In this article, we discuss the current understanding of how the intestinal mucosa may exert control over luminal bacteria, and how intestinal inflammation could ensue when this control is lost. We shall review research showing how Paneth cells, and evolutionarily conserved innate immune mechanisms, are emerging as key mediators of intestinal mucosal defence.
THE SMALL BOWEL CHALLENGE
Maintenance of a sterile environment in the small intestinal lumen represents a formidable challenge for the host. The multitude of villi and crypts create an expansive epithelial surface of approximately 400 m2, allowing efficient nutrient absorption but a wealth of potential entry sites for invading microbes. To heighten the challenge, the intestinal mucosa comprises a single layer of epithelial cells, unlike the multiple layers found at other mucosal surfaces. This aids nutrient absorption and water and electrolyte transport, yet spreads defensive strategies thinly. The nutrient rich luminal content would appear to provide an ideal culture medium, and there is constant exposure to a large population of micro-organisms, both ingested along with food and from the adjacent colon with its heavy bacterial load. In addition, epithelial cells are replaced every 2–5 days from pluripotential stem cells in the base of the crypts1 and so continuous antimicrobial protection for these stem cells is of paramount importance as damage to or parasitisation of stem cells would have severe consequences for the maintenance of the normal digestive epithelium. Against all the odds, microbial density in the healthy proximal small intestine (duodenum, jejunum, proximal ileum) is low.2 In contrast, in the distal ileum and colon, there is extensive resident bacterial flora (total ∼1014) consisting of ∼400 different species of anaerobic and aerobic bacteria.3 Mucosal defence mechanisms in the proximal small bowel are able to maintain a crucial barrier to microbial invasion yet allow efficient nutrient absorption.
ADAPTIVE VERSUS INNATE IMMUNITY
The immune system has many facets, which can be grouped into adaptive and innate components.4 Adaptive immunity, which is only found in vertebrates, is mediated by T and B cells which display structurally unique receptors that are generated by gene rearrangement. On binding of receptor to its specific antigen, clonal expansion of lymphocytes results to elicit a directed immune response. However, it takes three to five days for a sufficient number of lymphocytes to be produced and to differentiate into effector cells, which is more than enough time for most pathogens to invade and damage the host. In contrast, innate immunity refers to inbuilt mechanisms, many of which are relatively conserved throughout the animal and plant kingdoms, that respond immediately to a wide variety of micro-organisms and can be seen as a first line of defence to control an invasion before clonal lymphocytes can mount a specific attack. We shall discuss the role of adaptive and innate immune mechanisms in the defence of the gut’s epithelial monolayer.
Mucosal adaptive immunity
The adaptive mucosal immune system can principally be divided into inductive sites, where antigens from mucosal surfaces stimulate naïve T and B lymphocytes, and effector sites, where antigen sensitised cells extravasate and differentiate. Effector sites include the lamina propria and epithelium where synthesis and secretion of secretory IgA (sIgA) antibodies occurs. Whereas systemic immunity depends on antigenic supply to the lymph nodes and spleen via lymph and peripheral blood, the mucosal immune system actively samples antigen from mucosal surfaces and is tolerant of innocuous substances and commensal bacteria. Inductive sites for mucosal immunity consist of organised lymphatic follicles termed mucosa associated lymphatic tissue, which may occur singly or may congregate in Peyer’s patches (or the appendix), as well as draining lymph nodes5 Follicle associated epithelium, which covers organised lymphoid tissue in the intestine, contains specialised M cells that transport luminal antigens into the dome area of the follicle where antigen presenting dendritic cells (DC) and lymphocytes coexist. DCs also send processes between gut epithelial cells without disturbing tight junction integrity and sample commensal and pathogenic gut bacteria.5
When T and B cells are activated in Peyer’s patches, they express the α4β7 integrin and migrate to the blood.6 Gut endothelial cells express MADCAM-1, the ligand for α4β7, which allows Peyer’s patch derived cells to migrate from the blood to the lamina propria.6 The lamina propria is filled with plasma cells that secrete 2–5 g of sIgA into the gut lumen daily.7 Dimeric sIgA is one of the most important defence factors on mucosal surfaces. It is resistant to proteolysis, and its task is to prevent both the adherence of bacteria to mucosa and their penetration into the internal environment.
Mucosal innate immunity
Innate immune mechanisms protecting the gut mucosa comprise mechanical, cellular, and chemical components working at three levels: the extra-epithelial, epithelial, the subepithelial levels.
The mechanical element is the physical barrier of the epithelium, along with mucus coating, enterocyte shedding, and peristalsis. The chemical element includes pathogen recognition molecules, proteins, or peptides that induce microbial killing and cytokines that orchestrate an immune response. The cellular element includes epithelial cells, mast cells, dendritic cells, phagocytic cells, such as macrophages and granulocytes, natural killer cells, and γδ T cells.
The extra-epithelial defence barrier includes antimicrobial proteins and peptides, such as lysozyme and defensins, which disrupt microbial cell walls, mucus coating that traps bacteria to be removed by peristalsis, and commensal flora that provides resistance to colonisation.
Defence at the level of the epithelial cell includes the mechanical barrier to penetration of the epithelial monolayer and tight junctions, and pattern recognition receptors (PRRs) which recognise highly conserved motifs, designated pathogen associated molecular patterns (PAMPs), which are present in large groups of micro-organisms, but not their hosts, and are usually essential for the pathogenicity or survival of the microbe. Examples of PAMPs are lipopolysaccharide or peptidoglycan, which are both components of bacterial, but not host, cell walls. Unlike lymphocyte receptors in adaptive immunity, receptors for the innate immune system are germline coded and, therefore, are not altered by prior exposure to pathogens.8,9 Binding to PRRs triggers the secretion of chemokines that lead to the recruitment of cellular components of the innate immune response.
Toll-like receptors (TLRs) and nucleotide binding oligomerisation domain (NOD) proteins are two classes of PRRs in mammals.10,11 TLRs are transmembrane molecules which link the extracellular compartment, where recognition of microbial pathogens occurs, and the intracellular compartment, where signalling cascades leading to cellular responses are initiated (fig 1 ▶). NOD proteins are cytosolic and so recognise microbial components after invasion into the cell.
Figure 1.
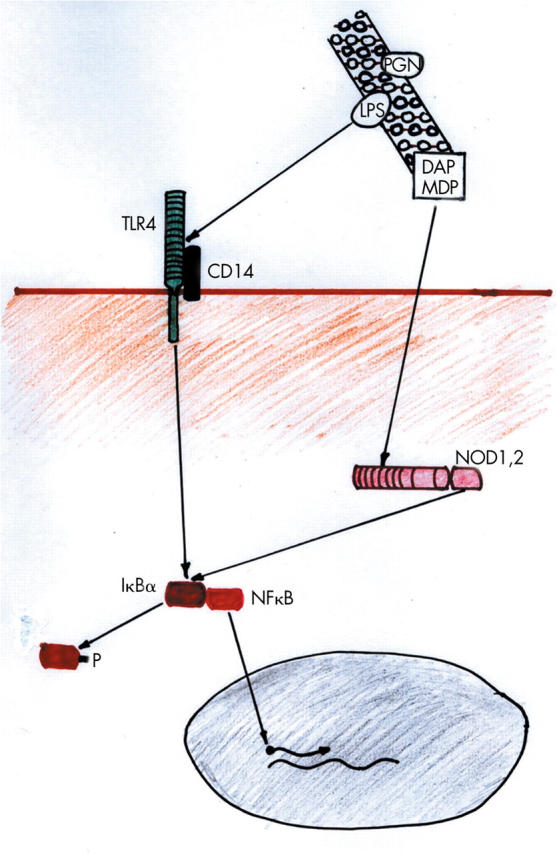
Schematic diagram of Toll-like receptor (TLR) and nucleotide binding oligomerisation domain (NOD) protein interactions with components of the bacterial cell wall and subsequent nuclear factor κB (NFκB) activation. Lipopolysaccharide (LPS), diaminopimelate (DAP), and muramyl dipeptide (MDP) from the peptidoglycan (PGN) bacterial cell wall bind to TLR4, NOD1, and NOD2, respectively. Phosphorylation (P) of IκBα leads to release of NFκB which migrates to the nucleus to promote transcription of specific genes.
Toll-like receptors
The first discovery of Toll related proteins in mammals in 1998 was quickly followed by demonstration that mammalian TLR4 was the long sought after signalling receptor in a protein complex responsible for the recognition of lipopolysaccharide leading to the cellular responses that result in endotoxic shock.12,13 In mammals, there are at least 11 members of the TLR family, and TLR1–9 are conserved in humans and mice.11 Many of the PAMPs recognised by individual TLRs have been elucidated (see table 1 ▶).
Table1.
Host recognition of microbial components
| Receptor | Ligand |
| TLR2 | Lipoteichoic acid from Gram positive bacteria |
| TLR2+TLR 1 | Triacyl lipoproteins |
| TLR2+TLR6 | Diacyl lipoproteins |
| TLR3 | dsRNA |
| TLR4 | Lipopolysaccharide |
| TLR5 | Flagellin |
| TLR7 | ssRNA recognition on endosomes (mouse) |
| TLR8 | ssRNA recognition on endosomes (human) |
| TLR9 | CpG (bacterial) DNA |
| NOD1 | D-Glu-meso-DAP (diaminopimelate; Gram negative peptidoglycan motif) |
| NOD2 | Muramyl dipeptide from peptidoglycan |
TLR, Toll-like receptor; NOD, nucleotide binding oligomerisation domain.
Ligand binding to the extracellular leucine-rich repeat portion of the TLRs sets up an intracellular cascade of recruitment of adaptor molecules and activation of kinases, which culminates in the phosphorylation of IκBα, the inhibitory molecule for nuclear factor κB (NFκB).10 NFκB encompasses inducible transcription factors that are important activators of genes involved in inflammatory and immunological responses.14 The active form of NFκB consists of heterogenous dimers that share a homologous region responsible for DNA binding, nuclear localisation, and cytoplasmic interaction with IκB proteins. The p60:p50 heterodimer is the predominant form of active NFκB. It is stored in an inactive state in the cytoplasm bound to inhibitory proteins of the IκB family (of which IκBα is the best characterised). IκB masks the nuclear localisation signal of NFκB, thereby retaining it in the cytoplasm. Following stimulation (by, for example, activated TLR, NOD protein, or proinflammatory cytokines such as interleukin 1 or tumour necrosis factor α), IκB is degraded to release active NFκB, which migrates to the nucleus to bind to its sequence recognition motif on promoters of target genes, leading to transcriptional upregulation of a number of genes involved in inflammatory responses.
NOD proteins
The NOD proteins, NOD1 and NOD2, have recently been shown to represent an intracellular pathogen sensing system in mammals, both recognising bacterial peptidoglycan, although each responding to distinct motifs of this molecule (see table 1 ▶).15 Like TLRs, NOD1 and NOD2 have a C terminal series of leucine rich repeats that facilitate PAMP recognition. At the N terminus, NOD1 has one caspase activating and recruitment domain (CARD) while NOD2 had two such CARD domains. NOD1 and NOD2 are also designated CARD4 and CARD15, respectively. Like TLRs, activation of NOD proteins sets up an intracellular cascade of events culminating in NFκB activation via IκBα phosphorylation.10,16
Recently, research on NOD2 has received considerable attention as a genetic approach has identified NOD2 as the first susceptibility gene for Crohn’s disease, as well as for Blau syndrome, a rare autoinflammatory disease affecting the eye and joints.17–19
Subepithelial components, including macrophages, dendritic cells, and myofibroblasts, recognise pathogens and their components that have breached the epithelial layer. Activation of cell associated receptors leads to release of cytokines and chemokines to recruit innate immune effector cells, and antigen presentation to T cells to elicit an adaptive immune response.
Key points: innate immune mechanisms.
Germline coded mechanisms that respond immediately to microbes and do not require prior exposure to antigen
Pattern recognition receptors (PRRs) include Toll-like receptors (TLRs) and nucleotide binding oligomerisation domain (NOD) proteins
PRRs recognise pathogen associated molecular patterns (PAMPs)
PAMPs are present on microbes but not hosts
PAMP recognition leads to microbial killing by various means
Antimicrobial peptides, such as defensins, are important effectors
Antigen presenting cells facilitate an adaptive immune response
PANETH CELLS
Paneth cells were first described over a century ago as granulated cells at the base of the small intestinal “crypts of Lieberkühn”.20 While their function remained an enigma until recent years, they are now considered to be important in innate intestinal defence as regulators of microbial density in the small intestine and in the protection of nearby stem cells.
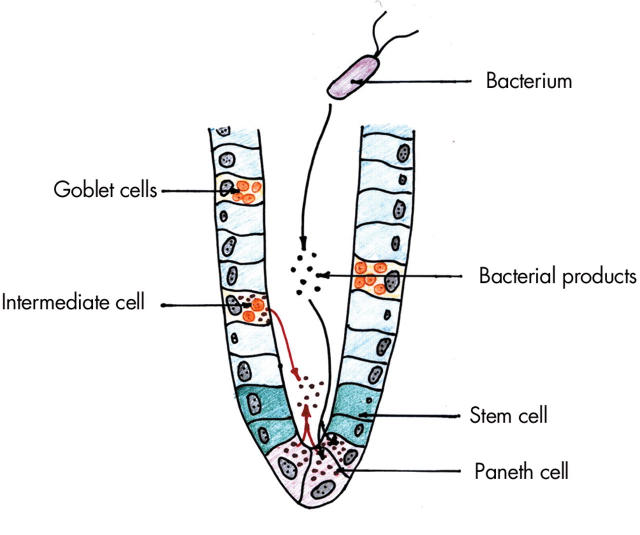
There are on average 5–12 Paneth cells in each small intestinal crypt and, in contrast with other epithelial cell types, they migrate down from the stem cell zone to the crypt base where they are relatively long lived (20 days compared with 3–5 days for enterocytes).21,22 Stem cells also give rise to three other cell lineages—enterocytes, goblet cells, and enteroendocrine cells—most of which migrate upwards and populate the villi. Paneth cells are filled with numerous prominent apical cytoplasmic granules which, on cell stimulation, can be released into the crypt lumen (fig 2 ▶).23
Figure 2.
Small intestinal crypt showing Paneth cells with secretory granules, stem cells, enterocytes, mucin secreting goblet cells, and an intermediate cell (features of Paneth and goblet cells). Exposure to bacterial products leads to release of antimicrobial peptides and proteins from Paneth and intermediate cells.
Various histochemical stains, including eosin, periodic acid Schiff’s stain, and phloxine-tartrazine (fig 3 ▶),24 intensely stain the basic Paneth cell granules whereas more recently precise staining has been achieved with immunohistochemistry employing antibodies against Paneth cell specific components, including lysozyme,25 defensins (fig 4 ▶),26 and secretory phospholipase A2 (sPLA2).27 Sometimes, cells with morphological features of Paneth cells and goblet cells are observed in the villi and are termed intermediate cells (fig 3 ▶).26,28 Although the function of intermediate cells is not clear, they have been shown to express defensins (figs 3 ▶, 4 ▶).26,28
Figure 3.
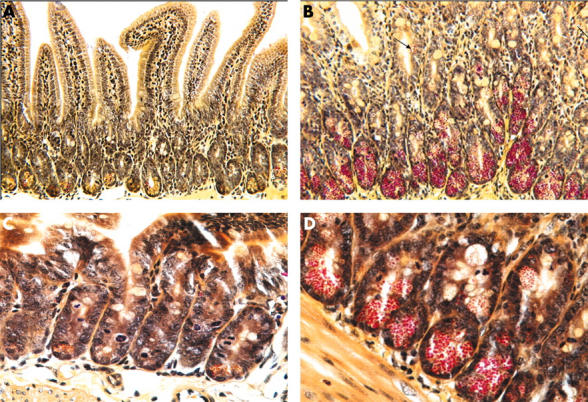
Low (A, B) and high (C, D) power views of phloxine-tartrazine stained sections of uninfected control (A, C) and T spiralis infected (B, D) murine small intestine. A few Paneth cells (with predominantly yellow granules) are present at the base of uninfected crypts. In T spiralis infected intestine, there is a marked increase in the number of Paneth cells (with red granules). Moreover, intermediate cells (arrowed) are seen in some villi. Two (A, B) of the figures are reproduced from Kamal and colleagues,28 with permission from Blackwell Publishing Ltd.
Figure 4.
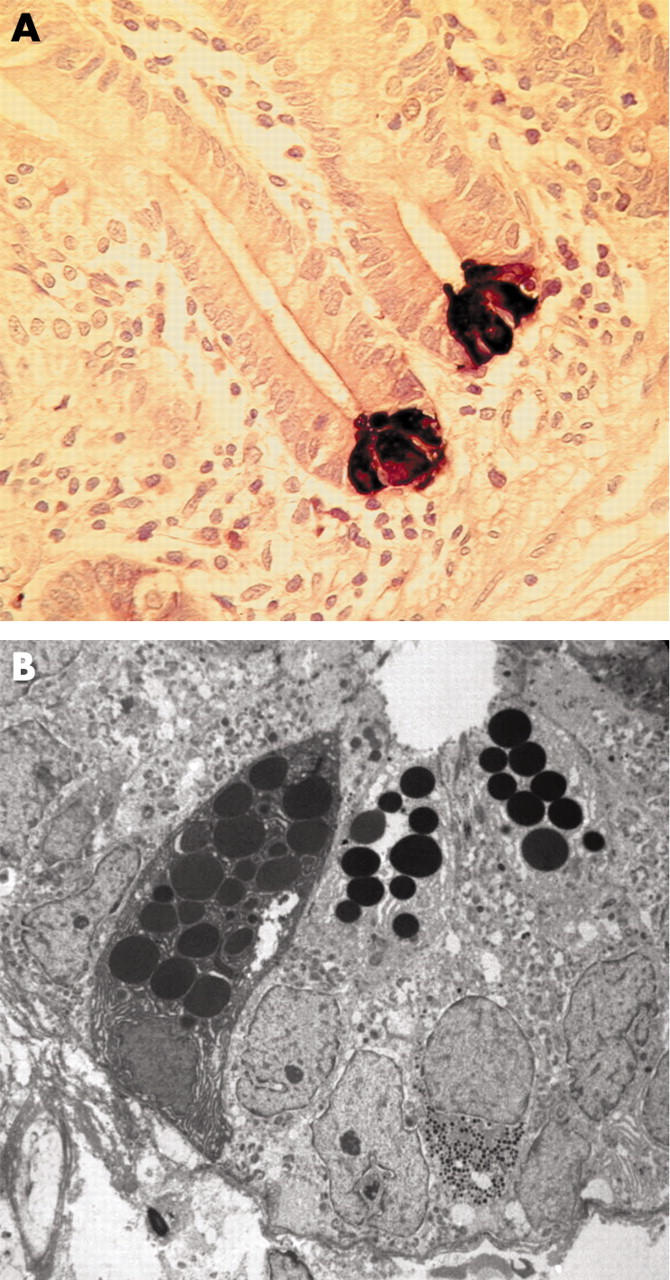
(A) Section of human jejunal mucosa showing human defensin 5 immunoreactive Paneth cells at the base of crypts. (B) Transmission electron micrograph of human jejunal crypt showing three Paneth cells with electron dense granules in the apical cytoplasm.
Paneth cell pattern recognition receptors
Studies in Paneth cell containing isolated murine small intestinal crypts have shown secretion of microbicidal peptides (predominantly cryptdins) following exposure to Gram negative or Gram positive bacteria or their products, lipopolysaccharide, lipoteichoic acid, lipid A, and muramyl dipeptide.29 It is of interest that fungi or protozoa did not stimulate Paneth cell degranulation. Whether the bacterial products interact with Paneth cells directly or via crypt non-Paneth cells remains to be determined. Lipid A is the biologically active component of lipopolysaccharide, and isolated Paneth cells have been shown to respond equivalently to different forms of lipid A and lipoteichoic acid, implying recognition of common components of these glycoproteins.30 Surprisingly, murine Paneth cells do not express mRNA transcripts for TLR4. Lipopolysaccharide and lipid A mediated Paneth cell responses, independent of TLR4, were demonstrated in TLR4 deficient C3H/HeJ mice.30 Murine Paneth cells do express mRNA transcripts for TLR 1–3 and TLR 5–9. The functional activity of TLR9 has recently been demonstrated by Paneth cell degranulation in response to its ligand, CpG (bacterial) DNA.31
NOD2 protein, which is an intracellular receptor for muramyl dipeptide, has recently been shown to be expressed in the cytoplasm of Paneth cells.32,33 The potential role of Paneth cells in the pathogenesis of Crohn’s disease in patients with NOD2 mutations is discussed below.
Paneth cell antimicrobial peptides
Several proteins and peptides, including lysozyme, sPLA2, and enteric α-defensins, cryptdin related sequence peptides, and angiogenin 4, with well documented antimicrobial activity, have been localised to Paneth cell granules.26,27,34,35 On exposure to viable or heat killed bacteria or to microbial products, such as lipopolysaccharide or lipoteichoic acid, Paneth cells release their granules resulting in increased concentrations of antimicrobial peptides in the intestinal lumen.29 This is believed to prevent microbial invasion into the crypt microenvironment, providing protection for the stem cell zone, and also contribute towards the control of microbial density in the small intestinal lumen.
Lysozyme
Lysozyme is an antibacterial protein that is found at significant concentrations in many human secretions, including tears, breast milk, saliva, and gastric and small intestinal secretions. It is expressed in the intestinal tract by gastric and pyloric glands, duodenal Brunner’s glands, small intestinal Paneth cells, macrophages, and granulocytes, but not in the normal colon.36 Lysozyme is predominantly active against Gram positive bacteria. It cleaves glycosidic bonds which stabilise peptidoglycan, resulting in bacterial lysis. Gram negative bacteria have an outer membrane which protects the underlying peptidoglycan cell wall and so are relatively resistant to lysozyme. The likely importance of lysozyme in intestinal innate defence has been suggested by its lack in Paneth cells in newborn infants with necrotising enterocolitis.37 The lack of lysozyme may render these infants susceptible to bacterial translocation and subsequent sepsis syndrome.
Secretory phospholipase A2
sPLA2 is a component of Paneth cell granules27 and is released into the intestinal lumen on stimulation by bacterial products, including lipopolysaccharide.38 sPLA2 purified from murine small intestinal Paneth cells has been shown to have bactericidal activity against Salmonella typhimurium and Listeria monocytogenes, indicating its role in small intestinal mucosal defence.39
Enteric α-defensins—of mice and men
Defensins are small (29–45 amino acids in length) cationic peptides that have been divided into two main families, the α- and β-defensins, on the basis of the disulphide bond pairing pattern.40,41 The defensins are synthesised as precursor polypeptides that are post-translationally processed into mature active peptides. The antimicrobial activity of these peptides is believed to be due to the formation of a membrane spanning pore that eventually leads to lysis of the bacterium.
In humans, four neutrophil defensins (HNP-1, -2, -3, -4) were identified first, followed by two enteric α-defensins (HD-5, -6), expression of which is normally restricted to Paneth cells in the small intestine. Enteric α-defensins have been well characterised in murine Paneth cells and are termed cryptdins (“crypt defensins”). In contrast with enteric α-defensins, human β-defensin 1 (HBD-1) and other members of the β-defensin family appear to be expressed by most epithelial cells of the small and large intestine. HBD-1 is expressed constitutively while HBD-2 is induced by stimuli that activate the transcriptional factor NFκB.41
Defensins may have multiple roles, dependent on concentration. Murine cryptdins reach concentrations of 15–100 mg/ml in the crypt microenvironment.29 These concentrations are at least 1000 times higher than minimal bactericidal concentrations in vitro, indicating the potential for α-defensins to maintain sterility in the crypt and protect the stem cells. It is plausible that lower concentrations of α-defensins that reach the small intestinal lumen have an appreciable effect on resident microflora. In keeping with this, mice deficient in the processing enzyme matrilysin that do not express active cryptdins are susceptible to small intestinal colonisation by non-invasive Escherichia coli species.42
Cryptdin 2 and 3 have also been shown to form pores in intestinal epithelial cells and to lead to a chloride secretory response,43 which would be expected to flush the epithelial surface of bacteria. More recently, cryptdin 3 (but not the non-pore forming cryptdin 4) has been reported to induce expression of the potent polymorphonuclear chemoattractant interleukin 8 via calcium dependent activation of p38 mitogen activated protein kinase (MAPK) and NFκB signalling pathways.44 These studies suggest that in addition to antimicrobial activity, some enteric α-defensins are capable of inducing biological effects in epithelial cells to enhance mucosal protection against luminal micro-organisms.
Cryptdin related sequence (CRS) peptides
CRS peptides have recently been described as a group of highly potent antimicrobial peptides in mice.34 They are a family of covalently linked dimeric antimicrobial molecules, a feature that might contribute to their diversity and potentiate efficient protection of the intestinal mucosa. They are processed in a similar way to cryptdins, with highly similar pro-regions of the peptide, with two possible processing sites for matrilysin (enzyme that processed cryptdin precursors, see below). CRS peptides have also been shown to bind and reduce the immunostimulatory activity of LPS.34
Angiogenins
Angiogenins have recently been established as a family of endogenous antimicrobial proteins.35 Although implicated in the growth of human tumour cells in mice, human angiogenin has been shown to exhibit microbicidal activity against systemic bacterial and fungal pathogens. A likely role for this class of microbicidal proteins in the defence of the intestinal mucosa has been demonstrated by expression of angiogenin 4 by mouse Paneth cells.35 Bacterial products stimulate the release of the microbicidal angiogenin 4 from Paneth cell granules into the gut lumen.
Paneth cell: bacterial interactions
Paneth cells respond to bacterial stimulation by releasing antimicrobial peptides/proteins stored within granules into the crypt lumen. Of these antimicrobial peptides, α-defensins have been the most thoroughly studied.
Mouse α-defensins
Enteric α-defensins (cryptdins) from mouse small intestinal Paneth cells were the first non-leucocyte defensins to be identified,45 and six have now been characterised and found to exhibit broad spectrum antimicrobial activity. They are active against E coli, Listeria monocytogenes, Staphylococcus aureus, and Giardia lamblia, although individual cryptdins exhibit antimicrobial activity of varying range and potency.46 Antibody to cryptdins 1, 2, 3, and 6 has been shown in vitro to neutralise 70% of the bactericidal activity secreted by mouse crypts, indicating the importance of α-defensins in mucosal defence.29
The mechanisms by which precursor forms of cryptdins are processed into mature active peptides have recently been characterised. Paneth cell granules in the mouse have been found to contain matrilysin (MMP-7), a matrix metalloproteinase enzyme which cleaves the propeptide so that Paneth cell degranulation releases active defensin into the crypt lumen.42 Prosegment and mature cryptdins have been demonstrated in Paneth cell granules, and analyses suggest that the majority of procryptdin is processed by MMP-7 within the granules.29 The in vivo importance of cryptdin activation has been shown by MMP-7 null mice, which accumulate procryptdin in their Paneth cell granules and are more susceptible to oral administration of the enteric pathogen Salmonella typhimurium.42
Inhibition of Paneth cell cryptdin and lysozyme expression has been reported after oral inoculation of mice with wild-type Salmonella typhimurium.47 Studies using heat killed and mutant S typhimurium showed that direct interactions between live wild-type bacteria and epithelial cells are required to inhibit cryptdin expression. Moreover, it was demonstrated that the epithelial cell responses are mediated via the p38 MAPK pathway. The effect of S typhimurium on cryptdin and lysozyme expression appears to be pathogen specific as Listeria monocytogenes, which also invades murine (and human) small intestine, did not have any effect.
Human enteric α-defensins
Whereas six enteric α-defensins have been characterised in mice, only two (HD-5 and HD-6) have been identified in humans.48 These, like cryptdins, are predominantly expressed in Paneth cells of the small intestine, and HD-5 has been isolated from ileal tissue and characterised.26,49 Recombinant HD-5 has been shown to have a wide spectrum of killing activity, being active against both Gram positive and Gram negative bacteria as well as fungi (Listeria monocytogenes, Escherichia coli, Salmonella typhimurium, and Candida albicans).50,51
The important contribution of human α-defensins to enteric mucosal immunity has been demonstrated by a recent in vivo study in which transgenic mice expressing HD-5 in small intestinal Paneth cells profoundly influenced mucosal protection against virulent Salmonella typhimurium.52 This was manifest by a reduction in the bacterial burden in the intestinal lumen and faeces, decreased bacterial translocation to the spleen, and higher survival rates after lethal Salmonella challenge.52 However, HD-5 transgenic mice did not have a survival advantage when intraperitoneally inoculated with Salmonella, indicating that the effect of HD-5 is mediated in the intestinal lumen. The mechanism of increased intestinal defence against Salmonella in the HD-5-transgenic mice is not clear, as HD-5 and mouse cryptdins have similar in vitro activity against this pathogen.
In contrast with mice, human Paneth cell granules contain only the proform of HD-5 (amino acids 20–94; fig 5 ▶), and they do not contain matrilysin.26,49 In vitro, pro-HD-5 can be processed to the mature form (amino acids 63–94) by trypsin, which together with α1-antitrypsin and pancreatic secretory trypsin inhibitor (Kazal-type trypsin inhibitor) are expressed in Paneth cells.51,53,54 Analysis of terminal ileal fluid taken at endoscopy has confirmed that the predominant in vivo form of luminal HD-5 is indeed the mature (amino acids 63–94) peptide predicted by in vitro trypsin cleavage.51 Luminal aspirates also yielded a slightly longer HD-5 form (amino acids 56–94). Of note, both of these isoforms are C terminal to an arginine residue, which is typical of trypsin cleavage. It has therefore been proposed that following secretion by Paneth cells, enzymatically active trypsin processes pro-HD-5 to the mature form in vivo.51
Figure 5.
Amino acid sequence of human defensin (HD)-5. Precursor form of HD-5 (amino acids 20–94) is stored in Paneth cell granules, and has been shown to be processed to three predominant forms (amino acids 63–94, 56–94, 36–94) during and/or after release into the lumen (see text for details).
Key points: enteric α-defensins.
Two forms exist in humans: HD-5 and HD-6
Secreted into the crypt lumen by Paneth cells
Selectively attack microbial cell membranes
Mouse defensin isoforms provide 70% of crypt antimicrobial activity
Transgenic mice expressing HD-5 are protected against orally administered virulent Salmonella typhimurium.
However, a further luminal form of HD-5 has been isolated from ileal neobladder urine.49 In these subjects, after radical cystectomy for transitional cell carcinoma of the bladder, an ileal neobladder was fashioned. Enteric defensins were then isolated from urine. The 56–94 and 63–94 mature HD-5 forms found in ileal aspirates were also found in neobladder urine, but a longer form, 36–94, was also present. This form has been shortened from pro-HD-5 (20–94) but not at a trypsin cleavage site. This same form (36–94) was also found in vitro when isolated small intestinal crypts were stimulated with either the cholinergic agonist carbamyl choline or bacterial lipopolysaccharide.26 It may therefore be that alternative mechanisms, or additional steps, for the processing of pro-HD-5 to the mature form exist.
Paneth cell: parasite interactions
Paneth cell granule depletion has been observed in subjects with human immunodeficiency virus related cryptosporidiosis55 and mice infected with the nematode Trichinella spiralis28 but the mechanism remains to be determined. In parasite infected mice, there is also an increase in the number of Paneth cells (fig 3 ▶), which is maximal around the time of worm expulsion.28,56 Transfer experiments in T cell deficient mice suggest that mucosal T cells play an important role in inducing an increase in the number of Paneth cells in parasite infected mice.28 Expression profiling studies in the small intestine of T spiralis infected mice have shown that Paneth cell specific cryptdins are strongly represented.56
Paneth cells: a link with inflammatory bowel disease
Metaplastic Paneth cells in colitis
Although in the normal gastrointestinal tract Paneth cells are confined to the small intestine, they can appear in aberrant sites in various disease states. Expression of small intestinal epithelium elsewhere in the gastrointestinal tract is termed intestinal metaplasia, and the term complete metaplasia is used if Paneth cells are present.57 Formation of intestinal metaplasia is usually preceded by chronic inflammation—such as in Barrett’s oesophagus or in the stomach following chronic Helicobacter pylori infection.58 Paneth cell metaplasia occurs in chronic inflammatory conditions of the colon, most notably ulcerative colitis and colonic Crohn’s disease,26 but also diverticulitis59 and radiation colitis.60 Metaplastic Paneth cells seen in inflammatory bowel disease colitis are usually seen in the crypt region, and are morphologically identical to small intestinal Paneth cells.26 Immunohistochemical studies indicate that they express the antimicrobial proteins lysozyme,61 sPLA2,62 and α-defensins.26 It is likely that in the colon affected by inflammatory bowel disease, metaplastic Paneth cells help protect the damaged colonic epithelium against bacterial invasion.
NOD2 protein is expressed in Paneth cells
NOD2 protein is a cytosolic PRR of the innate immune system that recognises muramyl dipeptide, a constituent of peptidoglycan which is present in bacterial cell walls.63 Mutations in the NOD2 gene, on chromosome 16, have been associated with Crohn’s disease.17,18 They lead to a deficiency in the gene product to sense muramyl dipeptide.64 Three main mutations in this gene account for 80% of the total mutations associated with Crohn’s disease, and 25–43% of Caucasian Crohn’s patients carry at least one of these mutations.65,66 Two of these single nucleotide polymorphisms result in single amino acid changes (R702W, G908R), and the third a frameshift mutation leading to a truncated protein (1007 fs). All mutations affect the C terminal leucine-rich repeat, or muramyl dipeptide receptor, domain. Genotype-phenotype correlations have demonstrated that NOD2 mutations are associated with ileal disease, a tendency to develop strictures, and with a younger age at onset.67
As the NOD2 gene has recently been shown to be expressed in the cytoplasm of Paneth cells,32,33 there is considerable current interest in a possible link between the presence of “defective” NOD2 protein in Paneth cells and the development of small bowel Crohn’s disease. It may be that defective recognition of bacterial products via NOD2 leads to dysregulation of Paneth cell mediated responses against intestinal bacteria in Crohn’s disease. This reduced bacterial sensing may lead to an increased susceptibility of the intestinal mucosa to invasion by luminal bacteria, and to the development of chronic inflammation.
NOD2 protein-antimicrobial peptides: is there a link within Paneth cells?
Altered expression of antimicrobial peptides secondary to expression of the mutant NOD2 gene in Paneth cells could lead to abnormal colonisation of the small bowel with microbes, which may provoke chronic inflammation via adaptive immune mechanisms.
There is some recent evidence of a link between the NOD2 gene and expression of α-defensins in Paneth cells. NOD2 deficient (NOD2−/−) mice have been found to show reduced Paneth cell expression of transcripts of the enteric α-defensins cryptdin 4 and cryptdin 10 sequence and there was further reduction in expression of these transcripts after intragastric infection with the Gram positive intracellular bacterium Listeria monocytogenes.68 Interestingly, NOD2−/− mice are outwardly healthy and display no overt symptoms or histological evidence of intestinal inflammation when observed for up to six months.68,69 However, when challenged with L monocytogenes via the intragastric route, NOD2−/− mice had greater susceptibility to infection, as shown by significantly greater numbers of bacteria recovered from the liver and spleen compared with wild-type controls. In contrast, NOD2−/− mice showed no difference to wild-type mice when inoculated with L monocytogenes, either by intravenous or intraperitoneal injection, indicating that NOD2 may play a key role in mediating protection of the intestinal mucosa against bacterial infection.
Key points: NOD2 protein.
Mutations in NOD2 are associated with Crohn’s ileitis
NOD2 is found in Paneth cells, which are prominent in the ileum
Paneth cells release α-defensins to maintain crypt sterility
α-Defensin expression may be reduced in Crohn’s ileitis
Expression of enteric α-defensin mRNA has recently been shown to be reduced in terminal ileal biopsies from patients with Crohn’s disease, with reduced expression being more pronounced in the presence of NOD2 mutations.70 Although this result adds further credence to the hypothesis that Crohn’s disease may be associated with a reduction in mucosal innate immunity, there is much yet to be explained. For example, the mechanism by which NOD2 may regulate expression of Paneth cell α-defensins remains to be determined.
A number of recent studies have reported the effect of NOD2 mutations in responses by cells other than Paneth cells but are not considered further as they are beyond the scope of this article.
SUMMARY
A variety of mechanisms are needed to mediate host protection against micro-organisms in the intestine prior to induction of highly specific adaptive immune responses. Recent studies suggest that Paneth cells play an important role in innate host defence via their ability to secrete antimicrobial peptides and proteins. There is considerable current interest in host and microbial factors that may regulate Paneth cell function. As they are able to respond to components of the commensal microbial flora, there is also significant current interest in the contribution of these cells to the pathogenesis of inflammatory bowel disease.
Conflict of interest: None declared.
REFERENCES
- 1.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest 2000;105:1493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Justesen T, Nielsen OH, Jacobsen IE, et al. The normal cultivable microflora in upper jejunal fluid in healthy adults. Scand J Gastroenterol 1984;19:279–82. [PubMed] [Google Scholar]
- 3.Smith G, Gorbach S. Normal alimentary tract flora. In: Blaser M, Smith P, Ravidin J, eds. Infections of the gastrointestinal tract. New York: Raven Press, 1995:53–69.
- 4.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med 2000;343:338–44. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Pabst R. Let’s go mucosal: communication on slippery ground. Trends Immunol 2004;25:570–7. [DOI] [PubMed] [Google Scholar]
- 6.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev 2003;195:58–71. [DOI] [PubMed] [Google Scholar]
- 7.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol 2003;3:63–72. [DOI] [PubMed] [Google Scholar]
- 8.Fernie-King B, Seilly DJ, Davies A, et al. Subversion of the innate immune response by micro-organisms. Ann Rheum Dis 2002;61 (suppl 2) :ii8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science 1996;272:50–3. [DOI] [PubMed] [Google Scholar]
- 10.Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 2004;41:1099–108. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol 2005;17:1–14. [DOI] [PubMed] [Google Scholar]
- 12.Rock FL, Hardiman G, Timans JC, et al. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA 1998;95:588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–8. [DOI] [PubMed] [Google Scholar]
- 14.Mahida YR, Johal S. NF-kappa B may determine whether epithelial cell-microbial interactions in the intestine are hostile or friendly. Clin Exp Immunol 2001;123:347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamaillard M, Girardin SE, Viala J, et al. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol 2003;5:581–92. [DOI] [PubMed] [Google Scholar]
- 16.Ogura Y, Inohara N, Benito A, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappa B. J Biol Chem 2001;276:4812–18. [DOI] [PubMed] [Google Scholar]
- 17.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001;411:599–603. [DOI] [PubMed] [Google Scholar]
- 18.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411:603–6. [DOI] [PubMed] [Google Scholar]
- 19.Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet 2001;29:19–20. [DOI] [PubMed] [Google Scholar]
- 20.Paneth J. Uber Die Secernierenden Zellen das Dunndarm-Epithels. Archiv fur mikroskepisch anatomie 1888;31:113–92. [Google Scholar]
- 21.Bry L, Falk P, Huttner K, et al. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc Natl Acad Sci USA 1994;91:10335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng H. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. IV. Paneth cells. Am J Anat 1974;141:521–35. [DOI] [PubMed] [Google Scholar]
- 23.Ouellette AJ, Satchell DP, Hsieh MM, et al. Characterization of luminal Paneth cell alpha-defensins in mouse small intestine: Attenuated antimicrobial activities of peptides with truncated amino termini. J Biol Chem 2000;275:33969–73. [DOI] [PubMed] [Google Scholar]
- 24.Lendrum A. The phloxine-tartrazine method as a general histological stain and for the demonstration of inclusion bodies. J Pathol Bacteriol 1947;59:399–404. [Google Scholar]
- 25.Erlandsen SL, Parsons JA, Taylor TD. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J Histochem Cytochem 1974;22:401–13. [DOI] [PubMed] [Google Scholar]
- 26.Cunliffe RN, Rose FR, Keyte J, et al. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 2001;48:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nevalainen TJ, Gronroos JM, Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Invest 1995;72:201–8. [PubMed] [Google Scholar]
- 28.Kamal M, Wakelin D, Ouellette AJ, et al. Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T. spiralis-infected mice. Clin Exp Immunol 2001;126:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayabe T, Satchell DP, Wilson CL, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 2000;1:113–18. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe H, Ayabe T, Bainbridge B, et al. Mouse paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect Immun 2005;73:2312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumio C, Besusso D, Palazzo M, et al. Degranulation of paneth cells via toll-like receptor 9. Am J Pathol 2004;165:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lala S, Ogura Y, Osborne C, et al. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology 2003;125:47–57. [DOI] [PubMed] [Google Scholar]
- 33.Ogura Y, Lala S, Xin W, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 2003;52:1591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornef MW, Putsep K, Karlsson J, et al. Increased diversity of intestinal antimicrobial peptides by covalent dimer formation. Nat Immunol 2004;5:836–43. [DOI] [PubMed] [Google Scholar]
- 35.Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat Immunol 2003;4:269–73. [DOI] [PubMed] [Google Scholar]
- 36.Klockars M, Reitamo S. Tissue distribution of lysozyme in man. J Histochem Cytochem 1975;23:932–40. [DOI] [PubMed] [Google Scholar]
- 37.Coutinho HB, Carmona da Mota H, Coutinho VB, et al. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. J Clin Pathol 1998;51:512–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harwig SSL, Tan L, Qu XD, et al. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest 1995;95:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu XD, Lloyd KC, Walsh JH, et al. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect Immun 1996;64:5161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouellette AJ, Bevins CL. Paneth cell defensins and innate immunity of the small bowel. Inflamm Bowel Dis 2001;7:43–50. [DOI] [PubMed] [Google Scholar]
- 41.Cunliffe RN, Mahida YR. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J Leukoc Biol 2004;75:49–58. [DOI] [PubMed] [Google Scholar]
- 42.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999;286:113–7. [DOI] [PubMed] [Google Scholar]
- 43.Lencer WI, Cheung G, Strohmeier GR, et al. Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proc Natl Acad Sci USA 1997;94:8585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin PW, Simon PO Jr, Gewirtz AT, et al. Paneth cell cryptdins act in vitro as apical paracrine regulators of the innate inflammatory response. J Biol Chem 2004;279:19902–7. [DOI] [PubMed] [Google Scholar]
- 45.Ouellette AJ, Greco RM, James M, et al. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol 1989;108:1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouellette AJ, Hsieh MM, Nosek MT, et al. Mouse Paneth cell defensins: Primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun 1994;62:5040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salzman NH, Chou MM, De Jong H, et al. Enteric Salmonella infection inhibits paneth cell antimicrobial peptide expression. Infect Immun 2003;71:1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 1992;267:23216–25. [PubMed] [Google Scholar]
- 49.Porter EM, Poles MA, Lee JS, et al. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Letters 1998;434:272–6. [DOI] [PubMed] [Google Scholar]
- 50.Porter EM, Van Dam E, Valore EV, et al. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun 1997;65:2396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghosh D, Porter E, Shen B, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol 2002;3:583–90. [DOI] [PubMed] [Google Scholar]
- 52.Salzman NH, Ghosh D, Huttner KM, et al. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003;422:522–6. [DOI] [PubMed] [Google Scholar]
- 53.Bohe M, Borgstrom A, Lindstrom C, et al. Pancreatic endoproteases and pancreatic secretory trypsin inhibitor immunoreactivity in human Paneth cells. J Clin Pathol 1986;39:786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molmenti EP, Perlmutter DH, Rubin DC. Cell-specific expression of alpha1-antitrypsin in human intestinal epithelium. J Clin Invest 1993;92:2022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly P, Feakins R, Domizio P, et al. Paneth cell granule depletion in the human small intestine under infective and nutritional stress. Clin Exp Immunol 2004;135:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knight PA, Pemberton AD, Robertson KA, et al. Expression profiling reveals novel innate and inflammatory responses in the jejunal epithelial compartment during infection with Trichinella spiralis. Infect Immun 2004;72:6076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong WM, Stamp GWH, Elia G, et al. Proliferative populations in intestinal metaplasia: Evidence of deregulation in Paneth and goblet cells, but not endocrine cells. J Pathol 2000;190:107–13. [DOI] [PubMed] [Google Scholar]
- 58.Smith VC, Genta RM. Role of Helicobacter pylori gastritis in gastric atrophy, intestinal metaplasia, and gastric neoplasia. Microsc Res Tech 2000;48:313–20. [DOI] [PubMed] [Google Scholar]
- 59.Sandow MJ, Whitehead R. The Paneth cell. Gut 1979;20:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe H. Experimentally induced intestinal metaplasia in Wistar rats by X-ray irradiation. Gastroenterology 1978;75:796–9. [PubMed] [Google Scholar]
- 61.Klockars M, Reitamo S, Reitamo JJ, et al. Immunohistochemical identification of lysozyme in intestinal lesions in ulcerative colitis and Crohn’s disease. Gut 1977;18:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haapamaki MM, Gronroos JM, Nurmi H, et al. Gene expression of group II phospholipase A2 in intestine in ulcerative colitis. Gut 1997;40:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003;278:8869–72. [DOI] [PubMed] [Google Scholar]
- 64.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 2003;278:5509–12. [DOI] [PubMed] [Google Scholar]
- 65.Cuthbert AP, Fisher SA, Mirza MM, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology 2002;122:867–74. [DOI] [PubMed] [Google Scholar]
- 66.Lesage S, Zouali H, Cezard JP, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet 2002;70:845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmad T, Armuzzi A, Bunce M, et al. The molecular classification of the clinical manifestations of Crohn’s disease. Gastroenterology 2002;122:854–66. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005;307:731–4. [DOI] [PubMed] [Google Scholar]
- 69.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol 2003;23:7531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 2004;53:1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]