Abstract
Background and aims: Flares are a well known phenomenon during antiviral treatment for chronic hepatitis B. Little is known about the effect of flares on response. We investigated the timing and characteristics of flares, in relation to treatment response (hepatitis B e antigen loss).
Patients: A total of 266 patients, participating in a global randomised controlled study, were assigned to 52 weeks of 100 μg pegylated (Peg)-interferon α-2b weekly, combined with either daily lamivudine 100 mg or placebo.
Results: Sixty seven patients (25%) exhibited 75 flares, with 38 (51%) flares in the combination therapy and 37 (49%) in the monotherapy groups. Overall, 30% of patients with and 38% of patients without a flare responded to therapy (p = 0.25). In 24 patients (36%) the flare was followed by a decrease in hepatitis B virus (HBV) DNA (host induced flare). In 25 (38%) patients the flare was preceded by an increase in HBV DNA (virus induced flare). In 17 (26%) patients the flare did not meet one of these criteria (indeterminate flare). Of patients with host induced flare, 58% responded whereas only 20% of patients with virus induced flares responded (p = 0.008). Hepatitis B surface antigen loss (n = 8) was exclusively seen in patients experiencing a host induced flare. Multivariate logistic analysis showed that host induced flares was an independent predictor of response (p = 0.043).
Conclusion: Flares are not more common in responders than in non-responders to Peg-interferon α-2b therapy. Virus induced flares, which occur after an increase in HBV DNA level, and most probably are indicative for increased expression of viral antigens, did not lead to treatment response. In contrast, host induced flares which were followed by a HBV DNA decrease were highly associated with treatment response.
Keywords: hepatitis B virus, virus, chronic hepatitis B, Peg-interferon α-2b, hepatitis e antigen, hepatitis B surface antigen
Approximately 400 million people worldwide are chronically infected with the hepatitis B virus (HBV). Chronic infection with HBV can lead to progression of liver diseases with increased risk of cirrhosis, liver failure, and hepatocellular carcinoma.1
Currently, interferon α (IFN), lamivudine, and adefovir are the only registered drugs for treating chronic hepatitis B (CHB). During treatment with IFN and after withdrawal of lamivudine therapy, flares of inflammatory activity are a well known phenomenon in CHB patients. Flares can be life threatening but have also been associated with virological response.
IFN induced flares affect 25–40% of patients and have been attributed to the stimulatory effect of IFN, which is capable of increasing T cell cytolytic activity and natural killer cell function.2 Typically, these flares are thought to occur in hepatitis B e antigen (HBeAg) positive patients during the second to third month of therapy, and may precede HBeAg seroconversion.2–5 In our previous observation, flares during IFN were accompanied by an increased number of CD8+ specific T lymphocytes.6
Lamivudine related flares are seen during treatment but they do not occur more often than in the natural course of CHB.3 More important appear to be the flares found after withdrawal of lamivudine, which occur in approximately 10–20% of patients.3,7 These flares are probably caused by re-occurrence of HBV replication, and have been associated with decompensation of liver disease.
To clarify the role of flares during and after cessation of therapy, and to determine their relation with treatment response, we analysed 266 HBeAg positive CHB patients who received pegylated (Peg)-IFN α-2b alone or in combination with lamivudine.
PATIENTS AND METHODS
Patients and study design
Data were extracted from a global multicentre randomised controlled trial comparing Peg-IFN α-2b combined with either lamivudine or placebo in CHB.8 Patients were assigned in a 1:1 ratio to receive 100 μg Peg-IFN α-2b weekly with 100 mg lamivudine daily (combination therapy) or 100 μg Peg-IFN α-2b weekly with placebo (monotherapy). Duration of therapy was 52 weeks. After 32 weeks, the dose of Peg-IFN α-2b was halved to 50 μg per week. Post-treatment follow up lasted 26 weeks.
Patients were eligible for treatment if they were 16 years of age or older, had been positive for hepatitis B surface antigen (HBsAg) for at least six months, had been HBeAg positive on two occasions within eight weeks prior to randomisation, and had two episodes of elevated serum alanine aminotransferase (ALT) levels (at least twice the upper limit of normal (ULN)) on two occasions within eight weeks prior to randomisation.
Patients were excluded for the following reasons: treatment with antiviral medication within six months or any investigational drug within 30 days of entry to the protocol, or presence of serum antibodies against hepatitis C virus, hepatitis D virus, or human immunodeficiency virus. Other exclusion criteria were: alcoholic hepatitis or other causes of liver disease; pre-existing leucopenia (white blood cell count ⩽3000/mm3), thrombocytopenia (platelets ⩽100 000/mm3), or granulocytopenia (granulocyts ⩽1800/mm3); decompensated liver disease (prothombin time prolonged by ⩾3 seconds, serum albumin <35 g/l, ascites, encephalopathy, history of variceal bleeding), or hypo- or hyperthyroidism. Patients were also excluded in the event of any contraindication specified for IFN. The ethics committee at the participating centres approved the protocol, and all patients provided written informed consent.
Monitoring
All patients were seen monthly during therapy and follow up. At each visit, patients attended the outpatient clinic for ALT measurement and other laboratory assessments. Transaminases were assessed locally and expressed as ×ULN. In accordance with Honkoop and colleagues,7 a flare was defined as a threefold increase in serum ALT compared with baseline levels. The time point of the flare was defined as the time of the peak level of serum ALT. Multiple peak levels of thrice baseline serum ALT levels were considered as different flares if they were separated by at least two measurements of ALT. In addition to ALT, HBV DNA (detection limit 400 copies/ml, using inhouse Taqman PCR based on the Eurohep standard9) was assessed at the same time points. Other virological parameters, such as HBeAg (AxSYM; Abbott, Chicago, Illinois, USA) and HBsAg (AxSYM; Abbott) were assessed at baseline, and at weeks 32, 52 (end of treatment), and 78 (end of follow up). HBV genotype was assessed by Inno-Lipa assay (Innogenetics, Gent, Belgium). Response to therapy was defined as serum HBeAg loss at the end of follow up.
Statistical analysis
The χ2 or Fisher’s exact test was used for categorical variables, and the Mann-Whitney U test was performed for continuous data. In order to determine independent predictors for the event flare, the baseline characteristics age, race, sex, mode of transmission, pre-existing cirrhosis, ALT, log HBV DNA, HBV genotype, and previous IFN were included in the univariate analysis. All tested variables with a p value <0.15 were entered in the multivariate time dependent Cox regression analysis. In order to determine independent predictors for response within the flare population, we included the above mentioned baseline variables plus timing of the flare, peak value of ALT during exacerbation, and flare type (host induced versus virus induced) in a univariate and multivariate analysis. In the event of multiple flares, the first flare was analysed for response to therapy. All data were analysed using SPSS (version 10.1; SPSS Inc., Chicago, Illinois, USA). A p value of 0.05 was considered significant (all two tailed).
RESULTS
Flare versus non-flare
Among the 266 patients analysed, 75 flares were recorded in 67 (25%) patients. Six patients experienced two flares and one patient three flares during treatment or follow up. Median time point of flare was at week 60 (range 4–78), and median peak of ALT during flares was 12.3×ULN (range 2.3–60.0). Of the 75 flares, 35 (47%) occurred during treatment and 40 (53%) after treatment discontinuation. In three patients, of whom two had pre-existing cirrhosis, the flare was reported as a serious adverse event ; in one patient this led to early cessation of treatment. One patient had signs of diminished liver function (bilirubin 62 μmol/l) during the flare episode which resolved after normalisation of ALT values. During the flare there were no other signs of hepatic decompensation.
Characteristics of patients with and without flare are given in table 1 ▶. Except for ALT and cirrhosis, all variables at baseline were comparable in the flare and non-flare groups. Ten patients (19%) in the flare group and 14 (9%) patients in the non-flare group had pre-existing cirrhosis (p = 0.06). Pre-existing cirrhosis (p = 0.046; relative risk 2.0 (95% confidence interval (CI) 1.0–4.0)) and lower ALT at baseline (p<0.0001; relative risk 1.4 (95% CI 1.2–1.6)) were also the only two independent predictors for experiencing a flare during therapy or follow up.
Table 1.
Characteristics at baseline of 266 patients with or without a flare of chronic hepatitis B
| Baseline | Flare (n = 67) | No Flare (n = 199) | p Value |
| Age* | 34 (12) | 35 (13) | 0.804 |
| ALT* (×ULN) | 2.9 (1.4) | 4.8 (3.8) | <0.001 |
| Log HBV DNA* | 9.1 (1.1) | 9.1 (0.9) | 0.764 |
| Male (%) | 52 (78) | 153 (77) | 0.947 |
| Transmission (%) | 0.132 | ||
| Vertical | 18 (27) | 42 (21) | |
| (Homo)sexual | 7 (10) | 22 (11) | |
| Parenteral | 8 (12) | 21 (11) | |
| Transfusion | 4 (6) | 3 (2) | |
| Unknown | 30 (45) | 111 (56) | |
| Genotype (%) | 0.738 | ||
| A | 19 (28) | 71 (36) | |
| B | 7 (10) | 16 (8) | |
| C | 11 (16) | 28 (14) | |
| D | 26 (39) | 77 (39) | |
| Race (%) | 0.432 | ||
| Caucasian | 47 (70) | 149 (75) | |
| Asian | 13 (19) | 39 (20) | |
| Cirrhosis (%) | 10 (19) | 14 (7) | 0.060 |
| Previous lamivudine (%) | 8 (12) | 31 (16) | 0.459 |
| Previous IFN (%) | 12 (18) | 47 (24) | 0.311 |
| Monotherapy (%) | 32 (48) | 104 (52) | |
| Combination therapy (%) | 35 (52) | 95 (48) | 0.566 |
*Mean (SD).
ALT, alanine aminotransferase; ULN, upper limit of normal; HBV, hepatitis B virus; IFN, interferon.
Among the 75 flares, we recorded 37 (49%) in the monotherapy group (35 patients) and 38 (51%) in the combination therapy group (32 patients). Baseline characteristics and response to therapy were not significantly different between patients with a flare undergoing monotherapy or combination therapy (table 2 ▶). In five patients who exhibited a flare during or after treatment with a combination of Peg-IFN α-2b and lamivudine, a YMDD mutant was detected. None of the flares was related to emergence of a YMDD mutant.
Table 2.
Characteristics of patients who had a flare, according to therapy
| PEG-IFN placebo (n = 32 (48%)) | PEG-IFN Lamivudine (n = 35 (52%)) | p Value | |
| Age (y)* | 36 (13.1) | 33 (10.8) | 0.44 |
| Male (%) | 24 (75) | 28 (80) | 0.77 |
| Race (%) | 0.73 | ||
| Caucasian | 21 (66) | 26 (74) | |
| Asian/Mongoloid | 7 (22) | 6 (17) | |
| ALT* (×ULN) | 2.9 (1.3) | 2.9 (1.5) | 0.94 |
| Log HBV DNA* | 8.9 (1.3) | 9.2 (0.9) | 0.25 |
| Genotype (%) | 0.46 | ||
| A | 9 (28) | 10 (29) | |
| B | 2 (6) | 5 (14) | |
| C | 7 (22) | 4 (11) | |
| D | 11 (34) | 15 (43) | |
| Pre-existing cirrhosis (%) | 5 (16) | 5 (14) | 0.99 |
| Dose reduction (%) | 11 (34) | 13 (37) | 0.81 |
| Discontinuation of treatment (%) | 4 (13) | 5 (14) | 0.83 |
| Flares during treatment (%) | 20 (63) | 14 (40) | 0.067 |
| Time of flare† | 36 (4–78) | 60 (4–78) | 0.27 |
| Peak value flare* (×ULN) | 13.7 (6.9) | 16.4 (13.8) | 0.89 |
| Response (%) | 10 (31) | 10 (29) | 0.81 |
*Mean (SD).
†Median (range).
Peg, pegylated; IFN, interferon; ALT, alanine aminotransferase; ULN, upper limit of normal; HBV, hepatitis B virus.
Flares in relation to response to treatment and genotype
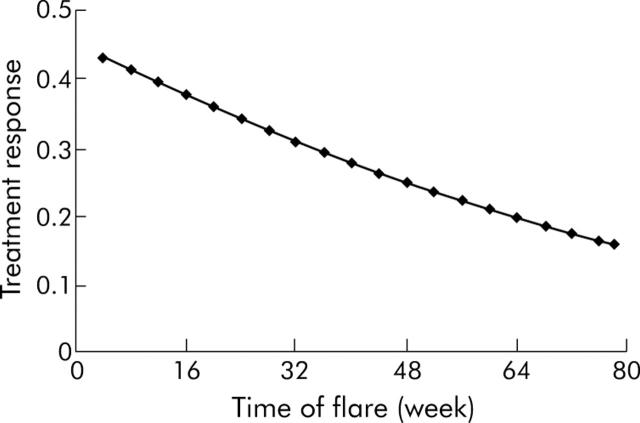
Among the 67 flare patients, 20 (30%) responded to therapy, 10 (31%) in the monotherapy and 10 (29%) in the combination group. Eight patients (12%) exhibited loss of HBsAg at the end of follow up. On-treatment flares led more often to treatment response (41%) than post-treatment flares (21%) (p = 0.081, fig 1 ▶).
Figure 1.
Proportion of response in relation to the time of the flare. Early presence of a flare increased the chance of response (p = 0.081). Probability of response is shown on the y axis.
The frequency of flares was comparable for patients with different HBV genotypes (fig 2A ▶). However, the timing of flares and response to therapy differed among the HBV genotypes. A total of 17 (74%) flares were recorded on-treatment in patients harbouring genotype A, versus three (43%) in genotype B, four (33%) in genotype C, and eight (27%) in genotype D (genotype A v other genotypes, p = 0.046) (fig 2B ▶). Treatment response in the flare population was 47% for genotype A, 28% for B, 18% for C, and 19% for D (genotype A v genotype D, p = 0.05) (fig 2C ▶). In addition to the timing of the flares and HBV genotype, the magnitude of the ALT elevation was associated with treatment response. Mean ALT during the flare within responders was 20.1×ULN versus 13.2×ULN in non-responders (p = 0.036).
Figure 2.
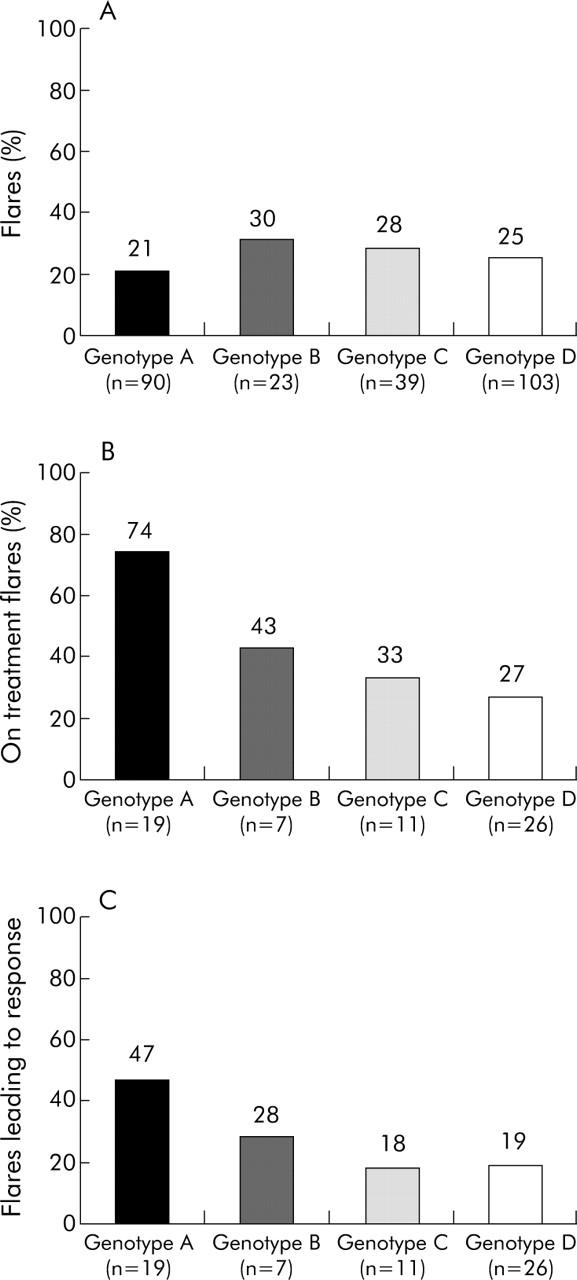
(A) Frequency of flares according to hepatitis B virus (HBV) genotype. Among the most important genotypes in our study (HBV genotypes A, B, C and D (n = 255)), no significant difference in the frequency of flares was found. (B) Proportion of flares recorded during treatment among the flare population according to HBV genotype (n = 63). On-treatment flares predominantly occurred within genotype A. Genotype A versus genotype D, p = 0.029. (C) Flares leading to response according to genotype (n = 63). Genotype A versus genotype D, p = 0.050.
Host induced versus virus induced flares
Close online monitoring of serum ALT and HBV DNA levels revealed different patterns of exacerbation (fig 3 ▶). A flare was defined as “virus induced” when preceded by an increase of least 1 log HBV DNA within four months. In general, these flares did not lead to a decline in serum HBV DNA. A flare was defined as “host induced” when the preceding HBV DNA levels were stable and when the flare was followed by a decline of 1 log HBV DNA or more within the four months thereafter. Flares that did not meet one of these criteria were classified as indeterminate. Only the first occurring flares were classified. For both host and virus induced flares, a minimum of 1 log HBV DNA alternation was chosen to exclude random oscillation of serum HBV DNA as a basis for our flare criteria.
Figure 3.
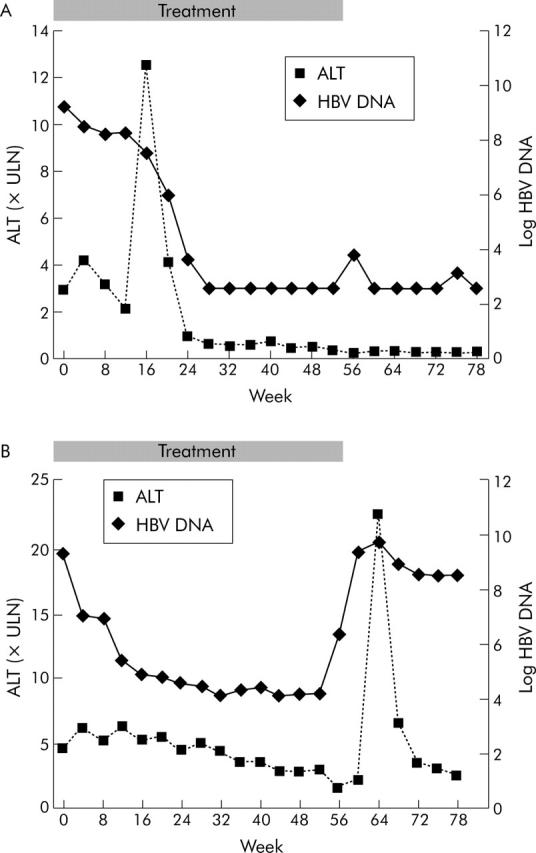
(A) Case with host induced flare. The elevation in serum alanine aminotransferase (ALT) was followed by a decrease in viral load (log hepatitis B virus (HBV) DNA). (B) Case with virus induced flare. Serum ALT elevation was preceded by a sharp increase in serum HBV DNA.
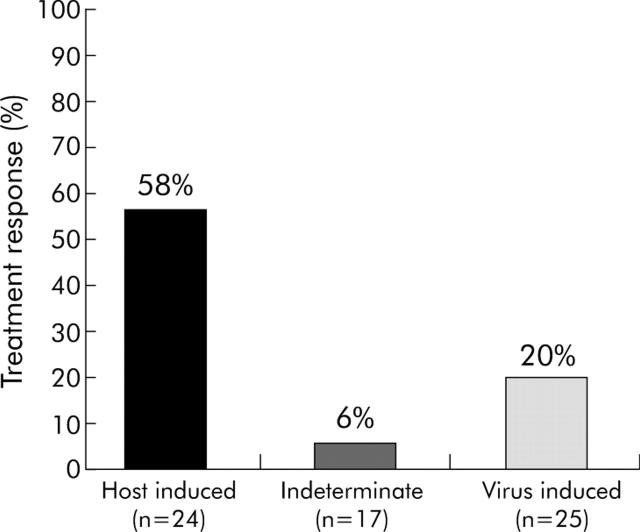
Twenty four flares (36%) were characterised as host induced, 25 (38%) were virus induced, and 17 (26%) were indeterminate. One flare could not be classified due to missing HBV DNA levels. Among the 67 flare patients, a host induced flare was strongly related to response to therapy (fig 4 ▶). Fourteen of the 24 patients (58%) with a host induced flare responded to therapy compared with five of 25 patients (20%) with a virus induced and one patient (6%) with an indeterminate flare. Moreover, eight patients (33%) with a host induced flare, but none of those with a virus induced or indeterminate flare, were HBsAg negative at the end of follow up. Seventy five per cent of host induced flares versus 16% of virus induced flares occurred during treatment (p<0.0001). Median peak of ALT of host induced flares was 13.8×ULN (range 5.3–60) and 12.1×ULN (range 3–45) for virus induced flares. Eleven patients (61%) with a host induced flare during treatment and three (50%) with a host induced flare after treatment responded to therapy. One patient (25%) with a virus induced flare during treatment and four (19%) with a virus induced flare after treatment responded to therapy. Within the flare population, multivariate analysis showed that the occurrence of host induced flare (p = 0.043; relative risk 3.5; 95% CI 0.9–13.9) and the magnitude of the ALT elevation (p = 0.031; RR 1.1; 95% CI 1.0–1.1) were the only factors independently predictive of response (serum HBeAg loss). On-treatment flares were not significantly related to response in this analysis (p = 0.65; RR 1.4; 95% CI 0.3–6.7).
Figure 4.
Host induced, indeterminate, and virus induced flares in relation to treatment response. Host induced versus virus induced, p = 0.008.
After entering the occurrence of host induced flares in the previously described multivariate analysis8 of the total study population (n = 266), it remained a significant variable predicting response (relative risk 2.4; 95% CI 1.0–5.8; p = 0.05).
DISCUSSION
Spontaneous or treatment induced flares of inflammation are frequently observed in CHB. These abrupt elevations in serum ALT are the result of an increase in intrahepatic necroinflammation associated with expanded numbers of intrahepatic lymphocytes, in particular cytotoxic T lymphocytes. Cytotoxic T lymphocytes are important to control HBV but can also induce liver damage, depending on the environment and functional capability.10–15 Therapy with IFN is based on its stimulating effect on cytotoxic T lymphocyte and natural killer cell function. Flares during standard IFN treatment occur typically during the second and third month, and are thought to herald virological response and disease remission.2–5,16 Probably, these flares represent an attempt of the immune system to clear the HBV infection.
In the current study, 29% of patients experienced a flare during therapy (n = 34) or follow up (n = 33). We did not find a significant difference between the number of flares in patients treated with Peg-IFN α-2b alone versus those treated with Peg-IFN α-2b in combination with lamivudine. Patients with low baseline ALT or pre-existing cirrhosis were more prone to having flares. Cirrhotics tended to experience flares with high ALT values. These patients should be monitored carefully during treatment with Peg-IFN α-2b, not only because of their increased risk of flares but also because of their diminished residual liver function and the consequent risk of developing decompensated liver disease. In the current study, no permanent or life threatening signs of liver failure were encountered.
Overall, flares were not associated with response to therapy. However, flares during treatment were more often associated with response than flares after treatment (fig 1 ▶). In addition to the timing of flares, response was dependent on HBV genotype and the magnitude of the flare. Previously, a strong association between the severity of flares and HBsAg seroconversion was found both in the natural history of CHB and in the setting of IFN therapy.16,17 In these studies, different definitions of flares were used. Nair and Perrillo16 defined a flare as an increase in ALT of at least twice the ULN compared with baseline values while Yuen and colleagues17 defined a flare as elevated transaminases above twice the ULN. As our patients already had high baseline serum ALT levels (ALT levels above twice the ULN was used as an entry criterion in this study population), these definitions were less suitable. For a clear distinction between flares and relative mild elevations in serum ALT, we based our definition on our previous experience, in which a threefold increase in serum ALT from baseline was used.7
An important finding of the current study was the distinct patterns of flares occurring, with stable viral load followed by a decrease in viral load (host induced flares) versus flares preceded by an increase in viral load and variable viral loads afterwards (virus induced flares). Patients with host induced flares responded significantly better to therapy than those with a virus induced flare. Multivariate analysis revealed host induced flare as the only independent factor predicting treatment response. Interestingly, all patients undergoing HBsAg seroconversion had a host induced flare. This further supports the hypothesis that full control and elimination of the virus, as indicated by clearance of HBeAg and HBsAg, is achieved by a vigorous host immune response rather than by direct suppression of the virus. Previous studies have shown that both spontaneous or IFN-α induced exacerbations of hepatocellular necrosis in CHB are associated with induction of a virus specific CD4+ T cell response.18,19 Under IFN-α therapy such a hepatitis flare preceding sustained HBeAg seroconversion requires a substantial increase in IL-12 production, along with induction of the Th1 cytokines IFN-α and IL-2.20
Virus induced flares, which emerged after increasing levels of HBV DNA, were related to treatment with the combination with lamivudine, and more frequently seen after therapy. These flares were attributed to reactivation of HBV after withdrawal of lamivudine. In general, they did not lead to disease remission but have been associated with clinical exacerbation and disease progression.7 In our study, virus induced flares did not usually lead to response, and even appeared to be detrimental rather than beneficial for treatment response. Virus induced flares are not restricted to CHB patients treated with IFN and or lamivudine, but also occur during the natural history of the disease. Liu et al described several patients in whom significant flares were preceded by an increase in HBV DNA.21 Studies in anti-HBe positive patients also showed episodes of flares as a result of sudden reactivation of HBV.22–24
In general, flares during treatment with IFN or Peg-IFN should not be treated with nucleoside analogues, and IFN should only be discontinued in case of impending liver failure. Particular care should be taken in patients with cirrhosis who have the highest risk of developing liver failure. On-treatment flares are likely to be host or IFN induced flares, and could well herald loss of HBeAg or even HBsAg. In contrast, flares after treatment, especially after lamivudine, are in general detrimental flares. These flares are typically seen after an increase in HBV DNA and seldom lead to treatment response. Retreatment with a nucleoside analogue should then be considered.
In conclusion, flares play an important role in the treatment with Peg-IFN α-2b alone or in combination with lamivudine, and patients with pre-existing cirrhosis are at greater risk for experiencing a flare. Furthermore, host induced flares but not virus induced flares may herald a response to therapy. For optimisation of treatment, it remains to be investigated which virological and immunological mechanisms induce the specific flare patterns described in our study.
Acknowledgments
The study was organised and sponsored by the Foundation for Liver Research (SLO), Rotterdam, the Netherlands. Financial support and study medication were provided by Schering-Plough International, Kenilworth, NJ, USA and GlaxoSmithKline, Research and Development, Greenford, UK. Monitoring was coordinated by Denys Research Consultants bvba, De Haan, Belgium. Data collection and data management were done by Elke Verheij and Eva Leeuwenhoek, Clinical Research Bureau, Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Centre Rotterdam, the Netherlands. Dr Janssen is a Clinical Research Fellow from the Netherlands Organisation of Scientific Research (NWO). D Sprengers is a clinical research trainee of the Netherlands Organisation of Scientific Research (NWO, grant No 920–03–244).
Abbreviations
HBV, hepatitis B virus
HBeAg, hepatitis B e antigen
HBsAg, hepatitis B surface antigen
IFN, interferon
ALT, alanine aminotransferase
Peg, pegylated
CHB, chronic hepatitis B
ULN, upper limit of normal
APPENDIX
In addition to the authors, the HBV 99-01 Study Group includes the following investigators:
The Netherlands: HGM Niesters, PE Zondervan (University Medical Centre Rotterdam), BCM Vroom (University Medical Centre Utrecht), CMJ van Nieuwkerk (VU University Medical Centre Amsterdam), RA de Vries (Rijnstate Hospital Arnhem), J Jansen, J Drenth, SJ van den Hazel (University Medical Centre Radboud Nijmegen), JW den Ouden-Muller (St Franciscus Hospital Rotterdam), AC Tan (Canisius Wilhelmina Hospital Nijmegen); Belgium: DM Adler (Hopital Erasme Brussels), P Michielsen (University Hospital Antwerp), H van Vlierberghe (University Hospital Gent), F Nevens (University Hospital Leuven), J Delwaide (Centre Hospitalier Universitaire Liège), J Henrion (Hopital de Jolimont, Haine St Paul); Germany: S Zeuzem (Saarland University Hospital, Homburg/Saar), G Gerken, S Bein, U Treichel (University Hospital Essen), J Trojan (JW Goethe Universität Frankfurt), MP Manns, J Hadem (Medizinische Hochschule Hannover), C Niederau (St Jozef Hospital Oberhausen); Denmark: MR Buhl, IM Hansen (Skejby Hospital, Arhus), K Krogsgaard (Copenhagen University Hospital Hvidovre); Poland: C Simon (Medical University, Wroclaw), J Cianciara, J Jablonska, J Kozlowska (Medical Academy of Warsaw), D Prokopowicz, R Flisiak (Medical Academy of Bialystok), T Mach (Collegium Medicum UJ Kraków); Spain: M Buti, A Valdes, R Esteban (Hospital Valle Hebron, Barcelona), M Rodriguez, M Garcia Espiga (Hospital Central de Asturias, Oviedo); Italy: A Andriulli, G Stornaiulo, GB Gaeta (Ospe Gesù e Maria, Napoli), G Montalto, F D’Antona (Università di Palermo); Greece: GE Kitis, P Xiarchos Panagiotis (George Papanikolaou General Regional Hospital, Thessaloniki), NC Tassopoulos (West Attica Hospital Athens); Turkey: US Akarca, G Ersöz (Ege University Faculty of Medicine Izmir), S Karayalcin, C Yurdayin, H Bozkaya (Medical School Cebeci Kampusu Ankara), H Simsek, Y Balaban (Hacettepe University Faculty of Medicine Ankara), H Senturk, F Tabak (Istanbul University Cerrahpasa Medical School, Istanbul), Y Cakaloglu (Medical Faculty, University of Istanbul, Istanbul); Israel: Y Lurie (Sauraski Medical Centre Tel-Aviv); Canada: J Heathcote (Toronto Western Hospital, Toronto); SV Feinman (Mount Sinai Hospital Toronto); S Greenbloom (General Hospital Etobicoke); Indonesia: DA Sulaiman (Ciptomangunkusomo Hospital Jakarta); Singapore: R Guan (Mount Elizabeth Medical Center Singapore); Malaysia: I Merican (Institute for Medical Research Kuala Lumpur); China: TMK So (Princess Margaret Hospital, Hong Kong).
Published online first 29 May 2005
Other members of the HBV 99-01 study group are listed in the appendix.
Conflict of interest: None declared.
REFERENCES
- 1.Lee W. Hepatitis B virus infection. N Eng J Med 1997;337:1733–45. [DOI] [PubMed] [Google Scholar]
- 2.Peters M, Davis GL, Dooley JS, et al. The interferon system in acute and chronic viral hepatitis. Prog Liver Dis 1986;8:453–67. [PubMed] [Google Scholar]
- 3.Perrillo RP. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 2001;120:1009–22. [DOI] [PubMed] [Google Scholar]
- 4.Perrillo RP, Schiff ER, Davis GL, et al. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med 1990;323:295–301. [DOI] [PubMed] [Google Scholar]
- 5.Alexander GJ, Brahm J, Fagan EA, et al. Loss of HBsAg with interferon therapy in chronic hepatitis B virus infection. Lancet 1987;2:66–9. [DOI] [PubMed] [Google Scholar]
- 6.Tang TJ, Kwekkeboom J, Mancham S, et al. Combined cytolytic and noncytolytic intrahepatic CD8 T-lymphocyte reactivity is important for response to antiviral therapy in chronic hepatitis B patients. Hepatology 2003;38 (suppl 1) :254A. [Google Scholar]
- 7.Honkoop P, de Man RA, Niesters HG, et al. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology 2000;32:635–9. [DOI] [PubMed] [Google Scholar]
- 8.Janssen H, Van Zonneveld M, Senturk H, et al. Pegylated interferon alpha-2b alone or in combination with lamivudine as the treatment for HBeAg-positive chronic hepatitis B. Lancet 2005;365:123–9. [DOI] [PubMed] [Google Scholar]
- 9.Pas SD, Fries E, De Man RA, et al. Development of a quantitative real-time detection assay for hepatitis B virus DNA and comparison with two commercial assays. J Clin Microbiol 2000;38:2897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowin B, Hahne M, Mattmann C, et al. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994;370:650–2. [DOI] [PubMed] [Google Scholar]
- 11.Milich DR, McLachlan A, Stahl S, et al. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol 1988;141:3617–24. [PubMed] [Google Scholar]
- 12.Yang PM, Su IJ, Lai MY, et al. Immunohistochemical studies on intrahepatic lymphocyte infiltrates in chronic type B hepatitis, with special emphasis on the activation status of the lymphocytes. Am J Gastroenterol 1988;83:948–53. [PubMed] [Google Scholar]
- 13.Tsai SL, Chen PJ, Lai MY, et al. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Invest 1992;89:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertoletti A, Maini MK. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr Opin Microbiol 2000;3:387–92. [DOI] [PubMed] [Google Scholar]
- 15.Bertoletti A, Maini M, Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Res 2003;60:61–6. [DOI] [PubMed] [Google Scholar]
- 16.Nair S, Perrillo RP. Serum alanine aminotransferase flares during interferon treatment of chronic hepatitis B: is sustained clearance of HBV DNA dependent on levels of pretreatment viremia? Hepatology 2001;34:1021–6. [DOI] [PubMed] [Google Scholar]
- 17.Yuen MF, Yuan HJ, Hui CK, et al. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut 2003;52:416–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohr HF, Weber W, Schlaak J, et al. Proliferative response of CD4+ T cells and hepatitis B virus clearance in chronic hepatitis with or without hepatitis B e-minus hepatitis B virus mutants. Hepatology 1995;22:61–8. [DOI] [PubMed] [Google Scholar]
- 19.Marinos G, Torre F, Chokshi S, et al. Induction of T-helper cell response to hepatitis B core antigen in chronic hepatitis B: a major factor in activation of the host immune response to the hepatitis B virus. Hepatology 1995;22:1040–9. [DOI] [PubMed] [Google Scholar]
- 20.Rossol S, Marinos G, Carucci P, et al. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J Clin Invest 1997;99:3025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CJ, Chen PJ, Lai MY, et al. A prospective study characterizing full-length hepatitis B virus genomes during acute exacerbation. Gastroenterology 2003;124:80–90. [DOI] [PubMed] [Google Scholar]
- 22.Perrillo RP, Campbell CR, Sanders GE, et al. Spontaneous clearance and reactivation of hepatitis B virus infection among male homosexuals with chronic type B hepatitis. Ann Intern Med 1984;100:43–6. [DOI] [PubMed] [Google Scholar]
- 23.Mels GC, Bellati G, Leandro G, et al. Fluctuations in viremia, aminotransferases and IgM antibody to hepatitis B core antigen in chronic hepatitis B patients with disease exacerbations. Liver 1994;14:175–81. [DOI] [PubMed] [Google Scholar]
- 24.Davis GL, Hoofnagle JH, Waggoner JG. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology 1984;86:230–5. [PubMed] [Google Scholar]