Morbus esuriendi semper inexplebili avidate uni animalium homini* Naturalis Historia, Book XI, CXVIII, Plinius Maior, AD 77&;
SUMMARY
Ghrelin is the endogenous ligand for the growth hormone secretagogue receptor (GHS-R), present on pituitary cells secreting growth hormone. Ghrelin and motilin, and GHS-R and the motilin receptor, are structurally related. Surprisingly, ghrelin is most abundant in the stomach, and GHS-R is also present in the stomach and in other organs and tissues, suggesting effects beyond stimulation of growth hormone in the pituitary, and in particular in the regulation of gastrointestinal function. However, as yet ghrelin seems rather a signal by which the digestive system regulates functions other than the digestive process itself. The most important role of ghrelin appears to be stimulation of appetite and regulation of energy homeostasis, favouring adiposity, and thus contributing to obesity. As recently suggested, ghrelin may therefore be called the “saginary” (fattening) peptide. Ghrelin may affect gastric acid secretion and gastroprotection but the suggested role of ghrelin in Helicobacter pylori infection implicates again the saginary effect. Ghrelin is functionally related to motilin as it also stimulates gastrointestinal motility. In rodents, ghrelin may have taken over the function of motilin, as rodents are natural motilin knockouts. Ghrelin appears to be an endocrine signal, possibly reaching the central nervous system via the bloodstream. However, it also uses neural pathways, in particular the vagus. A better understanding of the physiology of ghrelin may lead to new therapeutic approaches in the treatment of obesity and hypomotility syndromes.
CASABLANCA REVISITED
The hypothalamus has been called the “Casablanca of the central nervous system.” A place “plenty of intrigue … where mysterious messages from the brain are sorted out and scrambled into a new language of peptide hormones”.1 It is the language of the releasing hormones which start a signalling cascade first to the pituitary then via the tropins to the endocrine glands and by way of the hormones to the different organs and tissues. The story of the identification of the first one, thyrotropin releasing hormone, in 1969, has some of the qualities of the 1943 classic spy movie “Casablanca,” to which the quote refers. The process which led to the discovery of ghrelin, as yet the last one, also went through a few unexpected turns.
The hypothalamus is where it all started. In 1976, in an effort to learn more about the elusive hypothalamic factor responsible for stimulation of growth hormone secretion by the pituitary, the endocrinology group led by Bowers at Tulane University in New Orleans started to develop synthetic peptides, analogues of the enkephalins, as these were known to be weak growth hormone releasers. A similar strategy had been part of the search for thyrotropin releasing hormone. Eventually, compounds were synthesised, such as hexarelin and “growth hormone releasing peptide-6” (GHRP-6), devoid of opioid activity, but potent releasers of growth hormone from the pituitary. They became known as growth hormone secretagogues (GHSs). When growth hormone releasing hormone (GHRH) was isolated in 1982, interest in GHSs declined until it was realised in 1984 that GHSs act via a receptor other than GHRH. This stimulated the pharmaceutical industry to develop non-peptide GHSs and one of them, MK-0677, enabled the MSD group led by R Smith to clone the GHS receptor (GHS-R) in 1996. An extensive review of these developments was written in 1998 by Bowers who played a most prominent role in it.2
Cloning of GHS-R was a remarkable step in a process which has been coined “reverse pharmacology” because, in contrast with the normal discovery process in which first the endogenous agonist is identified, next the receptor is characterised, and then synthetic agonists developed, in this case the synthetic agonists (GHSs) came first and led to characterisation of the receptor. The final step of this reverse process, identification of the endogenous agonist, was realised by a cardiac physiology group. They were interested into GHSs because reports emerged that they had protective effects against cardiovascular dysfunction in growth hormone deficient rats.3 Using a cell line expressing the GHS-R to monitor receptor activation, Kojima and colleagues4 succeeded in a series of brilliant experiments in identifying the endogenous ligand for the GHS-R which they named ghrelin, from “ghre” the Indo-European root for “to grow”. The name can also be seen as the abbreviation for growth hormone, GH, followed by “relin” a suffix used for releasing substances (for example, in the synthetic GHS hexarelin). The key to success for Kojima and colleagues4 was that they did not look for the ligand only in the hypothalamus but also in extracts from many tissues. To their surprise the largest source of ghrelin was in the stomach, and it was from the gastric mucosa that ghrelin was isolated.
Growth hormone releasing hormone (GHRH) and ghrelin stimulate the release of growth hormone by pituitary cells, via distinct receptors.
Ghrelin acts via the previously discovered growth hormone secretagogue receptor (GHS-R).
Ghre-lin, from the ghre Indo-European root meaning to grow, or also GH-relin, growth hormone releasing.
From the hypothalamus, via the heart, to the stomach; from synthetic agonists via the receptor to the endogenous ligand. Steps illustrating that in research, milestones cannot always be written down five years ahead of time, as funding agencies nowadays would like. It would have been difficult to predict the next step, although in fact someone did.
FAMILY REUNION
Based on sequence similarities, gut peptides can be grouped into families, such as the gastrin-CCK family. The discoverers of ghrelin noted that their peptide “had no sequence homology to any known biologically active peptides…”4 but this was soon corrected in a rather remarkable way. Seven months after the discovery of ghrelin, another group described the “motilin related peptide” (MTLRP).5 These authors were interested in the self renewal process of the gastric epithelium and looked for peptides uniquely expressed in the gastric wall, hoping they may provide insight into this process. Using the molecular biology technique of “differential screening”, they identified a peptide which they named MTLRP because they noted sequence similarities with motilin. What they did not realise was that they had actually “rediscovered” the sequence of ghrelin. Still more remarkable, neither they nor the discoverers of ghrelin were aware that a similar sequence had already been submitted under the name of “motilin homologue” as part of a patent application in 1998, ahead of MTLRP and even ahead of ghrelin!6
The amino acid sequences of ghrelin, MTLRP, and motilin homologue are shown in fig 1 ▶ and it is obvious that they refer to the same substance. There is a difference of one residue, due to the use of another species, and there are differences in length because Tomasetto and colleagues5 and Sheppard and Deisher6 deduced the amino acid sequence from the nucleotide sequence and misjudged the processing of the precursor. More importantly, they could not know that serine3 is octanoylated, a post-translational modification which is unique to ghrelin and which is also crucial for its biological activity. For these reasons only the name ghrelin should be used, but the motilin homologue and MTLRP remind us of the limitations of molecular biology and genomic information.
Figure 1.
Amino acid sequences of ghrelin, motilin related peptide (MTLRP), and motilin homologue. Ghrelin was discovered three times, as is obvious from the similarity between the three sequences. The differences, shown in purple, are due to a species difference (RK in motilin homologue), the different interpretation of precursor processing (length indicated in superscript), and the octanoylation of serine,3 which could only be detected when the peptide was actually isolated.
Ghrelin is produced from a precursor but the details will not be dealt with here. Suffice it to say that the sequence and overall structure of the precursor show similarities with the motilin precursor. The amino acid sequence of the bioactive peptides produced in humans is shown in fig 2 ▶. It can be seen that motilin and ghrelin have identical residues in six positions. This number increases to eight for des-Gln14-ghrelin, a variant now known to be produced in minor quantities and which is the result of alternative splicing during transcription.7 It is of interest to note that motilin and ghrelin share the peculiarity that in their genes two exons are used to code for the bioactive peptide and that the boundary between these two exons is in both peptides at this residue 14.
Figure 2.
Similarity between the amino acid sequence of ghrelin and motilin. Alignment of part of the sequence of ghrelin (1–21) and of part of the des-Gln14-ghrelin sequence with the complete motilin sequence, demonstrating the relationship between them.
Between species the amino acid sequence of ghrelin is well conserved, especially in the N terminal region, and the same is true for motilin. This suggests that the biological activity is determined by the N terminus, in contrast with most other peptides where it is the C terminus, and this is indeed the case for both peptides. As already mentioned, a unique and crucially important feature of ghrelin is the octanoylation of serine.3 If the octanoyl group is removed, potency decreases dramatically: more than 2300-fold8 and the peptide can be shortened to an N terminal fragment of only five residues without appreciable loss of biological activity.9
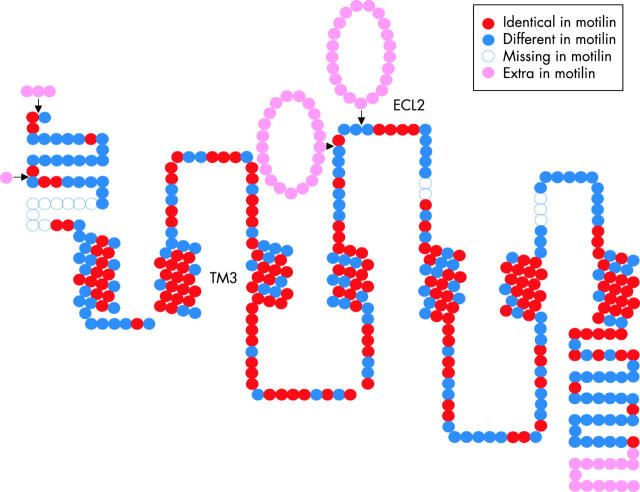
That ghrelin and motilin form a new family of peptides is strengthened by the observation that not only the peptides but also their receptors are structurally related. Both are classical G protein coupled seven transmembrane domain receptors but they are unrelated to other subfamilies of G protein coupled receptors (GPCRs) and therefore form a new receptor subfamily. The relationship between the amino acid sequence of the motilin receptor and GHS-R (that is, the ghrelin receptor) is illustrated in fig 3 ▶. The correspondence is striking, especially in the transmembrane domains, as 87% of the residues are identical. For GHS-R, the activation domain involves TM2 and TM3, and this domain has been remarkably conserved over more than 400 million years of evolution. Indeed, it is already present in related receptors in the pufferfish.10 For the motilin receptor it has been claimed that the second extracellular loop is critical for binding,11 and as a large part of this loop is absent in the ghrelin receptor, this would suggest that both peptides may not cross react. Indeed, in the classical in vitro assay to test motilin bioactivity (muscle strips from rabbit duodenum), ghrelin is inactive.12
Figure 3.
Topology of the ghrelin receptor (that is, GHS-R), illustrating the homology with the motilin receptor. Shown is the general two dimensional layout of the putative seven transmembrane domains and the three extracellular loops of the GHS-R. Each dot represents an amino acid residue, using the colour code indicated. The third transmembrane domain (TM3) and the second extracellular loop (ECL2) involved in the activation of, respectively, the ghrelin and motilin receptor are labelled. The figure is based on the alignment published as supplementary material to the paper by Feighner and colleagues13 and which can be found at http://www.sciencemag.org/feature/data/1039909.shl.
The relationship between the ghrelin receptor (GHS-R) and the motilin receptor was already known before ghrelin was isolated. Following the cloning of the GHS-R in 1996, other structurally related receptors were identified for which no ligand was known. In July 1999, one of these “orphan” receptors, GPR38, was identified as the motilin receptor and renamed MTL-R1A. The authors concluded their paper as follows: “The high amino acid sequence identity between MTL-R1A and the GHS-R implies that motilin and a natural ligand for the GHS-R, which has yet to be identified may also be related”13 (emphasis added). Although it was not immediately realised at the time, only six months later this prophecy was fulfilled when after almost 25 years of separation the story lines of motilin and ghrelin suddenly merged in just a few months when the peptide without receptor (motilin), the receptor without peptide (GHS-R), an orphan receptor (GPR38), and a new peptide (ghrelin) were united into one family (table 1 ▶).
Table 1.
Milestones in the discovery of ghrelin*
| Date | Ghrelin |
| 1971 | Motilin discovered using as bioassay the contractile effect of extracts of the duodenal mucosa from pigs on the canine stomach. |
| 1976 | In analogy with the release mechanism of other pituitary hormones, growth hormone releasing hormone (GHRH) is postulated to exist. Because enkephalins are weak releasers of growth hormone, it is proposed that GHRH may be structurally related to enkephalins. |
| 1977 | Analogues of enkephalins lacking opiate activity, but with enhanced potency to release growth hormone, are developed. They are referred to as growth hormone secretagogues (GHS). |
| 1982 | GHRH is isolated. Interest in GHSs declines. |
| 1984 | Synthesis of the GHS peptide GHRP-6, a potent releaser of growth hormone. |
| 1984 | It becomes clear that GHSs act via a receptor other than GHRH. Interest in GHS increases again. |
| 1993 | The first non-peptide GHS, L-692,429, is developed by Merck. |
| 1995 | Development of a more potent and orally active GHS, L-163,191 (MK-0677). |
| 1996 | Cloning of the GHS receptor (GHS-R) using expression cloning and MK-0677 as agonist. |
| 1997 | Two receptors related to GHS-R are cloned and named GPR38 and GPR39. Their ligand/agonist is unknown.14 |
| 1998 | A patent is submitted for “motilin homologues”. Much later it will be realised that it is related to ghrelin. |
| 1999, July | Orphan receptor GPR38 is identified as the motilin receptor and renamed MTL-R1a. It is predicted that the natural ligand for GHS-R could be related to motilin. |
| 1999, December | Ghrelin discovered using the Ca response of a cell line expressing GHS-R and isolated from extracts of the rat stomach. |
| 2000, August | Ghrelin “re-discovered” as “motilin related peptide”. |
The amino acid sequences of ghrelin and motilin show similarities.
In motilin and ghrelin bioactivity resides in the N terminus.
The amino acid sequences of their receptors also show similarities.
Ghrelin and motilin seem to use a different activation domain.
Ghrelin and motilin form a new family of peptides.
URBI ET ORBI
Considering the original studies on GHSs, one would expect to find GHS-R in the pituitary and ghrelin in the hypothalamus. But the fact that ghrelin was isolated from the gastric mucosa suggests that ghrelin may affect other organs and that both GHS-R and ghrelin could be widespread. This is indeed the case. The following summary of the distribution of ghrelin and GHS-R is extracted from two very recent and extensive reviews.16,17
Ghrelin is mainly produced by endocrine cells of the oxyntic mucosa of the stomach. Formerly known as X/A cells, the content of their granules was unknown until the discovery of ghrelin and these cells should be renamed “ghrelin cells”. A substantial amount is also present in the intestine, although gradually decreasing from the duodenum to the colon. In the pancreas, ghrelin is produced by a newly identified islet cell type, ɛ cells, especially numerous in the fetal pancreas, suggesting a role for ghrelin in the development of the endocrine pancreas. Ghrelin is also present in the lung, kidney, testis, placenta, and in immune cells. Ghrelin is found in the hypothalamus although levels are very low, and it has not been proven to be locally synthetised. Ghrelin is present in the pituitary where it may affect growth hormone secretion in an autocrine or paracrine manner.
GHS-R is, as expected, present in the pituitary, but also in the hypothalamus, in particular in the arcuate and ventromedial nuclei, and in several other brain regions, among them the dentate gyrus, hippocampus, substantia nigra, ventral tegmental area, and raphe nuclei. GHS-R is also present in many peripheral organs including the heart, lung, liver, kidney, pancreas, stomach, intestines, adipose tissue, and immune cells.
About two third of the ghrelin circulating in plasma is derived from the stomach, and the remaining one third from the small intestine, as can be deduced from the decrease following gastrectomy and small bowel resection.18,19 Plasma levels of ghrelin can be measured by immunoassay. Most studies published so far use the commercial radioimmunoassay kit distributed by Phoenix Pharmaceuticals (Belmont, California, USA) which finds normal values between 300 and 800 pg/ml (between 90 and 240 fmol/ml). However, it should be realised that this assay uses an antibody directed against the C terminus and measures acylated as well as deacylated ghrelin or “total” ghrelin. An antibody directed against the N terminus specific for non-acylated ghrelin would be better as it would measure “bioactive” ghrelin.
In addition to this technical issue is the effect of meal intake. Plasma levels of ghrelin rise before a meal and sharply decline as soon as the meal starts.20 The decline seems to be proportional to caloric intake. As the ratio between peak and trough is more than 2, sampling time may strongly affect the result. A good correlation has been found between integrated 24 hour release and trough values at about 6 am and about 80 minutes after breakfast, suggesting that a single measurement is enough to estimate ghrelin secretion.20 Nevertheless, meal related fluctuations introduce a degree of variability, and as short term fluctuations may determine some effects (for example appetite), a single measurement is not enough to evaluate such an effect.
Little is known about the mechanism controlling the release of ghrelin. A number of conditions have been described in which ghrelin is either increased or increased and these are summarised in table 2 ▶. The most important factor, feeding, has already been mentioned, but how fasting increases ghrelin secretion and how a meal reduces it is unclear. Blood glucose, insulin, leptin, and perhaps other peptide levels may play a role.
Table 2.
Conditions affecting ghrelin plasma levels*
| Comment | |
| Plasma ghrelin increases: | |
| With fasting | |
| In anorexia patients | Suggests tolerance to ghrelin |
| With decreasing body mass index | Suggests tolerance to ghrelin |
| Following leptin administration | |
| After vagotomy and hypophysectomy | |
| In renal insufficiency | Catabolism of ghrelin probably in the kidney |
| Plasma ghrelin decreases: | |
| Postprandially | Related to passage of foods, caloric intake |
| After gastrectomy and small bowel resection | Stomach is main source of ghrelin |
| With increasing body mass index | |
| After gastric bypass operation | May contribute to weight reduction |
SAGINARY JIT-PEPTIDE
The widespread distribution of ghrelin suggests a wide spectrum of biological activity and two different mechanisms of action: on the one hand local regulatory mechanisms due to locally produced ghrelin acting via a paracrine or neurocrine effect on effector cells bearing ghrelin receptors and on the other hand endocrine effects related to the release of ghrelin from the endocrine cells in the stomach. Table 3 ▶ gives an overview of what has been described so far. In this table the effects have been organised in the order of an estimate of the publication volume associated with them and, as can be seen, the effect on growth hormone secretion, which was at the root of the discovery of ghrelin, is less important in the literature than the effect on food intake and energy metabolism. Gastrointestinal physiology is presently close to the bottom of the list but the large number of abstracts submitted at recent gastroenterology meetings suggests this may soon change. “Gastrointestinal function” would now move immediately to the top of the list if the stimulatory effect of ghrelin on appetite were included. Not truly a gastrointestinal function, appetite is probably the most fascinating aspect of the physiology of ghrelin and it is an excellent example of how the gastrointestinal system may affect the rest of the body, including the brain. For this reason it will also be briefly considered.
Table 3.
Effects of ghrelin arranged in the order of an estimate of the number of publications related to them
| Effect† | No of &;studies‡ |
| Central nervous system | 133 |
| Increases appetite and food intake | |
| Metabolic effects | 108 |
| Increases blood glucose levels | |
| Stimulates fat deposit in adipose tissue | |
| Growth hormone secretion | 95 |
| More potent growth hormone* releaser than GHRH | |
| Endocrine pancreas. | 60 |
| Relation to insulin* secretion is unclear and may depend on plasma glucose level | |
| Increases somatostatin* and PP* secretion | |
| Hypothalamic factors other than growth hormone | 46 |
| High doses also release ACTH, prolactin, cortisol | |
| Affects gonadotroph secretion | |
| Cardiovascular physiology | 26 |
| Decreases blood pressure | |
| Increases cardiac output and stroke volume | |
| Improves cardiac performance also when dysfunction is present | |
| Digestive system | 19 |
| Increases gastric acid secretion | |
| Accelerates gastric emptying | |
| Offers gastroprotection against ulcerogens | |
| Reproductive physiology* | 15 |
| Possible link between energy status and fertility | |
| Dose dependent inhibition or stimulation of cell proliferation of tumour cell lines. | 14 |
| Central nervous system (other than appetite) | 9 |
| Enhances anxiety | |
| Increases memory retention | |
| Promotes slow wave sleep |
It has been known for a long time that the hypothalamus contains centres controlling appetite and satiety, and it had been suggested decades ago that these centres somehow received information about body weight and energy stores. One such signal was known to be insulin, and in 1994 the discovery of leptin introduced another circulating hormone communicating the status of adipose tissue and reducing food take. Already during the development of the GHSs weight gain had been noted following chronic administration21 and an increase in appetite following acute administration in rats.22,23 In studies on the release of growth hormone, human volunteers reported an increase in appetite as a “side effect” after intravenous administration of hexarelin24 and of ghrelin.25 These observations prompted several animal studies, soon confirmed in humans,26 which opened up a new and exciting concept aptly summarised in the titles of two of the first papers “A role for ghrelin in the central regulation of feeding”27 and “Ghrelin is an appetite-stimulatory signal from the stomach with structural resemblance to motilin”.28
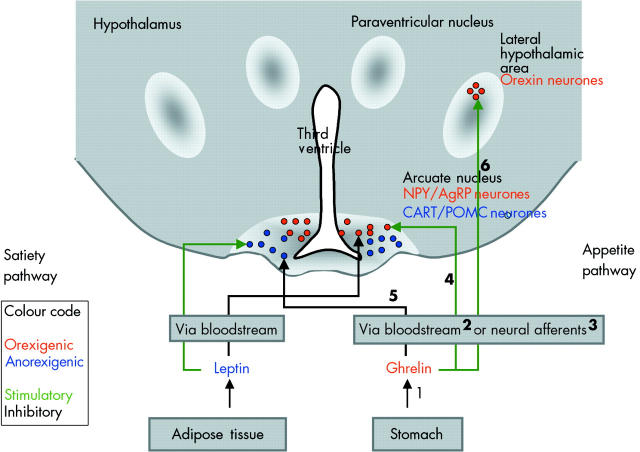
Thus a new concept emerged, as ghrelin is the first peripheral orexigenic signal.29 During fasting, secretion of ghrelin from the stomach is increased, perhaps in response to decreasing insulin and glucose levels, and blood plasma levels rise. By activating vagal afferents or via the bloodstream, the signal reaches the arcuate nucleus of the hypothalamus. Here neurones containing the orexigenic peptides neuropeptide Y and agouti related peptide are activated, while neurones containing the anorexigenic peptides cocaine and amphetamine related transcript and pro-opiomelanocortin are inhibited. Interestingly, leptin has opposite effects on the same neurones. The arcuate neurones project to other nuclei, among them orexin containing neurones in the lateral hypothalamic area, to stimulate appetite. The scheme is summarised in fig 4 ▶ and key findings in support of this scheme are listed in table 4 ▶.
Figure 4.
Schematic representation of the pathways involved in the stimulation of appetite. Oversimplified scheme based on possible pathways activated by ghrelin and leading to an increase in appetite. The different steps are numbered and the numbers refer to key observations supporting this scheme, as summarised in table 4 ▶. The leptin pathway leading to satiety is shown for comparison on the left side.
Table 4.
Experimental evidence supporting the scheme presented in fig 4 ▶
| Step | Event | Reference |
| 1 | During fasting plasma ghrelin increases | 20 |
| 1 | Following gastrectomy plasma ghrelin decreases dramatically | 18 |
| 2 | Small amounts of ghrelin may pass the blood brain barrier, and the arcuate nucleus does not have a blood brain barrier | 19 (review) |
| 3 | Ghrelin receptors are present in the nodose ganglion | 30 |
| 3 | Ghrelin (icv) activates neurones in the dorsomotor nucleus | 31 |
| 3 | Vagotomy or application of the neurotoxin capsaicin on vagal terminals inhibits the effect of ghrelin | 32 |
| 4 | Ghrelin (iv) activates NPY and AgRP neurones in the arcuate nucleus | 33 |
| 4 | The effect of ghrelin is blocked by NPY antagonists, and by antibodies to NPY &;and AgRP | 34 |
| 4 | In NPY or AgRP KO mice, the effect of ghrelin is reduced, in double KO is &;completely abolished | 35 |
| 6 | Ghrelin activates orexin neurones | 36 |
| 6 | The effect of ghrelin is reduced by antibodies to orexin | 36 |
| 6 | The effect of ghrelin is reduced in orexin KO mice | 36 |
NPY, neuropeptide Y; AgRP agouti related peptide; icv, intracerebroventricular.
The step numbers refer to fig 4 ▶.
The increased appetite will result in increased food intake and an increase in body weight. However, the effect on body weight involves more than this short term meal related event. Chronic administration and thus chronically increased plasma ghrelin levels affect energy homeostasis by reducing fat utilisation and inducing adiposity. For this reason a recent review proposed the neologism “saginary hormone” for ghrelin, from the Latin saginare, meaning “to fatten”.37 However, the link with obesity is less straightforward than was initially assumed as ghrelin levels correlate inversely with body adiposity38 and are actually low in obese individuals.39 Only in Prader-Willy syndrome is obesity accompanied by high ghrelin levels.40 Ghrelin also seems less important than leptin because while leptin null mice are obviously obese, ghrelin null mice have normal body weight, food intake, and appetite.41
The peaks of plasma ghrelin which occur before meals stimulate appetite.
A prolonged and sustained increase in plasma ghrelin induces adiposity and contributes to the development of obesity.
A compound blocking the effect of ghrelin could be helpful to treat obesity although one should realise that ghrelin is not the main factor in the development of obesity.
The possibility that ghrelin, by stimulating appetite, could be responsible for obesity made headlines in every major newspaper and magazine. Commenting on the fear that today’s babies may face obesity as a major health problem in adult life, The Guardian, obviously inspired by Dr SR Bloom, noted on 22 January 2005 “By the time the babies of 2005 reach early adulthood, far better obesity drugs will be available… the most likely drugs to be taken….will be those that mimic or block the natural control of appetite, by recently discovered hormones such as ghrelin.” Apparently ghrelin was discovered “just in time (JIT)” a popular concept nowadays in business jargon, where JIT is associated with “lean management.” Perhaps antagonists of the JIT-peptide ghrelin may be helpful in the “management of leanness!”
THE COMPLEXITY OF THE REGULATION OF GASTRIC ACID SECRETION
X/A cells of the acid producing part of the stomach are the largest source of ghrelin, suggesting that ghrelin may regulate gastric acid secretion. Few studies have as yet dealt with this issue and the results are not unequivocal, as stimulation,31,42–44 inhibition,45 and lack of effect46 have been reported. We will discuss these reports against the background of current concepts of the regulation of acid secretion.
After a meal gastrin is released from the antral mucosa, in response to the presence of food or a decreased pH in the lumen, and travels via the bloodstream to the oxyntic mucosa where gastrin activates the histamine producing enzyme of the ECL cells. Histamine stimulates parietal cells to produce hydrochloric acid. Parietal cells and ECL cells may also be stimulated by vagal cholinergic pathways in particular before the meal due to central effects caused by the thought, sight, or smell of food. On the other hand, luminal acidity exerts a feedback control as high acidity stimulates D cells in the antral mucosa to release somatostatin, which inhibits gastrin production by G cells.47
In isolated cell cultures, ghrelin had no effect on D cells, G cells, or ECL cells.46 In agreement with this observation, the same study found no effect of subcutaneous or intravenous administration of ghrelin on acid secretion. This seems to rule out paracrine or endocrine effects of ghrelin on gastric acid secretion, within or between parts of the stomach wall. However, other studies found that gastric acid secretion increased following intraperitoneal43 and intravenous42,44 ghrelin. In the last two studies the response was abolished by vagotomy and atropine, implying an effect of ghrelin on vagal pathways stimulating parietal cells. These may be the pathways also activated by intracerebroventricular administration of ghrelin, which was found to increase gastric acid secretion in one study.31 However, another study found inhibition following intracerebroventricular administration. It was suggested that conflicting intracerebroventricular data may reflect the presence of both stimulatory and inhibitory pathways, and that experimental conditions and models may determine how they balance out.45 To add to the complexity, it has been reported that ghrelin may cause the release of gastrin via presumably vagal pathways.48 This topic needs and deserves further study, as one study reported that ghrelin was almost equipotent to histamine.42
Although ghrelin secreting cells are found in the oxyntic mucosa in the proximity of the ECL and parietal cells, there does not seem to be a paracrine or endocrine effect.
Ghrelin may activate central pathways and via vagal efferents modulate gastric secretion with the dose determining whether there will be an increase or decrease.
HELICOBACTER: A POSSIBLE BENEFIT OF POOR HYGIENE
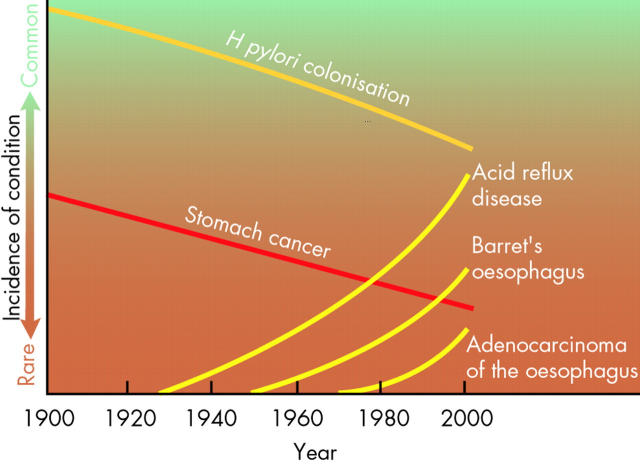
It is well known that Helicobacter pylori infection causes an increase in gastrin secretion from the antral mucosa. In contrast, in the oxyntic mucosa it impairs the secretion of histamine by ECL-like cells, of pepsin by chief cells, and of gastric acid by parietal cells.49 Therefore, an effect on ghrelin secreting cells could be envisaged also. While the first study tackling this issue found no difference between H pylori+ and H pylori− patients,50 a second one noted that curing H pylori infection was accompanied by a rise in plasma ghrelin.51 The authors proposed that this rise could promote the development of obesity. Citing evidence that obese individuals have a higher incidence of reflux disease, which increases the risk of developing Barrett’s oesophagus, which in turn increases the risk of oesophageal adenocarcinoma, they proposed that ghrelin could be “the missing link that explains the relative rarity of H pylori among patients with Barrett’s oesophagus and oesophageal adenocarcinoma”. The epidemiological data on which this hypothesis was based were recently summarised in a paper in Scientific American (fig 5 ▶) which also took over the hypothesis that ghrelin could be involved and that H pylori infection, by lowering plasma ghrelin, “would actually benefit some individuals”.52 Stated in a different way, the basic assumption is that eradication of H pylori, by increasing plasma ghrelin, contributes to the obesity epidemic in industrialised nations.53
Figure 5.
Trends in the incidence of Helicobacter pylori infection, stomach cancer, acid reflux, Barrett’s oesophagus, and adenocarcinoma of the oesophagus. In the past 100 years there has been a decline in H pylori infection and of stomach cancer, but a rise in three oesophageal pathologies in developed countries. A causal relation between these trends and involving ghrelin has been proposed (Nwokolo and colleagues51). The figure is from Blaser,52 with permission.
This is a sweeping hypothesis which was critically received as Nwokolo and colleagues51 extrapolated their data, perhaps with more enthusiasm than sound scientific reasoning.54,55 However, in view of its possible implications it should be explored further. It has already been confirmed that H pylori infection lowers expression of ghrelin, lowers the number of ghrelin producing cells, and lowers plasma ghrelin levels.56,57 However, this is not the crucial point. Apart from the fact that in underdeveloped countries with a high prevalence of H pylori infection there are factors that could cause low body weight other than low ghrelin levels, it is the rise in ghrelin following H pylori eradication which should trigger the suggested chain of events. The first question is whether this rise exists and the second question is whether it can be an important determinant of body weight increase. In relation to the first question, the rise reported by Nwokolo and colleagues51 is supported by the observation that ghrelin tissue levels increased after eradication.58 However, another study found that plasma levels were unaffected.56 It has been suggested that this may be explained by the different topography (antrum or fundus, where ghrelin cells are located) and duration of infection (reversible damage after a short infection, irreversible atrophy after long infection) in the different studies,53 so that only a subpopulation of infected patients may show a rise in ghrelin following eradication. Does body weight increase due to increased ghrelin production in this subgroup? A rise in body weight and appetite has been reported following H pylori eradication (see Cummings53 for more details) but no difference in body mass index was found between H pylori− and H pylori+ patients.59 The issue should be re-examined in the light of the ghrelin hypothesis, taking into account extent and duration of disease.
Eradication of H pylori may induce a rise in plasma ghrelin levels.
This rise may contribute to obesity.
It has been proposed that in this way H pylori eradication is the first step of a series of causally linked events: increase in reflux disease, increase in the risk of developing Barrett’s oesophagus, oesophageal adenocarcinoma.
GASTROPROTECTION
As mentioned previously, ghrelin was also discovered as a “motilin related peptide” by a team searching for factors involved in cell proliferation and differentiation in the gastric epithelium. Considering that growth factors contribute to maintenance of mucosal integrity and to the process of ulcer healing, a role for ghrelin in these processes could be envisaged. Surprisingly, a possible effect on the renewal of the gastric epithelium has received little attention, only in tumour cell lines (for example, prostatic cancer cell lines60) have effects on cell proliferation been described. However, a gastroprotective effect has been demonstrated, be it mainly in the context of the generation of nitric oxide and prostaglandins, which protect the mucosa by increasing blood flow.
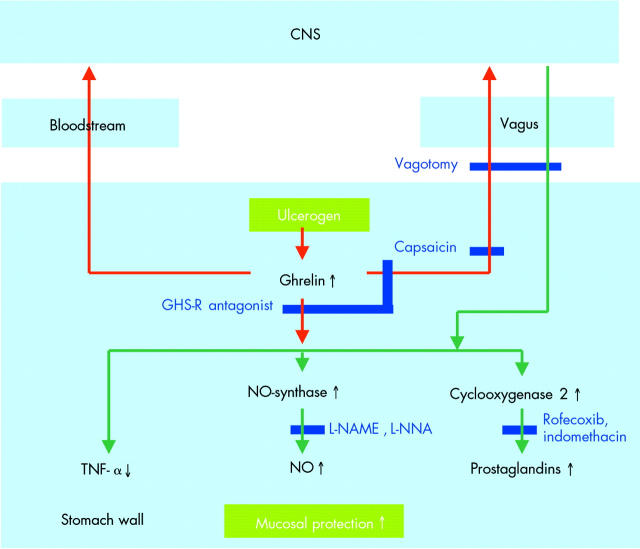
The first report related to ethanol induced gastric ulcers in rat.61 These observations have been confirmed in the same model and have also been extended to ulcers induced by water immersion or restraint stress.43,62 Only in indomethacin induced ulcers did ghrelin not have a beneficial effect,63 but when indomethacin, or the selective COX-2 inhibitor rofecoxib, was used in other models to block prostaglandin synthesis, the effect of ghrelin was reduced, suggesting that it is at least in part mediated via enhancement of prostaglandin synthesis. Other factors are also involved as there is a loss of effect of ghrelin in the presence of L-NAME or L-NNA, blockers of nitric oxide synthesis, and after deactivation of afferent sensory nerves with the neurotoxin capsaicin.43,61,62
The protective effect does not seem to be limited to the stomach as it has also been demonstrated in experimental colitis.64 It has also been observed after intraperitoneal,43,62 subcutaneous, or intracerebroventricular administration,61,63 suggesting that the mechanism is mediated via peripheral and central ghrelin receptors. Ghrelin released in the stomach wall may affect neighbouring cells or reach them via the bloodstream. Thus it has been shown that ghrelin acts directly on enteric neurones to produce nitric oxide.65 Exogenous ghrelin, or ghrelin produced in the stomach, may also reach the central nervous system via the bloodstream, or the ghrelin signal may be conveyed to the brain via vagal afferents. However, one study found that vagotomy did not affect the gastroprotective effects of centrally administered ghrelin, while sensory denervation did, and suggested involvement of spinal afferents.61 If the protective effect requires signalling to the brain, the efferent pathways and the cells responsible for the production of the protective factors remain to be identified. Figure 6 ▶ summarises these findings.
Figure 6.
Scheme summarising the observations made in relation to the gastroprotective effects of ghrelin. Red arrows indicate primary signalling by ghrelin, green arrows effector pathways.
If mucosal protection is a physiological role of ghrelin, one would assume that ulcerogens induce ghrelin secretion. Increased mucosal expression of ghrelin and increased plasma levels of ghrelin have indeed been reported in stress, ethanol, and cysteamine induced ulcers.43,62,66 However, the role of endogenous ghrelin has not yet been investigated. A ghrelin antagonist inhibited the protective effect of the GHS agonist hexarelin but had no effect on its own under control conditions.63 One may also hypothesise that ghrelin knockout mice would develop ulcers more easily but these studies have yet to be done.
Ghrelin may protect against mucosal damage.
MOTILITY: LEGACY OF A DISTANT RELATIVE?
In view of the structural relationships between ghrelin and motilin and of their receptors, it seems prudent to evaluate the motor effects of ghrelin. The best characterised effects of motilin are induction of the migrating motor complex (MMC) and the acceleration of gastric emptying. These two effects have now also been observed with ghrelin.
In fed rats ghrelin, given intravenously or intracerebroventricularly, the MMC cycle was shortened in the duodenum,67,68 and in healthy fasting volunteers intravenous infusion of ghrelin induced a premature phase III originating in the stomach.69 There is also ample evidence that ghrelin and the ghrelin agonist GHRP-6 accelerate gastric emptying in rats70,71 in mice28,72 and in dogs.73 In humans it has been noted that gastric emptying half time is correlated with fasting plasma ghrelin levels74 and a recent study reported acceleration of gastric emptying of liquids in patients with gastroparesis following intravenous administration of ghrelin.75 It should be mentioned that the first study in humans was negative. However, in this study continuous infusion was given for 270 minutes, and breakfast, for which emptying was evaluated, was eaten after 120 minutes.26 This protocol may have induced desensitisation by the time the meal was taken.
One may wonder how a peptide whose plasma level decreases as soon as the meal starts can play an important role in the regulation of gastric emptying. And how could a continuous preprandial rise regulate a periodic phenomenon such as MMC? The fact that ghrelin null mice, apart from a normal appetite, also have normal gastrointestinal motility76 suggests that ghrelin may not be a crucial physiological regulator of gastrointestinal motility. On the other hand, the ghrelin antagonist D-Lys3-GHRP-6 delays gastric emptying in mice,72,77 suggesting inhibition of endogenous ghrelin. However, this antagonist may cross react with other receptors12 weakening this argument. In any case, the effects of ghrelin exist and may have therapeutic potential. Indeed, the effect on emptying in humans was observed in gastroparesis patients, and animal studies showed that ghrelin reverses the delay in gastric emptying in postoperative ileus in the rat70 and in the dog,78 and in septic mice.79 It is therefore of interest to consider the mechanisms involved.
In rodents, the motor effects were observed after central as well as after peripheral administration of ghrelin. When administered centrally, the effect was blocked by central, but not by intravenous administration of ghrelin antagonist and by vagotomy, implying that the effect depends on activation of central receptors and efferent vagal pathways.28,67 Vagotomy also blocks the effect of peripheral administration of ghrelin. One could therefore envisage that ghrelin reaches the central nervous system via the bloodstream to activate central ghrelin receptors. However, central administration of ghrelin antagonist does not block the effect of peripheral administration in intact animals, suggesting that vagal afferents may also be involved. There is indeed evidence for GHS-R on vagal afferents.30
Alternatively, ghrelin may activate peripheral receptors in the enteric nervous system as recent studies, using a variety of techniques, have documented the presence of the ghrelin receptor in the myenteric plexus. GHS-R mRNA is present in the intestinal wall and in cultured myenteric neurones.80 GHS-R immunoreactivity is present in neurones of the myenteric plexus in the human and rat stomach and colon81 and GHS-R is colocalised with ChAT neurones in the guinea pig myenteric plexus.80 In vitro, ghrelin enhances contractions induced by electrical field stimulation in rat and mouse preparations72,81 and evokes cholinergically mediated contractions of unstimulated preparations of rat jejunum.68 Also in the guinea pig, a subset of myenteric plexus neurones responds with an increase in intracellular calcium on superfusion with ghrelin.82 Interestingly, in control rats, a ghrelin antagonist had no effect on fasted motor activity but when given to vagotomised rats completely blocked the MMC.67 These data demonstrate that local and central pathways exist but that in normal rats only central pathways are operational. However, when these pathways are eliminated by vagotomy, ghrelin is able to exert its effects via the myenteric plexus.
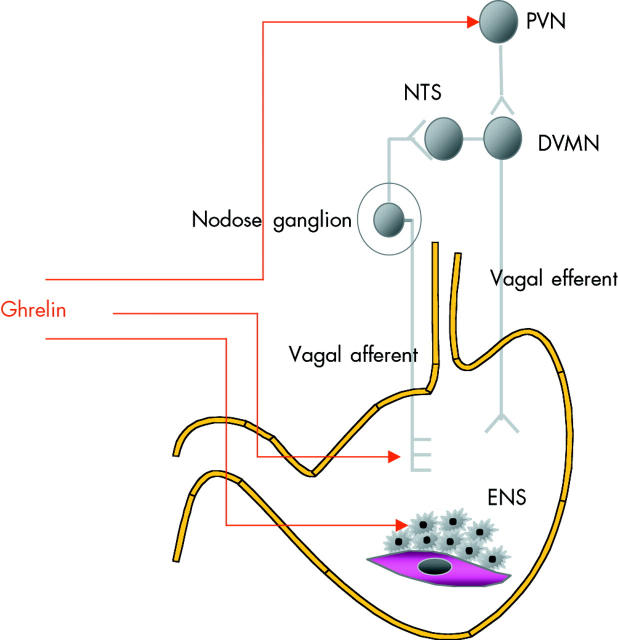
The scheme in fig 7 ▶ summarises the different pathways by which ghrelin may affect gastrointestinal motility. The scheme relies rather heavily on the excellent and detailed study of Fujino and colleagues67 yet it will need further verification and confirmation using better antagonists. As was already mentioned, the antagonist D-Lys3-GHRP-6, also used by these authors, may cross react with other receptors and is also quite weak.68 A possible interaction with the motilin receptor can be considered but as was already mentioned, there is no evidence for this in the classical motilin in vitro model.12 In fact, in rats and mice there is another reason to exclude cross reactivity. It is known that motilin has no effects in the mouse or rat, and it was thought that this could be due to differences in the structure of motilin and the structure of the motilin receptor in these species. Recent studies using bioinformatics came up with another answer, and with the explanation why attempts in the past to isolate rat or mice motilin had failed. While the rat and mouse genomes contain remnants of motilin and of the motilin receptor, both genes are non-functional and are not expressed. Rats and mice are therefore natural motilin and motilin receptor knockouts83,84 and it is tempting to speculate that in the rat and in the mouse the function of motilin has been taken over by ghrelin.
Figure 7.
Scheme illustrating the pathways involved in the motor effects of ghrelin. ENS, enteric nervous system; DMVN, dorsal vagal motor nucleus; NTS, nucleus tractus solitarius; PVN, paraventricular nucleus.
Like motilin, ghrelin induces the migrating motor complex and accelerates gastric emptying.
Ghrelin may increase motor activity by activating efferent central pathways, vagal afferents, or the enteric nervous system.
EPILOGUE
This review has looked at the new peptide ghrelin, from the perspective of the gastrointestinal system. The surprising finding that a peptide discovered as a factor stimulating growth hormone release from the hypothalamus is mainly produced in the stomach and secreted into the general circulation suggested that such effects would exist. Yet, while the discovery process led from the hypothalamus to the stomach, the study of the physiological role of the peptide seems to bring us back to the hypothalamus. Indeed, the most important role of ghrelin appears to be that of a satiety signal from the stomach to the hypothalamus. For those digestive functions that appear to be affected by ghrelin, gastric acid secretion, gastroprotection, and motility, the mechanism also brings us back to the hypothalamus. Ghrelin is therefore primarily an element of the gut-brain axis.
The physiological relevance of some findings may be limited but this does not exclude a possible therapeutic application. Appetite regulation, with ghrelin antagonists or drugs targeting the elusive enzyme responsible for octanoylation, and prokinetic activity, with ghrelin agonists, appear to be the most promising targets. However, in both cases the wide spectrum of possible side effects have to be kept in mind.
For the sake of clarity and brevity, several aspects of ongoing research on ghrelin have been omitted. For example, differences in the spectrum of activities of GHSs and ghrelin raise the possibility of receptor subtypes. In fact, the structural differences between motilin and the ghrelin receptor suggest that intermediate forms may exist, and if so they may offer a solution to avoid the side effects of new drugs developed from ghrelin. We may also have to consider that the motilin family has more than two members. A third peptide could be the deacylated form of ghrelin which is the main circulating form of “ghrelin.” As yet the effects of desacyl ghrelin are controversial.37 For example, it was recently reported that desacyl ghrelin decreased food intake and decreased gastric emptying85 while the patent on “motilin homologues” (an N terminal fragment of desacyl ghrelin) claimed stimulation of contractility.6 However, the effects of desacyl ghrelin require the existence of another receptor, most likely related to the motilin and ghrelin receptor, and they strengthen the concept of a family of peptides complementing each other in the regulation of appetite and motility. It is of interest that Itoh et al, in their first paper describing the relation between motilin and the migrating complex, commented that motilin was unique because it was active in the fasting state, inducing “hunger contractions”.86 The paper concluded by stating that “motilin may be considered the hunger hormone”. It is remarkable that the related peptide ghrelin emerges as a factor controlling appetite, eventually without requiring the contractions.
While ghrelin seems to be a factor related to what Plinius called the insatiable appetite of man, it is a factor we share with many, if not all mammals. However, what sets man really apart is his insatiable appetite for knowledge. Therefore, the coming years will lead to new insights, quite likely to new therapies, but perhaps also to the discovery of new members of the motilin-ghrelin family and/or their receptors. Meanwhile, we may now find applications for what is known today about the physiology of ghrelin. Ghrelin rises during fasting and also rises during sleep deprivation.87 On the other hand, ghrelin promotes the fourth stage or “delta wave” sleep.88 Therefore, it seems indicated to work not too late in the evening and to allow ghrelin to induce deep sleep, before it triggers a trip to the refrigerator.
Acknowledgments
The research of the author on motilin and ghrelin is supported by grants from the Belgian Ministry of Science (GOA 03/11 and IAP P5/20), the Flemish Foundation for Scientific Research (FWO grant No G.0144.04), and the Ministry of the Flemish Community (International Scientific and Technological Cooperation with the PR China grant BIL 01/13).
Conflict of interest: None declared.
Footnotes
“Man is the only animal liable to the disease of a continuously insatiable appetite.” Latin and English text from Pliny Natural History III Books VIII-XI translated by H Rackham, Loeb Classical Library, edited by GP Goold, Harvard University Press, Massachusetts, USA.
REFERENCES
- 1.Crapo L. Hormones. The messengers of life. New York: WH Freeman and Company, 1985.
- 2.Bowers C Y. Growth hormone-releasing peptide (GHRP). Cell Mol Life Sci 1998;54:1316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Gennaro Colonna V, Rossoni G, Bernareggi M, et al. Hexarelin, a growth hormone-releasing peptide, discloses protectant activity against cardiovascular damage in rats with isolated growth hormone deficiency. Cardiologia 1997;42:1165–72. [PubMed] [Google Scholar]
- 4.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–60. [DOI] [PubMed] [Google Scholar]
- 5.Tomasetto C, Karam S M, Ribieras S, et al. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology 2000;119:395–405. [DOI] [PubMed] [Google Scholar]
- 6.Sheppard P, Deisher T. Motilin homologs. Patent application WO 98/42840, 01.10. 1998.
- 7.Hosoda H, Kojima M, Matsuo H, et al. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem 2000;275:21995–2000. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Hosoda H, Kitajima Y, et al. Structure-activity relationship of ghrelin: pharmacological study of ghrelin peptides. Biochem Biophys Res Commun 2001;287:142–6. [DOI] [PubMed] [Google Scholar]
- 9.Bednarek M A, Feighner S D, Pong S S, et al. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 2000;43:4370–6. [DOI] [PubMed] [Google Scholar]
- 10.Palyha O C, Feighner S D, Tan C P, et al. Ligand activation domain of human orphan growth hormone (GH) secretagogue receptor (GHS-R) conserved from Pufferfish to humans. Mol Endocrinol 2000;14:160–9. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura B, Dong M, Miller L J. Differential determinants for peptide and non-peptidyl ligand binding to the motilin receptor. Critical role of second extracellular loop for peptide binding and action. J Biol Chem 2002;277:9834–9. [DOI] [PubMed] [Google Scholar]
- 12.Depoortere I, Thijs T, Thielemans L, et al. Interaction of the growth hormone-releasing peptides ghrelin and growth hormone-releasing peptide-6 with the motilin receptor in the rabbit gastric antrum. J Pharmacol Exp Ther 2003;305:660–7. [DOI] [PubMed] [Google Scholar]
- 13.Feighner S D, Tan C P, McKee K K, et al. Receptor for motilin identified in the human gastrointestinal system. Science 1999;284:2184–8. [DOI] [PubMed] [Google Scholar]
- 14.McKee K K, Tan C P, Palyha O C, et al. Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 1997;46:426–34. [DOI] [PubMed] [Google Scholar]
- 15.Casanueva F F, Dieguez C. Growth hormone secretagogues: physiological role and clinical utility. Trends Endocrinol Metab 1999;10:30–8. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev 2005;85:495–522. [DOI] [PubMed] [Google Scholar]
- 17.Ghigo E, Broglio F, Arvat E, et al. Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol (Oxf) 2005;62:1–17. [DOI] [PubMed] [Google Scholar]
- 18.Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 2001;86:4753–8. [DOI] [PubMed] [Google Scholar]
- 19.Krsek M, Rosicka M, Haluzik M, et al. Plasma ghrelin levels in patients with short bowel syndrome. Endocr Res 2002;28:27–33. [DOI] [PubMed] [Google Scholar]
- 20.Cummings D E, Purnell J Q, Frayo R S, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50:1714–19. [DOI] [PubMed] [Google Scholar]
- 21.Bowers C Y, Momany F A, Reynolds G A, et al. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology 1984;114:1537–45. [DOI] [PubMed] [Google Scholar]
- 22.Locke W, Kirgis H D, Bowers C Y, et al. Intracerebroventricular growth-hormone-releasing peptide-6 stimulates eating without affecting plasma growth hormone responses in rats. Life Sci 1995;56:1347–52. [DOI] [PubMed] [Google Scholar]
- 23.Torsello A, Luoni M, Schweiger F, et al. Novel hexarelin analogs stimulate feeding in the rat through a mechanism not involving growth hormone release. Eur J Pharmacol 1998;360:123–9. [DOI] [PubMed] [Google Scholar]
- 24.Korbonits M, Kaltsas G, Perry L A, et al. The growth hormone secretagogue hexarelin stimulates the hypothalamo-pituitary-adrenal axis via arginine vasopressin. J Clin Endocrinol Metab 1999;84:2489–95. [DOI] [PubMed] [Google Scholar]
- 25.Arvat E, Di Vito L, Broglio F, et al. Preliminary evidence that ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest 2000;23:493–5. [DOI] [PubMed] [Google Scholar]
- 26.Wren A M, Seal L J, Cohen M A, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001;86:5992. [DOI] [PubMed] [Google Scholar]
- 27.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001;409:194–8. [DOI] [PubMed] [Google Scholar]
- 28.Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001;120:337–45. [DOI] [PubMed] [Google Scholar]
- 29.Muccioli G, Tschop M, Papotti M, et al. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur J Pharmacol 2002;440 (2–3) :235–54. [DOI] [PubMed] [Google Scholar]
- 30.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002;123:1120–8. [DOI] [PubMed] [Google Scholar]
- 31.Date Y, Nakazato M, Murakami N, et al. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 2001;280:904–7. [DOI] [PubMed] [Google Scholar]
- 32.Williams D L, Grill H J, Cummings D E, et al. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology 2003;144:5184–7. [DOI] [PubMed] [Google Scholar]
- 33.Hewson A K, Dickson S L. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol 2000;12:1047–9. [DOI] [PubMed] [Google Scholar]
- 34.Shintani M, Ogawa Y, Ebihara K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 2001;50:227–32. [DOI] [PubMed] [Google Scholar]
- 35.Chen H Y, Trumbauer M E, Chen A S, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 2004;145:2607–12. [DOI] [PubMed] [Google Scholar]
- 36.Toshinai K, Date Y, Murakami N, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003;144:1506–12. [DOI] [PubMed] [Google Scholar]
- 37.Cummings D E, Foster-Schubert K E, Overduin J. Ghrelin and energy balance: focus on current controversies. Curr Drug Targets 2005;6:153–69. [DOI] [PubMed] [Google Scholar]
- 38.Tschop M, Smiley D L, Heiman M L. Ghrelin induces adiposity in rodents. Nature 2000;407:908–13. [DOI] [PubMed] [Google Scholar]
- 39.Cummings D E, Weigle D S, Frayo R S, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002;346:1623–30. [DOI] [PubMed] [Google Scholar]
- 40.Cummings D E, Clement K, Purnell J Q, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 2002;8:643–4. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Ahmed S, Smith R G. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 2003;23:7973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuda Y, Tanaka T, Inomata N, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 2000;276:905–8. [DOI] [PubMed] [Google Scholar]
- 43.Brzozowski T, Konturek P C, Konturek S J, et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul Pept 2004;120:39–51. [DOI] [PubMed] [Google Scholar]
- 44.Ro S, Tanaka T, Ochiai M, et al. The interaction between gastrin and ghrelin on acid secretion in rat stomach 2004;26 (suppl 2) :A147.
- 45.Sibilia V, Pagani F, Guidobono F, et al. Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric acid secretion in conscious rats. Neuroendocrinology 2002;75:92–7. [DOI] [PubMed] [Google Scholar]
- 46.Dornonville de la Cour C, Lindstrom E, Norlen P, et al. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept 2004;120:23–32. [DOI] [PubMed] [Google Scholar]
- 47.Walsh J H, Gastrin. In: Walsh J, Dockray G, eds. Gut peptides. New York: Raven Press, 1994:75–121.
- 48.Lee H M, Wang G, Englander E W, et al. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002;143:185–90. [DOI] [PubMed] [Google Scholar]
- 49.Blaser M J, Atherton J C. Helicobacter pylori persistence: biology and disease. J Clin Invest 2004;113:321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gokcel A, Gumurdulu Y, Kayaselcuk F, et al. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol 2003;148:423–6. [DOI] [PubMed] [Google Scholar]
- 51.Nwokolo C U, Freshwater D A, O’Hare P, et al. Plasma ghrelin following cure of Helicobacter pylori. Gut 2003;52:637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blaser M J. An endangered species in the stomach. Sci Am 2005;292:38–45. [DOI] [PubMed] [Google Scholar]
- 53.Cummings D E. Helicobacter pylori and ghrelin: Interrelated players in body-weight regulation? Am J Med 2004;117:436–9. [DOI] [PubMed] [Google Scholar]
- 54.Macadam R C, Borse V, Dodo I, et al. Helicobacter pylori, ghrelin, and obesity. Gut 2004;53:315–6. [PMC free article] [PubMed] [Google Scholar]
- 55.Murray C D, Emmanuel A V. Ghrelin and Helicobacter pylori. Gut 2004;53:315. [PMC free article] [PubMed] [Google Scholar]
- 56.Isomoto H, Nakazato M, Ueno H, et al. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med 2004;117:429–32. [DOI] [PubMed] [Google Scholar]
- 57.Osawa H, Nakazato M, Date Y, et al. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab 2005;90:10–16. [DOI] [PubMed] [Google Scholar]
- 58.Tatsuguchi A, Miyake K, Gudis K, et al. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol 2004;99:2121–7. [DOI] [PubMed] [Google Scholar]
- 59.Ioannou G N, Weiss N S, Kearney D J. Is Helicobacter pylori seropositivity related to body mass index in the United States? Aliment Pharmacol Ther 2005;21:765–72. [DOI] [PubMed] [Google Scholar]
- 60.Cassoni P, Ghe C, Marrocco T, et al. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur J Endocrinol 2004;150:173–84. [DOI] [PubMed] [Google Scholar]
- 61.Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology 2003;144:353–9. [DOI] [PubMed] [Google Scholar]
- 62.Konturek P, Nikiforuk A, Kukharskyy V, et al. Ghrelin in Barrett’s esophagus: expression and effect on cell growth. Gastroenterology 2004;126 (4 suppl 2) :A135. [Google Scholar]
- 63.Sibilia V, Torsello A, Pagani F, et al. Effects of hexarelin against acid-independent and acid-dependent ulcerogens in the rat. Peptides 2004;25:2163–70. [DOI] [PubMed] [Google Scholar]
- 64.Pawlik M, Brzozowski T, Kwiecien S, et al. Role of exogenous and endogenous leptin and ghrelin in healing of colonic damage in experimental ulcerative colitis. 2004;126:A575.
- 65.Tanaka T, Uneyama H, Yoshie S, et al. Nitric oxide generation by ghrelin and localization of GHS-R in the rat stomach. Gastroenterology 2004;126 (suppl 2) :A410. [Google Scholar]
- 66.Suzuki H, Fukuhara S, Masaoka T, et al. Ghrelin dynamics in rats with cysteamine-induced duodenal ulcer. Gastroenterology 2004;126 (suppl 2) :A144. [Google Scholar]
- 67.Fujino K, Inui A, Asakawa A, et al. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol 2003;550 (Pt 1) :227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edholm T, Levin F, Hellstrom P M, Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons, et al. Regul Pept 2004;121:25–30. [DOI] [PubMed] [Google Scholar]
- 69.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on inter digestive gastrointestinal motility in man. Gut. 2006 (in press). [DOI] [PMC free article] [PubMed]
- 70.Trudel L, Tomasetto C, Rio M C, et al. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol 2002;282:G948–52. [DOI] [PubMed] [Google Scholar]
- 71.Dornonville de la Cour C, Lindstrom E, Norlen P, et al. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept 2004;120:23–32. [DOI] [PubMed] [Google Scholar]
- 72.Kitazawa T, De Smet B, Verbeke K, et al. Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut 2005;54:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreaux B, Van den Berg J, Thielemans L, et al. Activation of the GHS-receptor accelerates gastric emptying in dogs. Gastroenterology 2004;126 (suppl 2) :A278. [Google Scholar]
- 74.Tschop M, Wawarta R, Riepl R L, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 2001;24:RC19–21. [DOI] [PubMed] [Google Scholar]
- 75.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on gastric emptying and meal-releated symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005 (in press). [DOI] [PubMed]
- 76.De Smet B, Depoortere I, Moreaux B, et al. Gastric emptying and food intake in ghrelin knockout mice. Gastroenterology 2004;126 (suppl 2) :A90. [Google Scholar]
- 77.Asakawa A, Inui A, Kaga T, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003;52:947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trudel L, Bouin M, Tomasetto C, et al. Two new peptides to improve post-operative gastric ileus in dog. Peptides 2003;24:531–4. [DOI] [PubMed] [Google Scholar]
- 79.De Winter B Y, De Man J G, Seerden T C, et al. Effect of ghrelin and growth hormone-releasing peptide 6 on septic ileus in mice. Neurogastroenterol Motil 2004;16:439–46. [DOI] [PubMed] [Google Scholar]
- 80.Xu L, Depoortere I, Tomasetto C, et al. Evidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regul Pept 2005;124:119–25. [DOI] [PubMed] [Google Scholar]
- 81.Dass N B, Munonyara M, Bassil A K, et al. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience 2003;120:443–53. [DOI] [PubMed] [Google Scholar]
- 82.Bisschops R, Vanden Berghe P, Depoortere I, et al. Ghrelin activates a subset of myenteric neurons in guinea-pig jejunum. Gastroenterology 2003;124 (suppl 1) :A1.12774765 [Google Scholar]
- 83.Peeters T L, Aerssens J, De Smet B, et al. The mouse is a natural knock-out for motilin and for the motilin receptor. Functionally they have been replaced by ghrelin. Neurogastroenterol Motil 2004;16:687. [Google Scholar]
- 84.Aerssens J, Depoortere I, Thielemans L, et al. The rat lacks functional genes for motilin and for the motilin receptor. Neurogastroenterol Motil 2004;16:841. [Google Scholar]
- 85.Asakawa A, Inui A, Fujimiya M, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005;54:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Itoh Z, Aizawa I, Takeuchi S, et al. In: Vantrappen G, ed. Hunger contractions and motilin. Porceedings of the Fifth International Symposium on Gastrointestinal Motility. Herentals, Belgium: Typoff-Press. 1975:48–55.
- 87.Spiegel K, Tasali E, Penev P, et al. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50. [DOI] [PubMed] [Google Scholar]
- 88.Weikel J C, Wichniak A, Ising M, et al. Ghrelin promotes slow-wave sleep in humans. Am J Physiol Endocrinol Metab 2003;284:E407–15. [DOI] [PubMed] [Google Scholar]