Abstract
Cirrhosis is associated with the development of a hyperdynamic circulation, which is secondary to the presence of systemic vasodilatation. Several mechanisms have been postulated to be involved in the development of systemic vasodilatation, including increased synthesis of nitric oxide, hyperglucagonaemia, increased carbon monoxide synthesis, and activation of KATP channels in vascular smooth muscle cells in the systemic and splanchnic arterial circulation. Hydrogen sulphide (H2S) has recently been identified as a novel gaseous transmitter that induces vasodilatation through activation of KATP channels in vascular smooth muscle cells. In this brief review, we comment on what is known about H2S, vascular and neurological function, and postulate its role in the pathogenesis of the vascular abnormalities in cirrhosis.
Keywords: hydrogen sulphide, hyperdynamic circulation, cirrhosis
Endogenous gaseous transmitters such as nitric oxide (NO) and carbon monoxide (CO) constitute a unique class of mediators which play an important role in cell physiology. The high membrane permeability of these gases enables their rapid transfer across the cell membrane where they bind directly to the haeme group of guanylate cyclase or cytochrome oxidase, resulting in cell signalling in a receptor independent manner. A number of other biologically active gases such as nitrous oxide, ammonia, and hydrogen sulphide (H2S) may also participate in the regulation of cell function. Among them, recent reports have proposed H2S as a novel endogenous transmitter with potential roles in both physiology and disease.
FORMATION AND METABOLISM OF H2S
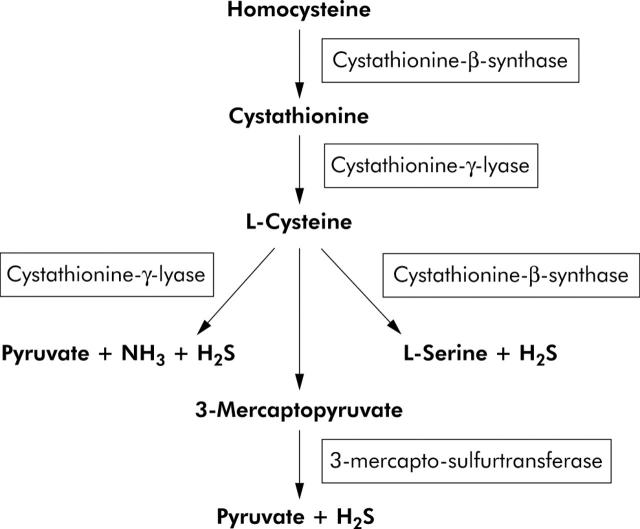
H2S is produced endogenously from desulphydration of cysteine (or cystine) by three different enzymes.1,2 The reaction is catalysed by cystathionine-γ-lyase (sometimes termed cystathionase), cystathionine-β-synthase, or 3-mercapto-sulphurtransferase (fig 1 ▶). The first two enzymes are cytosolic haeme proteins, and the latter is a zinc dependent protein which is present in both the cytoplasm as well as mitochondria.2 Cystathionase is currently the only identified H2S generating enzyme present in the vasculature3 whereas cystathionine-β-synthase is the only H2S generating system found in the nervous system.4 However, all three enzymes are present in the liver and kidney, with cystathionine-β-synthase being most prominent in the liver.5
Figure 1.
There are three enzymatic pathways involved in the synthesis of hydrogen sulphide (H2S) from cysteine in mammals. Of these only cystathionine-γ-lyase is found in the vasculature. All three enzymes are present in the liver and kidney, with most activity residing in cystathionine-β-synthase.
H2S is permeable to plasma membranes as its solubility in lipophilic solvents is fivefold greater than in water.6 It can be hydrolysed to hydrosulphide and sulphide ions in the following sequential reactions:
H2S ↔ H+ + HS− ↔ 2H+ + S2−
However, even in an aqueous solution, approximately one third of H2S remains undissociated at pH 7.4.6 Cellular concentrations of H2S are reported to be in the micromolar range (50–160 μM reported in the brain and 45 μM in plasma)4,7 with a short half life due to its rapid reaction with haeme groups or disulphide containing proteins, or its oxidation to thiosulphate (S2O3) and sulphate.2,6 These relatively high concentrations, together with its short half life, suggest that generation or flux of H2S is high. The amounts of urinary thiosulphate as well as sulphaemoglobin in erythrocytes are currently believed to be among the best markers of H2S formation in vivo,2,6 although these do have limitations, and recent studies have suggested that fluxes of H2S can be measured using polarographic techniques.8
PHYSIOLOGICAL ACTIONS OF H2S AND UNDERLYING MECHANISMS
The first and most important evidence for a physiological role of H2S was obtained in 1989 when endogenous sulphide levels in rat brain tissues (1.6 μg/g)9 and in normal human post mortem brainstem (0.7 μg/g) were reported.10 The study by Awata et al in 199511 provided the enzymatic mechanisms for this endogenous H2S in rat brain, in which the activities of cystathionine-β-synthase and cystathionine-γ-lyase in six different brain regions were measured, with the activity of cystathionine-β-synthase being >30-fold that of cystathionine-γ-lyase.
“There has been an explosion of interest in the biochemistry, physiology, and pharmacology of H2S, which is rapidly emerging as a new biological mediator”
Further evidence for a physiological role of H2S was reported by Abe and Kimura in 1996, who suggested that it may act as a neuromodulator as physiological concentrations of H2S enhance glutamate mediated transmission via N-methyl-D-aspartate (NMDA) receptors which promote neuronal long term potentiation.4 Since these initial publications there has been an explosion of interest in the biochemistry, physiology, and pharmacology of H2S, which is rapidly emerging as a new biological mediator.
At present, the functional role of endogenous H2S in the cardiovascular system is still the subject of much ongoing research and so far there have been no human studies on the physiological role of endogenously produced H2S in the cardiovascular system. However, expression of cystathionine-γ-lyase mRNA and endogenous production of H2S have been demonstrated in the aorta,3,7 mesenteric artery,12 portal vein,3 as well as cardiac tissue13 in rats. Hosoki et al have demonstrated that H2S could be produced in the portal vein, mesenteric artery, pulmonary artery, and thoracic aorta.3 H2S is only generated from vascular smooth muscle cells as no H2S generating enzyme systems are expressed in the endothelial layer.7 This is in contrast with NO and CO which can be produced from both endothelial and vascular smooth muscle cells. Moreover, unlike NO or CO, H2S relaxed vascular tissues independent of activation of the cGMP pathway.8
“H2S induces vasorelaxation through activation of ATP sensitive K+ channels in vascular smooth muscles in vitro and in vivo”
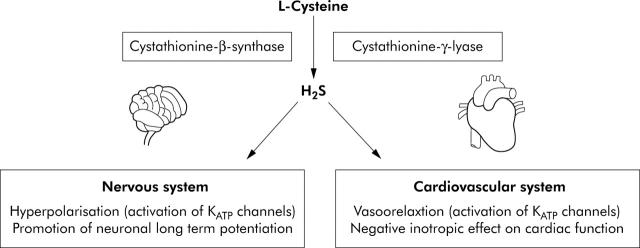
Whereas vasorelaxation induced by NO is virtually abolished by ODQ, a specific inhibitor of soluble guanylyl cyclase, H2S induced vasorelaxation is not inhibited by ODQ.7 The vasorelaxant activity of H2S is mimicked by ATP sensitive K+ channel (KATP) openers, and antagonised by glibenclamide (a KATP channel blocker).12 In a series of studies, Wang and colleagues have shown that H2S induces vasorelaxation through activation of ATP sensitive K+ channels (KATP) in vascular smooth muscles in vitro and in vivo.7,12,13 H2S may also exert effects on adjacent cell types. Thus H2S released from vascular smooth muscle cells may stimulate endothelial cells of small peripheral resistant arteries to release endothelium derived hyperpolarising factor which further hyperpolarises vascular smooth muscle cells and potentiates vascular relaxation.12,13 In vitro studies have also shown that H2S exerts a negative inotropic effect on cardiac function, primarily through activation of KATP channels.14 A summary of the major physiological effects of H2S is presented at fig 2 ▶.
Figure 2.
Major physiological actions of endogenously produced hydrogen sulphide (H2S). Activation of ATP sensitive K+ channels (KATP) is a common mechanism of H2S physiological effects, which induce vasorelaxation and neuronal hyperpolarisation in the cardiovascular system and nervous system, respectively. H2S can also promote glutamate medicated transmission via N-methyl-D-aspartate receptors which enhance neuronal long term potentiation.
INTERACTION OF H2S WITH NO AND CO
NO can regulate endogenous production of H2S in vascular tissues by increasing cystathionine-γ-lyase gene expression; this is evident by the fact that incubating cultured vascular smooth muscle cells with an NO donor significantly increased the transcriptional level of cystathionine-γ-lyase.7 On the other hand, H2S, even at a very low concentration, can enhance relaxation of smooth muscle induced by NO by approximately 10-fold.3 This effect is independent of free thiol groups as both cysteine and glutathione do not have such an effect.3
“There is a dynamic interplay between not only the H2S and NO pathways but also between the H2S and CO systems”
H2S has also recently been shown to upregulate CO synthesis through induction of haeme oxygenase.15 Altered synthesis of H2S may also affect the pulmonary circulation.15 Thus Qingyou et al have shown that administration of sodium hydrosulphide (a donor of H2S) causes a decrease in pulmonary artery pressure in rats with hypoxic pulmonary hypertension, and administration of an inhibitor of cystathionine-γ-lyase led to an increase in pulmonary artery pressure and a decrease in CO synthesis.15 This suggests that there is a dynamic interplay between not only the H2S and NO pathways but also between the H2S and CO systems.
POTASSIUM CHANNELS AND CONTROL OF VASCULAR FUNCTION IN CIRRHOSIS
Hypotension, low systemic vascular resistance, and reduced responsiveness to vasoconstrictors are all features of the hyperdynamic circulation in cirrhosis. These changes have been attributed to increased synthesis of NO, CO, anandamide, and calcitonin gene related polypeptide16–19; however, the precise mechanisms underlying the cardiovascular changes in cirrhotic subjects are not completely understood. In 1994, Moreau et al showed that there was activation of KATP channels in vascular smooth muscle cells in rats with cirrhosis, and that this was partly responsible for the development of systemic vasodilatation in this animal model.20,21 In arterial smooth muscle cells, plasmalemmal KATP channels play an important role in arterial vasodilatation by modulating membrane potential.22 In cirrhosis, activation of KATP leads to membrane hyperpolarisation which results in closure of the L-type Ca2+ channel and subsequent decrease in Ca2+ entry and vasorelaxation.20,21 One potential mechanism of KATP channel activation involves prostaglandins such as prostacyclin as KATP activation can be partially inhibited by cyclooxygenase inhibitors.20 However, the observation that H2S can cause KATP activation in a variety of experimental systems lends support to the idea that H2S may be involved in KATP channel activation in cirrhosis.
H2S AND THE HYPERDYNAMIC CIRCULATION
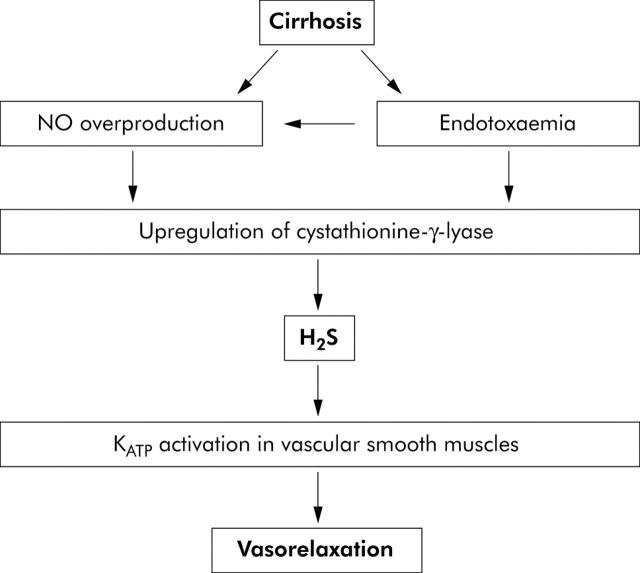
In this paper, we suggest that H2S may contribute to the pathogenesis of vascular dysfunction in cirrhosis (fig 3 ▶). This hypothesis is based on the following evidence.
Figure 3.
Postulated role of hydrogen sulphide (H2S) in the development of a hyperdynamic circulation in cirrhosis. Endotoxaemia leads to increased nitric oxide (NO) synthesis and upregulation of the enzyme responsible for H2S production (cystathionine-γ-lyase). H2S causes activation of KATP channels which causes vasodilatation in liver disease. Increased NO synthesis may also cause vasodilatation directly.
Plasma H2S concentrations increase in rats with endotoxaemia.23 Endotoxaemia is a common feature of cirrhosis24 and high concentrations of circulating endotoxins are observed in cirrhotic patients with no clinical evidence of infection, and this may be due to impaired clearance of gut bacteria in cirrhotic liver.24,25 Studies are emerging which increasingly link the development of extrahepatic complications of cirrhosis (for example, hyperdynamic circulation, cirrhotic cardiomyopathy, and hepatic encephalopathy) to the advent of endotoxaemia or sepsis in cirrhosis.26–28 As endotoxin can induce the synthesis of H2S, this may have two consequences. Firstly, there may be increased H2S synthesis leading to increased KATP activation in vascular smooth muscle cells and a resulting systemic vasodilatation. Secondly, increased H2S formation may lead to altered cardiac function as it has been shown that H2S exerts a negative inotropic effect on cardiac function, primarily through activation of KATP channels.14
Increased synthesis of NO is well recognised in cirrhosis and portal hypertension,16 and may lead to increased expression of cystathionine-γ-lyase, the main H2S producing enzyme in vascular smooth muscle cells.7 Thus increased NO synthesis may enhance the formation of H2S in cirrhosis, thus leading indirectly to activation of KATP channels.
Increased activity of serum cystathionine-γ-lyase has been demonstrated in rats with liver injury due to carbon tetrachloride.29 Whether this is applicable to other forms of liver injury is unknown but increased cystathionine-γ-lyase activity would be expected to increase H2S formation in this model.23
In conclusion, we propose a mechanism by which endotoxaemia, either alone or in combination with increased NO synthesis, leads to upregulation of cystathionine-γ-lyase activity and H2S synthesis. Increased synthesis of H2S leads to activation of KATP channels and systemic vasodilatation (fig 3 ▶). Studies in the future will determine the validity of this hypothesis in humans.
Abbreviations
H2S, hydrogen sulphide
NO, nitric oxide
CO, carbon monoxide
NMDA, N-methyl-D-aspartate
Conflict of interest: None declared.
REFERENCES
- 1.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphydration in liver and kidney of the rat. Biochem J 1982;206:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids 2004;26:243–54. [DOI] [PubMed] [Google Scholar]
- 3.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophysic Res Commun 1997;237:527–31. [DOI] [PubMed] [Google Scholar]
- 4.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996;16:1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 2003;81:848–53. [DOI] [PubMed] [Google Scholar]
- 6.Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002;16:1792–8. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Zhang J, Lu Y, et al. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 2001;20:6008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doeller JE, Isbell TS, Benavides G, et al. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 2005;341:40–51. [DOI] [PubMed] [Google Scholar]
- 9.Warenycia MW, Goodwin LR, Benishin CG, et al. Acute hydrogen sulfide poisoning: demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol 1989;38:973–81. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin LR, Francom D, Dieken FP, et al. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol 1989;13:105–9. [DOI] [PubMed] [Google Scholar]
- 11.Awata S, Nakayama K, Suzuki I, et al. Changes in cystathionine gamma-lyase in various regions of rat brain during development. Biochem Mol Biol Int 1995;35:1331–8. [PubMed] [Google Scholar]
- 12.Cheng Y, Ndisang JF, Tang G, et al. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 2004;287:H2316–23. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 2002;283:H474–80. [DOI] [PubMed] [Google Scholar]
- 14.Geng B, Yang J, Qi Y, et al. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 2004;313:362–8. [DOI] [PubMed] [Google Scholar]
- 15.Qingyou Z, Junbao D, Weijin Z, et al. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem Biophys Res Commun 2004;317:30–7. [DOI] [PubMed] [Google Scholar]
- 16.Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet 1991;337:776–8. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez M, Bonkovsky HL. Increased heme oxygenase-1 gene expression in liver cells and splanchnic organs from portal hypertensive rats. Hepatology 1999;29:1672–9. [DOI] [PubMed] [Google Scholar]
- 18.Batkai S, Jarai Z, Wagner JA, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med 2001;7:827–32. [DOI] [PubMed] [Google Scholar]
- 19.Bendtsen F, Schifter S, Henriksen JH. Increased circulating calcitonin gene-related peptide (CGRP) in cirrhosis. J Hepatol 1991;12:118–23. [DOI] [PubMed] [Google Scholar]
- 20.Moreau R, Komeichi H, Kirstetter P, et al. Altered control of vascular tone by adenosine triphosphate-sensitive potassium channels in rats with cirrhosis. Gastroenterology 1994;106:1016–23. [DOI] [PubMed] [Google Scholar]
- 21.Moreau R, Lebrec D. Endogenous factors involved in the control of arterial tone in cirrhosis. J Hepatol 1995;22:370–6. [DOI] [PubMed] [Google Scholar]
- 22.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997;77:1165–232. [DOI] [PubMed] [Google Scholar]
- 23.Hui Y, Du J, Tang C, et al. Changes in arterial hydrogen sulfide (H(2)S) content during septic shock and endotoxin shock in rats. J Infect 2003;47:155–60. [DOI] [PubMed] [Google Scholar]
- 24.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol 1995;22:165–72. [DOI] [PubMed] [Google Scholar]
- 25.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology 1988;8:232–6. [DOI] [PubMed] [Google Scholar]
- 26.Le Moine O, Soupison T, Sogni P, et al. Plasma endotoxin and tumor necrosis factor-alpha in the hyperkinetic state of cirrhosis. J Hepatol 1995;23:391–5. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Ma Z, Lee SS. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology 2000;118:937–44. [DOI] [PubMed] [Google Scholar]
- 28.Bigatello LM, Broitman SA, Fattori L, et al. Endotoxemia, encephalopathy, and mortality in cirrhotic patients. Am J Gastroenterol 1987;82:11–15. [PubMed] [Google Scholar]
- 29.Taguchi T, Awata S, Nishioka M, et al. Elevation of cystathionine gamma-lyase activity in the serum of rats treated with a single dose of carbon tetrachloride. Ind Health 1995;33:199–205. [DOI] [PubMed] [Google Scholar]