82:830–1. [PubMed] [Google Scholar]
- 27.Fortes C, Farchi S, Forastiere F, et al. Depressive symptoms lead to impaired cellular immune response. Psychother Psychosom 2003;72:253–60. [DOI] [PubMed] [Google Scholar]
- 28.Schleifer SJ, Bartlett JA, Keller SE, et al. Immunity in adolescents with major depression. J Am Acad Child Adolesc Psychiatry 2002;41:1054–60. [DOI] [PubMed] [Google Scholar]
- 29.Danner M, Kasl SV, Abramson JL, et al. Association between depression and elevated C-reactive protein. Psychosom Med 2003;65:347–56. [DOI] [PubMed] [Google Scholar]
- 30.Sajadieh A, Nielsen OW, Rasmussen V, et al. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J 2004;25:363–70. [DOI] [PubMed] [Google Scholar]
- 31.Marsland AL, Manuck SB, Fazzari TV, et al. Stability of individual differences in cellular immune responses to acute psychological stress. Psychosom Med 1995;57:295–8. [DOI] [PubMed] [Google Scholar]
- 32.Kirschbaum C, Klauer T, Filipp SH, et al. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom Med 1995;57:23–31. [DOI] [PubMed] [Google Scholar]
- 33.Valori RM, Kumar D, Wingate DL. Effects of different types of stress and of “prokinetic” drugs on the control of the fasting motor complex in humans. Gastroenterology 1986;90:1890–900. [DOI] [PubMed] [Google Scholar]
- 34.Pike JL, Smith TL, Hauger RL, et al. Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosom Med 1997;59:447–57. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen LS, Bonlokke L, Christensen NJ. Life strain, life events, and autonomic response to a psychological stressor in patients with chronic upper abdominal pain. Scand J Gastroenterol 1986;21:605–13. [DOI] [PubMed] [Google Scholar]
- 36.Gold SM, Zakowski SG, Valdimarsdottir HB, et al. Stronger endocrine responses after brief psychological stress in women at familial risk of breast cancer. Psychoneuroendocrinology 2003;28:584–93. [DOI] [PubMed] [Google Scholar]
- 37.Rohleder N, Wolf JM, Piel M, et al. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology 2003;28:261–73. [DOI] [PubMed] [Google Scholar]
- 38.Golden CJ. The measurement of creativity by the Stroop Color and Word Test. J Pers Assess 1975;39:502–6. [DOI] [PubMed] [Google Scholar]
- 39.Richter SD, Schurmeyer TH, Schedlowski M, et al. Time kinetics of the endocrine response to acute psychological stress. J Clin Endocrinol Metab 1996;81:1956–60. [DOI] [PubMed] [Google Scholar]
- 40.Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 1998;10:313–18. [DOI] [PubMed] [Google Scholar]
- 41.Goebel MU, Mills PJ, Irwin MR, et al. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med 2000;62:591–8. [DOI] [PubMed] [Google Scholar]
- 42.Ackerman KD, Martino M, Heyman R, et al. Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom Med 1998;60:484–91. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs R, Pawlak CR, Mikeska E, et al. Systemic lupus erythematosus and rheumatoid arthritis patients differ from healthy controls in their cytokine pattern after stress exposure. Rheumatology (Oxford) 2001;40:868–75. [DOI] [PubMed] [Google Scholar]
- 44.Steptoe A, Willemsen G, Owen N, et al. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond) 2001;101:185–92. [PubMed] [Google Scholar]
- 45.Schmid-Ott G, Jaeger B, Meyer S, et al. Different expression of cytokine and membrane molecules by circulating lymphocytes on acute mental stress in patients with atopic dermatitis in comparison with healthy controls. J Allergy Clin Immunol 2001;108:455–62. [DOI] [PubMed] [Google Scholar]
- 46.Herbert TB, Cohen S, Marsland AL, et al. Cardiovascular reactivity and the course of immune response to an acute psychological stressor. Psychosom Med 1994;56:337–44. [DOI] [PubMed] [Google Scholar]
- 47.Mawdsley JE, Jenkins DG, Macey MG, et al. Natural killer cell numbers are increased by stress and decreased by hypnotherapy in patients with ulcerative colitis Gut 2005;54 (suppl II) :A23.
- 48.Naesh O, Haedersdal C, Hindberg I, et al. Platelet activation in mental stress. Clin Physiol 1993;13:299–307. [DOI] [PubMed] [Google Scholar]
- 49.Wallen NH, Goodall AH, Li N, et al. Activation of haemostasis by exercise, mental stress and adrenaline: effects on platelet sensitivity to thrombin and thrombin generation. Clin Sci (Lond) 1999;97:27–35. [PubMed] [Google Scholar]
- 50.Malkoff SB, Muldoon MF, Zeigler ZR, et al. Blood platelet responsivity to acute mental stress. Psychosom Med 1993;55:477–82. [DOI] [PubMed] [Google Scholar]
- 51.Mawdsley JE, Macey MG, Rampton DS. Acute psychological stress increases platelet activation and platelet-leucocyte aggregate formation in patients with inactive ulcerative colitis. Gut 2005;54 (suppl Ii) :A91. [Google Scholar]
- 52.Collins CE, Cahill MR, Newland AC, et al. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology 1994;106:840–5. [DOI] [PubMed] [Google Scholar]
- 53.Collins CE, Rampton DS. Review article: platelets in inflammatory bowel disease—pathogenetic role and therapeutic implications. Aliment Pharmacol Ther 1997;11:237–47. [DOI] [PubMed] [Google Scholar]
- 54.Kawano TA, Aoki N, Homori M, et al. Mental stress and physical exercise increase platelet-dependent thrombin generation. Heart Vessels 2000;15:280–8. [DOI] [PubMed] [Google Scholar]
- 55.Steptoe A, Magid K, Edwards S, et al. The influence of psychological stress and socioeconomic status on platelet activation in men. Atherosclerosis 2003;168:57–63. [DOI] [PubMed] [Google Scholar]
- 56.Irving PM, Macey MG, Shah U, et al. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm Bowel Dis 2004;10:361–72. [DOI] [PubMed] [Google Scholar]
- 57.Pawlak CR, Jacobs R, Mikeska E, et al. Patients with systemic lupus erythematosus differ from healthy controls in their immunological response to acute psychological stress. Brain Behav Immun 1999;13:287–302. [DOI] [PubMed] [Google Scholar]
- 58.Lusyne P, Page H, Lievens J. Mortality following conjugal bereavement, Belgium 1991–96: the unexpected effect of education. Popul Stud (Camb) 2001;55:281–9. [DOI] [PubMed] [Google Scholar]
- 59.Kang DH, Coe CL, McCarthy DO. Academic examinations significantly impact immune responses, but not lung function, in healthy and well-managed asthmatic adolescents. Brain Behav Immun 1996;10:164–81. [DOI] [PubMed] [Google Scholar]
- 60.Engel GL. Psychological factors in ulcerative colitis in man and gibbon. Gastroenterology 1969;57:362–5. [PubMed] [Google Scholar]
- 61.Alexander t. An objective study of psychological factors in ulcerative colitis in children. Lancet 1965;85:22–4. [PubMed] [Google Scholar]
- 62.Robertson DA, Ray J, Diamond I, et al. Personality profile and affective state of patients with inflammatory bowel disease. Gut 1989;30:623–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helzer JE, Stillings WA, Chammas S, et al. A controlled study of the association between ulcerative colitis and psychiatric diagnoses. Dig Dis Sci 1982;27:513–18. [DOI] [PubMed] [Google Scholar]
- 64.Helzer JE, Chammas S, Norland CC, et al. A study of the association between Crohn’s disease and psychiatric illness. Gastroenterology 1984;86:324–30. [PubMed] [Google Scholar]
- 65.North CS, Clouse RE, Spitznagel EL, et al. The relation of ulcerative colitis to psychiatric factors: a review of findings and methods. Am J Psychiatry 1990;147:974–81. [DOI] [PubMed] [Google Scholar]
- 66.Mittermaier C, Dejaco C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: a prospective 18-month follow-up study. Psychosom Med 2004;66:79–84. [DOI] [PubMed] [Google Scholar]
- 67.Feagan BG, McDonald JW, Koval JJ. Therapeutics and inflammatory bowel disease: a guide to the interpretation of randomized controlled trials. Gastroenterology 1996;110:275–83. [DOI] [PubMed] [Google Scholar]
- 68.Meyers S, Janowitz HD. “Natural history” of Crohn’s disease. An analytic review of the placebo lesson. Gastroenterology 1984;87:1189–92. [PubMed] [Google Scholar]
- 69.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 70.Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paar GH, Bezzenberger U, Lorenz-Meyer H. The correlation of psychosocial stress and disease activity in patients with Crohn disease and ulcerative colitis. Z Gastroenterol 1988;26:648–57. [PubMed] [Google Scholar]
- 72.Fava GA, Pavan L. Large bowel disorders. I. Illness configuration and life events. Psychother Psychosom 1976;27:93–9. [DOI] [PubMed] [Google Scholar]
- 73.North CS, Alpers DH, Helzer JE, et al. Do life events or depression exacerbate inflammatory bowel disease? A prospective study. Ann Intern Med 1991;114:381–6. [DOI] [PubMed] [Google Scholar]
- 74.von Wietersheim J, Kohler T, Feiereis H. Relapse-precipitating life events and feelings in patients with inflammatory bowel disease. Psychother Psychosom 1992;58:103–12. [DOI] [PubMed] [Google Scholar]
- 75.Bitton A, Sewitch MJ, Peppercorn MA, et al. Psychosocial determinants of relapse in ulcerative colitis: a longitudinal study. Am J Gastroenterol 2003;98:2203–8. [DOI] [PubMed] [Google Scholar]
- 76.Mardini HE, Kip KE, Wilson JW. Crohn’s disease: a two-year prospective study of the association between psychological distress and disease activity. Dig Dis Sci 2004;49:492–7. [DOI] [PubMed] [Google Scholar]
- 77.Bach O, Wild HJ. Life stress events preceding illness episodes in multiple sclerosis and ulcerative colitis—a comparison. Z Gesamte Hyg 1990;36:442–3. [PubMed] [Google Scholar]
- 78.Duffy LC, Zielezny MA, Marshall JR, et al. Relevance of major stress events as an indicator of disease activity prevalence in inflammatory bowel disease. Behav Med 1991;17:101–10. [DOI] [PubMed] [Google Scholar]
- 79.Tocchi A, Lepre L, Liotta G, et al. Familial and psychological risk factors of ulcerative colitis. Ital J Gastroenterol Hepatol 1997;29:395–8. [PubMed] [Google Scholar]
- 80.Li J, Norgard B, Precht DH, et al. Psychological stress and inflammatory bowel disease: a follow-up study in parents who lost a child in Denmark. Am J Gastroenterol 2004;99:1129–33. [DOI] [PubMed] [Google Scholar]
- 81.Levenstein S, Prantera C, Varvo V, et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol 2000;95:1213–20. [DOI] [PubMed] [Google Scholar]
- 82.Levenstein S, Prantera C, Varvo V, et al. Psychological stress and disease activity in ulcerative colitis: a multidimensional cross-sectional study. Am J Gastroenterol 1994;89:1219–25. [PubMed] [Google Scholar]
- 83.Garrett VD, Brantley PJ, Jones GN, et al. The relation between daily stress and Crohn’s disease. J Behav Med 1991;14:87–96. [DOI] [PubMed] [Google Scholar]
- 84.Greene BR, Blanchard EB, Wan CK. Long-term monitoring of psychosocial stress and symptomatology in inflammatory bowel disease. Behav Res Ther 1994;32:217–26. [DOI] [PubMed] [Google Scholar]
- 85.Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology 1998;114:640–8. [DOI] [PubMed] [Google Scholar]
- 86.Peck OC, Wood JD. Brain-gut interactions in ulcerative colitis. Gastroenterology 2000;118:807–8. [DOI] [PubMed] [Google Scholar]
- 87.Kemler MA, Barendse GA, van Kleef M. Relapsing ulcerative colitis associated with spinal cord stimulation. Gastroenterology 1999;117:215–17. [DOI] [PubMed] [Google Scholar]
- 88.Bjorck S, Dahlstrom A, Johansson L, et al. Treatment of the mucosa with local anaesthetics in ulcerative colitis. Agents Actions. 1992; Spec NoC60–72. [PubMed]
- 89.Lechin F, van der DB, Insausti CL, et al. Treatment of ulcerative colitis with clonidine. J Clin Pharmacol 1985;25:219–26. [DOI] [PubMed] [Google Scholar]
- 90.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med 1994;330:811–15. [DOI] [PubMed] [Google Scholar]
- 91.Bjorck S, Dahlstrom A, Ahlman H. Treatment of distal colitis with local anaesthetic agents. Pharmacol Toxicol 2002;90:173–80. [DOI] [PubMed] [Google Scholar]
- 92.Straub RH, Vogl D, Gross V, et al. Association of humoral markers of inflammation and dehydroepiandrosterone sulfate or cortisol serum levels in patients with chronic inflammatory bowel disease. Am J Gastroenterol 1998;93:2197–202. [DOI] [PubMed] [Google Scholar]
- 93.Straub RH, Herfarth H, Falk W, et al. Uncoupling of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis in inflammatory bowel disease? J Neuroimmunol 2002;126:116–25. [DOI] [PubMed] [Google Scholar]
- 94.Straub RH, Antoniou E, Zeuner M, et al. Association of autonomic nervous system hyperreflexia and systemic inflammation in patients with Crohn’s disease and ulcerative colitis. J Neuroimmunol 1997;80:149–57. [DOI] [PubMed] [Google Scholar]
- 95.Rao SS, Hatfield RA, Suls JM, et al. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol 1998;93:985–90. [DOI] [PubMed] [Google Scholar]
- 96.Barclay GR, Turnberg LA. Effect of psychological stress on salt and water transport in the human jejunum. Gastroenterology 1987;93:91–7. [DOI] [PubMed] [Google Scholar]
- 97.Barclay GR, Turnberg LA. Effect of cold-induced pain on salt and water transport in the human jejunum. Gastroenterology 1988;94:994–8. [DOI] [PubMed] [Google Scholar]
- 98.Gonlachanvit S, Rhee J, Sun WM, et al. Effect of acute acoustic stress on anorectal function sensation in healthy human. Neurogastroenterol Motil 2005;17:222–8. [DOI] [PubMed] [Google Scholar]
- 99.Murray CD, Flynn J, Ratcliffe L, et al. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology 2004;127:1695–703. [DOI] [PubMed] [Google Scholar]
- 100.Greenwood-Van Meerveld B, Gibson MS, Johnson AC, et al. NK1 receptor-mediated mechanisms regulate colonic hypersensitivity in the guinea pig. Pharmacol Biochem Behav 2003;74 (4) :1005–13. [DOI] [PubMed] [Google Scholar]
- 101.Raithel M, Schneider HT, Hahn EG. Effect of substance P on histamine secretion from gut mucosa in inflammatory bowel disease. Scand J Gastroenterol 1999;34:496–503. [DOI] [PubMed] [Google Scholar]
- 102.O’Connor TM, O’Connell J, O’Brien DI, et al. The role of substance P in inflammatory disease. J Cell Physiol 2004;201:167–80. [DOI] [PubMed] [Google Scholar]
- 103.Wood JD, Peck OC, Tefend KS, et al. Evidence that colitis is initiated by environmental stress and sustained by fecal factors in the cotton-top tamarin (Saguinus oedipus). Dig Dis Sci 2000;45:385–93. [DOI] [PubMed] [Google Scholar]
- 104.Stout C, Snyder RL. Ulcerative colitis-like lesion in Siamang gibbons. Gastroenterology 1969;57:256–61. [PubMed] [Google Scholar]
- 105.Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry 2002;159:1265–83. [DOI] [PubMed] [Google Scholar]
- 106.Gue M, Bonbonne C, Fioramonti J, et al. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol 1997;272 (1 Pt 1) :G84–91. [DOI] [PubMed] [Google Scholar]
- 107.Collins SM, McHugh K, Jacobson K, et al. Previous inflammation alters the response of the rat colon to stress. Gastroenterology 1996;111:1509–15. [DOI] [PubMed] [Google Scholar]
- 108.Qiu BS, Vallance BA, Blennerhassett PA, et al. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med 1999;5:1178–82. [DOI] [PubMed] [Google Scholar]
- 109.Milde AM, Enger O, Murison R. The effects of postnatal maternal separation on stress responsivity and experimentally induced colitis in adult rats. Physiol Behav 2004;81:71–84. [DOI] [PubMed] [Google Scholar]
- 110.Sternberg EM, Young WS III, Bernardini R, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A 1989;86:4771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Million M, Tache Y, Anton P. Susceptibility of Lewis and Fischer rats to stress-induced worsening of TNB-colitis: protective role of brain CRF. Am J Physiol 1999;276 (4 Pt 1) :G1027–36. [DOI] [PubMed] [Google Scholar]
- 112.Elson CO. Genes, microbes, and T cells—new therapeutic targets in Crohn’s disease. N Engl J Med 2002;346:614–16. [DOI] [PubMed] [Google Scholar]
- 113.Kiliaan AJ, Saunders PR, Bijlsma PB, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol 1998;275 (5 Pt 1) :G1037–44. [DOI] [PubMed] [Google Scholar]
- 114.Santos J, Saunders PR, Hanssen NP, et al. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol 1999;277 (2 Pt 1) :G391–9. [DOI] [PubMed] [Google Scholar]
- 115.Soderholm JD, Yates DA, Gareau MG, et al. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol 2002;283:G1257–63. [DOI] [PubMed] [Google Scholar]
- 116.Saunders PR, Hanssen NP, Perdue MH. Cholinergic nerves mediate stress-induced intestinal transport abnormalities in Wistar-Kyoto rats. Am J Physiol 1997;273 (2 Pt 1) :G486–90. [DOI] [PubMed] [Google Scholar]
- 117.Santos J, Benjamin M, Yang PC, et al. Chronic stress impairs rat growth and jejunal epithelial barrier function: role of mast cells. Am J Physiol Gastrointest Liver Physiol 2000;278:G847–54. [DOI] [PubMed] [Google Scholar]
- 118.Eutamene H, Theodorou V, Fioramonti J, et al. Acute stress modulates the histamine content of mast cells in the gastrointestinal tract through interleukin-1 and corticotropin-releasing factor release in rats. J Physiol 2003;553 (Pt 3) :959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Velin AK, Ericson AC, Braaf Y, et al. Increased antigen and bacterial uptake in follicle associated epithelium induced by chronic psychological stress in rats. Gut 2004;53:494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soderholm JD, Yang PC, Ceponis P, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 2002;123:1099–108. [DOI] [PubMed] [Google Scholar]
- 121.Chen C, Brown DR, Xie Y, et al. Catecholamines modulate Escherichia coli O157:H7 adherence to murine cecal mucosa. Shock 2003;20:183–8. [DOI] [PubMed] [Google Scholar]
- 122.Green BT, Lyte M, Kulkarni-Narla A, et al. Neuromodulation of enteropathogen internalization in Peyer’s patches from porcine jejunum. J Neuroimmunol 2003;141 (1–2) :74–82. [DOI] [PubMed] [Google Scholar]
- 123.Ferrier L, Mazelin L, Cenac N, et al. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology 2003;125:795–804. [DOI] [PubMed] [Google Scholar]
- 124.Soderholm JD, Streutker C, Yang PC, et al. Increased epithelial uptake of protein antigens in the ileum of Crohn’s disease mediated by tumour necrosis factor alpha. Gut 1994;53:1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Enck P, Merlin V, Erckenbrecht JF, et al. Stress effects on gastrointestinal transit in the rat. Gut 1989;30:455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tache Y, Martinez V, Million M, et al. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors, Am J Physiol Gastrointest Liver Physiol 2001;280:G173–7. [DOI] [PubMed] [Google Scholar]
- 127.Saunders PR, Kosecka U, McKay DM, et al. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol 1994;267:G794–9. [DOI] [PubMed] [Google Scholar]
- 128.Castagliuolo I, Lamont JT, Qiu B, et al. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol 1996;271 (5 Pt 1) :G884–92. [DOI] [PubMed] [Google Scholar]
- 129.Wilson LM, Baldwin AL. Environmental stress causes mast cell degranulation, endothelial and epithelial changes, and edema in the rat intestinal mucosa. Microcirculation 1999;6:189–98. [PubMed] [Google Scholar]
- 130.Castagliuolo I, Wershil BK, Karalis K, et al. Colonic mucin release in response to immobilization stress is mast cell dependent. Am J Physiol 1998;274 (6 Pt 1) :G1094–100. [DOI] [PubMed] [Google Scholar]
- 131.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab 2002;13:436–44. [DOI] [PubMed] [Google Scholar]
- 132.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 2004;44:525–57. [DOI] [PubMed] [Google Scholar]
- 133.Tache Y, Perdu MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil 2004;16 (suppl 1) :137–42. [DOI] [PubMed] [Google Scholar]
- 134.Leu SJ, Singh VK. Stimulation of interleukin-6 production by corticotropin-releasing factor. Cell Immunol 1992;143:220–7. [DOI] [PubMed] [Google Scholar]
- 135.Singh VK, Leu SJ. Enhancing effect of corticotropin-releasing neurohormone on the production of interleukin-1 and interleukin-2. Neurosci Lett 1990;120:151–4. [DOI] [PubMed] [Google Scholar]
- 136.Singh VK. Stimulatory effect of corticotropin-releasing neurohormone on human lymphocyte proliferation and interleukin-2 receptor expression. J Neuroimmunol 1989;23:257–62. [DOI] [PubMed] [Google Scholar]
- 137.Leu SJ, Singh VK. Modulation of natural killer cell-mediated lysis by corticotropin-releasing neurohormone. J Neuroimmunol 1991;33:253–60. [DOI] [PubMed] [Google Scholar]
- 138.Cantarella G, Lempereur L, Lombardo G, et al. Divergent effects of corticotropin releasing hormone on endothelial cell nitric oxide synthase are associated with different expression of CRH type 1 and 2 receptors. Br J Pharmacol 2001;134:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wlk M, Wang CC, Venihaki M, et al. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology 2002;123:505–15. [DOI] [PubMed] [Google Scholar]
- 140.van Tol EA, Petrusz P, Lund PK, et al. Local production of corticotropin releasing hormone is increased in experimental intestinal inflammation in rats. Gut 1996;39:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawahito Y, Sano H, Mukai S, et al. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut 1995;37:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muramatsu Y, Fukushima K, Iino K, et al. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides 2000;21:1799–809. [DOI] [PubMed] [Google Scholar]
- 143.Schwarz SP, Blanchard EB. Evaluation of a psychological treatment for inflammatory bowel disease. Behav Res Ther 1991;29:167–77. [DOI] [PubMed] [Google Scholar]
- 144.Jantschek G, Zeitz M, Pritsch M, et al. Effect of psychotherapy on the course of Crohn’s disease. Results of the German prospective multicenter psychotherapy treatment study on Crohn’s disease. German Study Group on Psychosocial Intervention in Crohn’s Disease. Scand J Gastroenterol 1998;33:1289–96. [DOI] [PubMed] [Google Scholar]
- 145.Milne B, Joachim G, Niedhardt J. A stress management programme for inflammatory bowel disease patients. J Adv Nurs 1986;11:561–7. [DOI] [PubMed] [Google Scholar]
- 146.Whorwell PJ. Hypnotherapy in irritable bowel syndrome. Lancet 1989;1:622. [DOI] [PubMed] [Google Scholar]
- 147.Whorwell PJ, Prior A, Colgan SM. Hypnotherapy in severe irritable bowel syndrome: further experience. Gut 1987;28:423–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet 1984;2:1232–4. [DOI] [PubMed] [Google Scholar]
- 149.Shetty A, Kalantzis C, Polymeros D, et al. Hypnotherapy for inflammatory bowel disease—a randomised, placebo-controlled trial. Gut 2004;53 (suppl VI) :A226. [Google Scholar]