Abstract
Background and aims: Major liver resection incurs a risk of postoperative liver dysfunction and infection and there is a lack of objective evidence relating residual liver volume to these complications.
Patients and methods: Liver volumetry was performed on computer models derived from computed tomography (CT) angioportograms of 104 patients with normal synthetic liver function scheduled for liver resection. Relative residual liver volume (%RLV) was calculated as the relation of residual to total functional liver volume and related to postoperative hepatic dysfunction and infection. Receiver operator characteristic curve analysis was undertaken to determine the critical %RLV predicting severe hepatic dysfunction and infection. Univariate analysis and multivariate logistic regression analysis were performed to delineate perioperative predictors of severe hepatic dysfunction and infection.
Results: The incidence of severe hepatic dysfunction and infection following liver resection increased significantly with smaller %RLV. A critical %RLV of 26.6% was identified as associated with severe hepatic dysfunction (p<0.0001). Additionally, body mass index (BMI), operating time, and intraoperative blood loss were significant prognostic indicators for severe hepatic dysfunction. It was not possible to predict the individual risk of postoperative infection precisely by %RLV. However, in patients undergoing major liver resection, infection was significantly more common in those who developed postoperative severe hepatic dysfunction compared with those who did not (p = 0.030).
Conclusions: The likelihood of severe hepatic dysfunction following liver resection can be predicted by a small %RLV and a high BMI whereas postoperative infection is more related to liver dysfunction than precise residual liver volume. Understanding the relationship between liver volume and synthetic and immune function is the key to improving the safety of major liver resection.
Keywords: image analysis, innate immunity, liver function, liver surgery
Liver resection of primary and secondary malignancies has becoming increasingly important in recent decades.1–5 Based on promising survival results and a perioperative mortality rate of <5%, the frontiers of liver surgery are extending continuously towards more major liver resections leaving smaller fractions of residual liver.6–8 At the same time, a significant increase in postoperative morbidity due to hepatic dysfunction and infectious complications following extended liver resection has been reported, even by very specialised centres.7,9,10 The paradigm that at least a third of healthy liver tissue should be left to avoid hepatic failure following resection was developed long ago but few data exist to support this arbitrary value in patients with otherwise healthy livers. The expansion of major liver surgery as a treatment option for various liver tumours has presented new challenges to surgeons and physicians in terms of the assessment and management of postoperative complications, particularly those involving hepatic insufficiency and susceptibility to infection.
The liver contains the largest reserve of fixed tissue macrophages in the body (Kupffer cells) and regulates the synthesis of hepatic proteins responsible for recognition and opsonisation of pathogens as part of the innate immune system.11 It was shown previously that innate immunity is significantly impaired in acute and chronic liver failure and after liver surgery, suggesting a link between changes in innate immune response and postoperative infection following major liver resection.12–17 The contribution of the liver to maintain various pathways of innate immunity and its relation to liver volume and global hepatic function after liver resection have hitherto not been explored.
Measurement of total and partial liver volumes based on computed tomography (CT) and magnetic resonance image analysis has become popular to estimate actual graft size before resection in living related liver transplantation.18–20 Some studies have addressed the predictive value of residual liver volume regarding liver function and complications after major liver resection.21,22 However, most of this work has been done in patients with chronic liver disease.23–26 We have shown previously that preoperative estimation of residual liver volume by CT angioportography (CTAP) image based volumetry provides accurate measures of the actual amount of hepatic parenchyma left after liver resection.27 The aim of the present study was to use three dimensional hepatic volumetry and virtual resection to define the critical residual liver volume associated with the development of hepatic dysfunction in patients with a healthy liver undergoing liver resection. Furthermore, we wished to establish whether there is a relationship between residual liver volume, liver function, and the incidence of postoperative infection.
PATIENTS AND METHODS
Patients and surgical technique
Volumetric analysis of the liver was performed in patients who were admitted to the Hepatobiliary Unit, Royal Infirmary Edinburgh, for a liver resection and had CTAP done as part of their preoperative assessment. No patient had any background of chronic liver disease and all gave written informed consent. Selection of patients for surgery and the technique of hepatic resection have been described previously.28 Briefly, surgery for liver tumours was based on segment oriented anatomical resection.29 With the anaesthetist maintaining a low central venous pressure (3–5 cmH2O), liver transection was performed using a Cavitron ultrasonic surgical aspirator (CUSA System 200 Macrodissector; Cavitron Surgical Systems, Stamford, Connecticut, USA) without the need for extrahepatic vascular occlusion. The resected liver surface was sealed haemostatically using argon beam coagulation (Force GSU System; Valleylab, Boulder, Colorado, USA). The extent of liver resection was defined according to the number of liver segments removed and grouped into extended (five or more segments), standard (three or four segments), and minor (one or two segments or wedge resection) resections.
Liver volumetry and calculation of relative residual liver volume
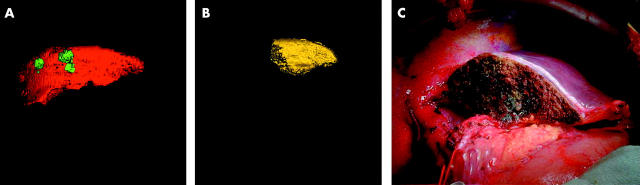
Liver volumetry was performed on sets of axial images which were obtained preoperatively during CTAP. The majority of images were taken from a spiral CT GE Hispeed Advantage scanner (General Electric Medical Systems, Milwaukee, Wisconsin, USA) using an image slice thickness of 7 mm. In the latest phase of the study, 1 mm image slices were obtained from a multi-slice CT Toshiba Aquilion 16 scanner (Toshiba Medical Systems, California, USA). Medical image analysis software GE Advantage Windows and Analyze 5.0 (Analyze Direct, Inc; Biomedical Imaging Resource, Mayo Foundation, Rochester, USA) were used for volumetry. Every slice from the GE scanner and every fifth slice from the Toshiba scanner were analysed in the following way. The outline of the region of interest was traced manually in each image section, excluding the gall bladder, retro hepatic vena cava, and main branches of the intrahepatic vascular structures. An automated process stacked all slices together to build a virtual model of the liver. Volumetric values were obtained by the inherent software volume rendering algorithm. Total liver volume (TLV) and tumour volume (TuV) were measured and total functional liver volume (TFLV) was calculated by subtracting tumour volume from total liver volume (TFLV = TLV − TuV) (fig 1A ▶). The model of the whole liver was then subjected to virtual hepatic resection according to the operative strategy for each individual patient and the volume of segments to be resected, and residual liver volume (RLV) was measured (fig 1B ▶, C). Relative residual liver volume (%RLV) was expressed as a percentage of TFLV. When the type of resection actually performed was different from that estimated preoperatively, volumetric analysis was repeated.
Figure 1.
(A–C) Three dimensional liver volumetry of an extended right hepatectomy with caudate excision. (A) Total liver volume (TLV = 1133 ml; red colour); tumour volume (TuV = 34 ml; green colour); and total functional liver volume (TFLV = 1099 ml). (B) Residual liver volume (RLV = 293 ml yellow colour) from three dimensional liver model after virtual resection; relative residual liver volume (%RLV = 26.7%). (C) Intraoperative view of the residual liver following resection.
Definition of postoperative complications, hepatic dysfunction, inflammation and infection, and body mass index
Postoperative complications included surgical complications (bleeding from the surgical site and bile leak), hepatic dysfunction, cardiovascular, respiratory, and renal system dysfunction, and infection. These complications were assessed on a daily basis starting from the day of surgery until discharge. Readmission due to problems related to the previous operation was also included. The definition of postoperative hepatic dysfunction was based on serum concentrations of total bilirubin and lactate, prothrombin time, and signs of encephalopathy, categorised into four grades (none/mild/moderate/severe) (table 1 ▶).7,30,31 No, 1, or 2 points were given for each parameter according to the actual results, and summation of all provided the actual score. Parameters were taken into account only when present for two consecutive observations within a 48 hour period. The highest score during the postoperative course determined the severity of hepatic dysfunction (table 1 ▶).
Table 1.
Definition of postoperative hepatic dysfunction based on results from blood tests and clinical observation
| Total serum bilirubin (µmol/l) | ⩽20 | 21–60 | >60 |
| Prothrombin time (seconds above normal) | <4 | 4–6 | >6 |
| Serum lactate (mmol/l) | ⩽1.5 | 1.6–3.5 | >3.5 |
| Encephalopathy grade | No | 1 and 2 | 3 and 4 |
| 0 | 1 | 2 | |
| Severity of hepatic dysfunction | None (0), mild (1–2), moderate (3–4), severe (>4) | ||
The definitions of cardiovascular, respiratory, and renal system dysfunction were taken from two consecutive reports by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conferences.32,33 Clinically significant infections only were taken into account and defined as coincidence of positive microbial culture together with either local or general symptoms of inflammation.32–34 Perioperative death was defined as death within 30 days or during the hospital stay following surgery if this was greater. Severe hepatic dysfunction and infection, but not moderate or mild hepatic dysfunction, had a significant adverse influence on the duration of hospital stay and were chosen as primary end points.
Statistical analysis
Statistical analysis was performed using SPSS 11.0 statistical analysis software (SPSS Inc., Chicago, Illinois, USA). One way between group ANOVA analysis of variance was performed in order to assess differences in %RLV between different types and extent of liver resection and between patients with and without different severities of hepatic dysfunction. The independent sample t test was used to assess differences in %RLV between patients with and without infection. Receiver operator characteristic (ROC) curve analysis was undertaken to identify the value of %RLV in predicting severe hepatic dysfunction and infection with a sensitivity of at least 90% and a specificity of not less than 85%. The positive predictive value of the critical %RLV for severe hepatic dysfunction was calculated for the study group. Univariate analysis of preoperative and intraoperative variables was performed by Pearson χ2 and Fisher’s exact test, respectively, for categorical variables and independent sample t test for continuous variables. Significant variables in univariate analysis were entered simultaneously (forced entry method) into multivariate logistic regression to evaluate their independent predictive value for severe hepatic dysfunction and infection.
RESULTS
Patient demography, diagnosis, and the extent of liver resection
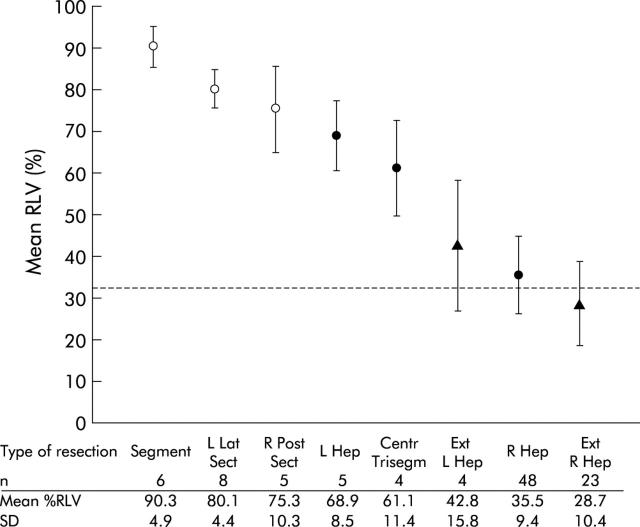
Volumetry of the liver was performed in 104 patients (59 males and 45 females; mean age 61 (SD 12) years) who consecutively underwent liver resection. The diagnosis was colorectal cancer liver metastasis in 92 (88.5%), other secondary malignancies in six (5.8%), adenoma in two (1.9%), and hepatocellular carcinoma, cholangiocarcinoma, haemangiosarcoma, and focal nodular hyperplasia in one (1.0%) patient each. In total, 27 (26.0%) extended resections, 57 (54.8%) standard resections, and 20 (19.2%) minor resections were performed (fig 2 ▶). Mean %RLV was 30.8 (SD 12.1)% after extended liver resection, 40.2 (14.5)% after standard resection, and 82.9 (9.2)% after minor resection (one way between groups ANOVA: p = 0.007 between standard and extended resection; p<0.0001 between minor and standard or extended resection). In 36 of 104 (34.6%) patients, less than 33% RLV was left after resection.
Figure 2.
Mean (SD) relative residual liver volume (%RLV) of different types of extended (Ext R Hep, extended right hepatectomy; Ext L Hep, extended left hepatectomy), standard (R Hep, right hepatectomy; Centr Trisegm, central trisegmentectomy; L Hep, left hepatectomy), and minor (R Post Sect, right posterior sectionectomy; L Lat Sect, left lateral sectionectomy; Segment, segmentectomy) liver resection. Reference line indicates 33% RLV.
Postoperative complications and their relation to residual liver volume
In 54 of 104 (51.9%) patients, one or more complications became evident following liver resection. Thirty three (31.7%) patients developed postoperative infection as the most common cause of complications. Mild, moderate, and severe hepatic dysfunction were evident in 42 (40.4%), 22 (21.2%), and 13 (12.5%) patients. Pleural effusions were found in 11 (10.6%), a bile duct leak in six (5.8%), renal dysfunction in four (3.8%), pulmonary embolism in three (2.9%), temporary atrial fibrillation in three (2.9%), bleeding from the surgical site requiring reoperation in two (1.9%), and portal vein thrombosis and upper gastrointestinal tract bleeding in one (1%) patient each. Two of 104 (1.9%) patients died; both developed liver failure (in one associated with sepsis) following extended liver resection. The incidence of postoperative hepatic dysfunction in general and severe hepatic dysfunction in particular increased with the extent of liver resection (table 2 ▶). Five of 57 (8.8%) patients after standard liver resection and eight of 27 (29.6%) patients after extended liver resection, but none after minor resection, developed severe hepatic dysfunction.
Table 2.
Hepatic dysfunction and infection following minor, standard, and extended liver resection
| Extent of liver resection | |||
| Minor (n = 20) | Standard (n = 57) | Extended (n = 27) | |
| Postoperative hepatic dysfunction**** | |||
| No | 17 (85.0) | 9 (15.8) | 1 (3.7) |
| Mild | 3 (15.0) | 28 (49.1) | 11 (40.7) |
| Moderate | 0 | 15 (26.3) | 7 (25.9) |
| Severe | 0 | 5 (8.8) | 8 (29.6) |
| Infection*** | 3 (15.0) | 14 (24.6) | 16 (59.3) |
***p = 0.001, ****p<0.0001 by Pearson χ2.
Values in parentheses are percentages of patients in each category by extent of liver resection.
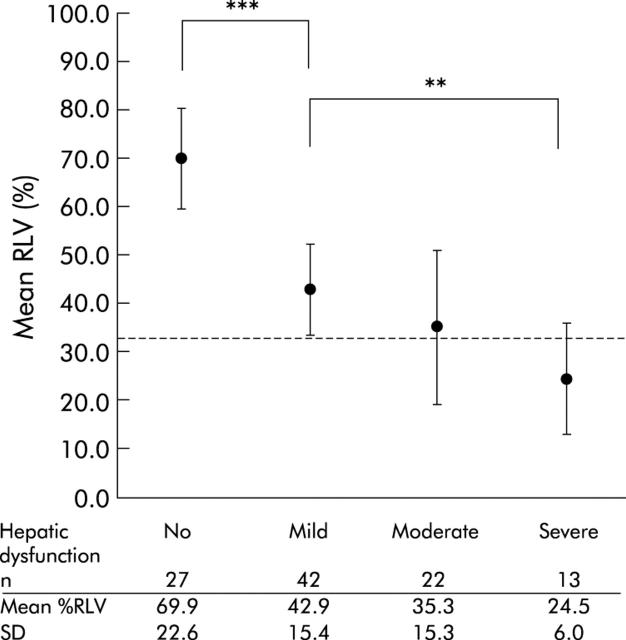
Mean %RLV was significantly smaller in patients who developed mild hepatic dysfunction compared with those without hepatic dysfunction (42.9 (15.4)% and 69.9 (22.6)%; p<0.0001) (fig 3 ▶). Patients who developed severe hepatic dysfunction had a significantly smaller mean %RLV compared with those with mild hepatic dysfunction (24.5 (6.0)% and 42.9 (15.4)%; p = 0.005) (fig 3 ▶). The number of patients who developed postoperative infection increased from minor to standard liver resection and was highest after extended liver resection (table 2 ▶). Mean %RLV was significantly smaller in patients with postoperative infection compared with those without infection (38.8 (18.5)% and 49.3 (23.6)%; independent sample t test, p = 0.016).
Figure 3.
Mean (SD) relative residual liver volume (%RLV) in patients with no, mild, moderate, and severe hepatic dysfunction following liver resection (one way between group ANOVA; **p = 0.005, ***p<0.0001). Reference line indicates 33% RLV.
Receiver operator characteristic (ROC) curve analysis of %RLV to predict severe hepatic dysfunction and infection following liver resection
ROC curve analysis revealed that a %RLV value of 26.6% identified patients at significant risk for severe hepatic dysfunction following liver resection (fig 4A ▶). Using this value it was possible to predict severe hepatic dysfunction in patients undergoing liver resection with 90.9% sensitivity and 87.1% specificity. The positive predictive value for the study group was 50.2%, the likelihood ratio for a positive test result (<26.6% RLV) associated with severe hepatic dysfunction was 7.0, and for a negative test result (⩾26.6% RLV) 0.1. Ten of 22 (45.5%) patients with a %RLV value of <26.6% developed severe hepatic dysfunction compared with one of 82 (1.2%) with a larger %RLV (p<0.0001) (fig 4B ▶). However, 12 of 22 (54.5%) patients with a %RLV value below the critical %RLV did not develop severe hepatic dysfunction. It was not possible to identify a precise %RLV value for predicting postoperative infection by ROC curve analysis (fig 5A ▶). Applying the critical %RLV of 26.6% to predict postoperative infection, sensitivity was 33.3% and specificity 84.5%. The likelihood ratio for a positive test result (<26.6% RLV) associated with postoperative infection was 2.1 and for a negative test result (⩾26.6% RLV) 0.8. Eleven of 22 (50.0%) patients with a %RLV below 26.6% developed postoperative infection compared with 22 of 82 (26.8%) with a larger %RLV (p = 0.069) (fig 5B ▶).
Figure 4.
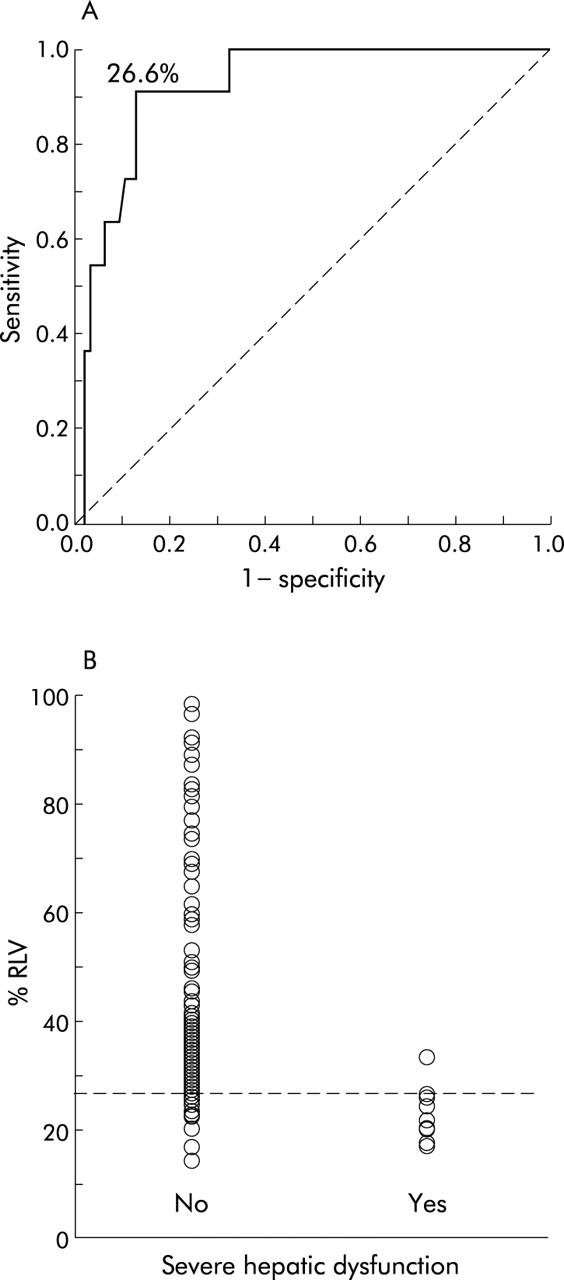
(A) Receiver operator characteristic (ROC) curve analysis of relative residual liver volume (%RLV) to predict postoperative severe hepatic dysfunction. A critical %RLV value of 26.6% was identified (area under the curve = 0.918 (95% confidence interval 0.854–0.983); p<0.0001). (B) Incidence of severe hepatic dysfunction following liver resection according to %RLV. Reference line indicates the critical %RLV of 26.6% associated with a significant risk of postoperative severe hepatic dysfunction (Fisher’s exact test; p<0.0001).
Figure 5.
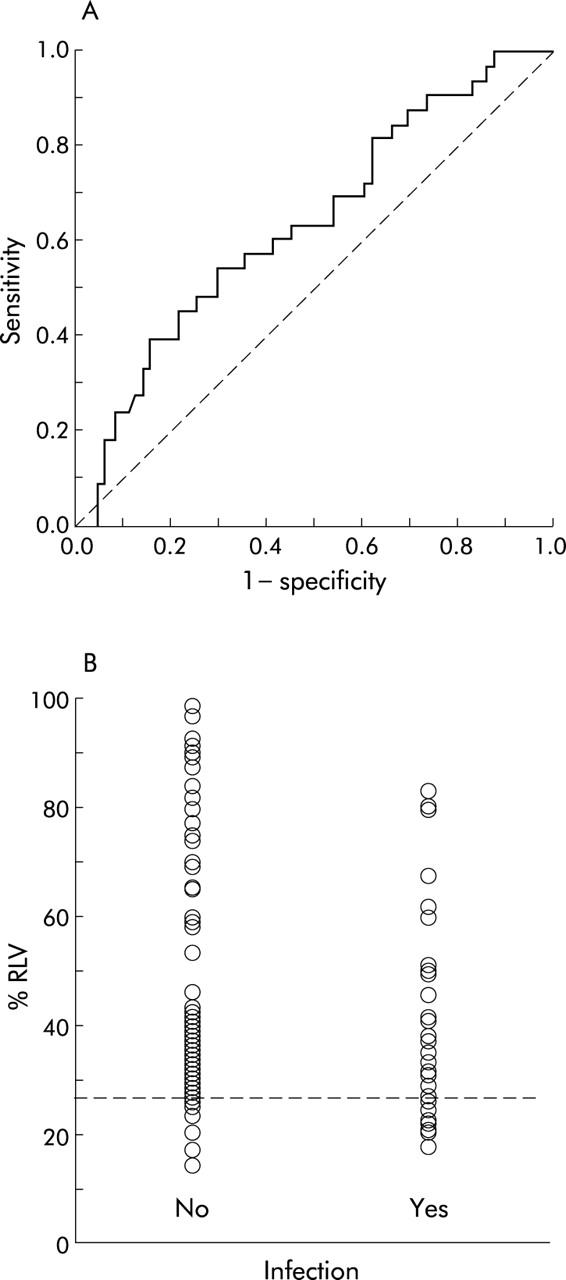
(A) Receiver operator characteristic (ROC) curve analysis of relative residual liver volume (%RLV) to predict postoperative infection. No critical %RLV was identified in predicting infection with precision (area under the curve = 0.641 (95% confidence interval 0.528–0.755); p = 0.021). (B) Incidence of infection following liver resection according to %RLV (Fisher’s exact test, p = 0.069). Reference line indicates 26.6% RLV.
Ability of %RLV in association with other preoperative and intraoperative parameters to predict postoperative severe hepatic dysfunction and infection
Univariate analysis revealed that small %RLV (p<0.0001), high body mass index (BMI) (p<0.0001), extended liver resection (p = 0.007), prolonged operating time (p = 0.001), increased blood loss during surgery (p = 0.007), and perioperative blood transfusion (p = 0.003) were significant predictors of severe hepatic dysfunction following liver resection (tables 3 ▶, 4 ▶). Extended liver resection (p = 0.001), small %RLV (p = 0.016), and prolonged operating time (p = 0.009) showed significant value in predicting postoperative infection (tables 3 ▶, 4 ▶). %RLV together with BMI, operating time, intraoperative blood loss, and perioperative blood transfusion were entered into a logistic regression model to identify variables with independent predictive value for severe hepatic dysfunction and infection. A small %RLV and high BMI were found to be significant independent predictors of severe hepatic dysfunction (table 5 ▶). However, prolonged operating time and large intraoperative blood loss increased the accuracy of this regression model. By applying BMI, operating time, and intraoperative blood loss to the risk assessment of patients with a small %RLV, it became possible to distinguish between patients who developed severe hepatic dysfunction and those who did not (fig 6 ▶). A small %RLV and prolonged operating time were the only two significant independent predictors of postoperative infection (table 5 ▶).
Table 3.
Preoperative and intraoperative categorical predictors for severe hepatic dysfunction and infection
| Severe hepatic dysfunction | p Value | Infection | p Value | ||
| Sex | |||||
| Male | 59 | 9 (15.3) | 0.109* | 22 (37.3) | 0.204* |
| Female | 45 | 2 (4.4) | 11 (24.4) | ||
| Extent of resection | |||||
| Minor | 20 | 0 | 0.007** | 3 (15.0) | 0.001** |
| Standard | 57 | 4 (7.0) | 14 (24.6) | ||
| Extended | 27 | 7 (25.9) | 16 (59.3) | ||
| Hepatic inflow occlusion | |||||
| No | 76 | 9 (11.8) | 1.000* | 25 (32.9) | 1.000* |
| Yes | 16 | 2 (12.5) | 5 (31.3) | ||
| Blood transfusion | |||||
| No | 69 | 3 (4.3) | 0.003* | 17 (24.6) | 0.080* |
| Yes | 25 | 7 (28.0) | 11 (44.0) | ||
*Fisher’s exact test or **Pearson χ2.
Table 4.
Mean (SD) values of preoperative and intraoperative predictors for severe hepatic dysfunction and infection (independent sample t test)
| Severe hepatic dysfunction | Infection | |||||
| Yes | No | p Value | Yes | No | p Value | |
| Age | 59.5 (14.7) | 61.1 (11.9) | 0.681 | 63.5 (8.9) | 59.8 (13.3) | 0.097 |
| BMI | 29.9 (6.1) | 24.6 (4.2) | <0.0001 | 26.2 (4.6) | 24.6 (4.7) | 0.121 |
| %RLV | 23.1 (4.9) | 48.7 (22.3) | <0.0001 | 38.8 (18.5) | 49.3 (23.6) | 0.016 |
| Operating time (min) | 264.6 (31.3) | 211.3 (53.1) | 0.002 | 237.1 (52.2) | 207.5 (52.1) | 0.009 |
| Blood loss (ml) | 2090.7 (1242.2) | 1059.5 (924.2) | 0.007 | 1419.7 (1029.7) | 1025.9 (949.4) | 0.085 |
BMI, body mass index; %RLV, relative residual liver volume.
Table 5.
Multivariate logistic regression analysis of variables to predict severe hepatic dysfunction and infection following liver resection
| B | SE | Wald | p Value | Exp(B) | 95% CI for Exp(B) | |||
| Severe hepatic dysfunction | ||||||||
| %RLV | −0.358 | 0.133 | 7.271 | 0.007 | 0.699 | 0.539–0.907 | ||
| BMI | 0.280 | 0.139 | 4.056 | 0.044 | 1.323 | 1.008–1.738 | ||
| Blood loss | 0.001 | 0.001 | 2.194 | 0.139 | 1.001 | 1.000–1.002 | ||
| Operating time | −0.008 | 0.013 | 0.386 | 0.534 | 0.992 | 0.967–1.018 | ||
| Infection | ||||||||
| %RLV | −0.029 | 0.010 | 9.282 | 0.002 | 0.971 | 0.953–0.990 | ||
| Operating time | 0.003 | 0.002 | 2.075 | 0.150 | 1.003 | 0.999–1.006 | ||
BMI, body mass index; %RLV, relative residual liver volume.
Figure 6.
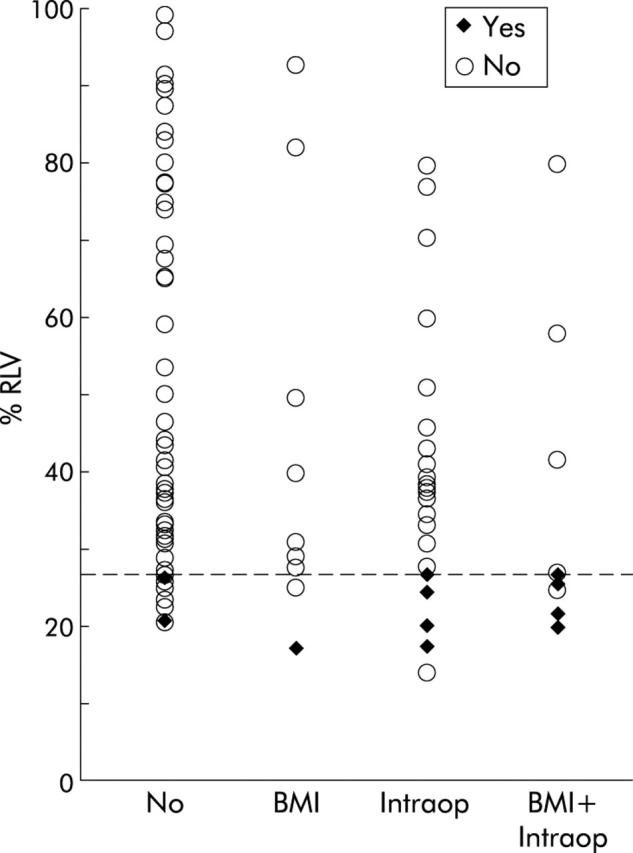
Incidence of severe hepatic dysfunction following liver resection in relation to relative residual liver volume (%RLV) and the presence of the additional risk factors body mass index >30 (BMI), operating time >240 minutes and/or blood loss >2000 ml (Intraop), or both (BMI+Intraop). Reference line indicates the critical %RLV of 26.6%.
Incidence of infection in patients with and without postoperative severe hepatic dysfunction
In order to analyse the relation between impaired liver function and susceptibility to infection, we compared the incidence of postoperative infection between patients who developed postoperative severe hepatic dysfunction and those who did not. The analysis was limited to patients with less than 26.6% RLV because severe hepatic dysfunction was almost exclusively seen in this group. Eight of 11 (72.7%) patients with postoperative severe hepatic dysfunction developed infection whereas this was the case in two of 11 (18.2%) without severe hepatic dysfunction (p = 0.030) (table 6 ▶).
Table 6.
Relation of severe hepatic dysfunction and infection following major liver resection in patients with a small (<26.6%) residual liver volume
| Severe hepatic dysfunction (%) | |||
| No | Yes | ||
| 11 | 11 | ||
| Infection | No | 9 (81.8) | 3 (27.3) |
| Yes | 2 (18.2) | 8 (72.7) | |
Fisher’s exact test; p = 0.030.
DISCUSSION
Liver resection is still accompanied by a certain risk of postoperative complications and the overall incidence of complications is significantly increasing towards extended liver resections.7,23 Hepatic dysfunction and infection are the two most common conditions necessitating prolonged treatment and hospital stay following liver resection.7,30,35,36 One key to improve the safety of liver resection is to understand the relationship between liver volume and function. There is a lack of evidence to support the assumption that at least one third of a healthy liver should be left to avoid significant hepatic dysfunction, and adequacy of RLV in the past has been based largely on guesswork or crude measures rather than precise measurements. Similarly, the relation between small residual liver, liver dysfunction, and postoperative infection has not been defined.
This study aimed to identify the critical residual liver volume able to predict postoperative severe hepatic dysfunction and to investigate the relationship between small residual liver and postoperative infection. We studied only patients without chronic liver disease to enable an estimate of the maximum capacity of the healthy liver before hepatic dysfunction or infection supervene. An accurate and validated technique of virtual resection in three dimensional liver models was used to calculate the precise volumes associated with liver resection.27 By subtracting tumour volume from total liver volume, the percentage of RLV in relation to functional liver volume rather than total volume was calculated, thus taking into account the extent of hepatic replacement by large tumours that clearly would not contribute to the functional liver volume. Using this approach, we have demonstrated that volumetric image analysis provides more precise information about the amount of liver tissue left after resection compared with other estimates based on either the type of resection or number of liver segments removed.
There is no consensus on how to define hepatic dysfunction after liver resection and several studies have used different estimates of liver function to describe hepatic functional impairment.21–23,37 Some of the variables used to define postoperative hepatic dysfunction, namely alanine aminotransferase, gamma-glutamyl transferase, and alkaline phosphatase, are influenced by the surgical insult to and regeneration of the remaining liver rather than reflecting hepatic function. For the purpose of this study, a novel scoring system of hepatic dysfunction following liver resection was introduced which was derived from routine blood tests and clinical observations. We found a good correlation between liver dysfunction scores and %RLV, and a critical minimum %RLV of 26.6% was identified below which serious hepatic dysfunction is likely to occur. According to our findings, this is the first study using ROC curve analysis to define a precise %RLV that is able to predict the individual likelihood of postoperative severe hepatic dysfunction. Two other studies reported a %RLV of less than 25% as being associated with significant liver dysfunction in patients with normal liver.21,22 However, the cut off for %RLV in these studies was not derived from precise analysis but rather it was set arbitrarily to compare results of liver function and overall complications between different extents of resection.
We believe that calculating %RLV before major liver resection provides useful information for planning hepatic surgery and in advising individual patients of the potential risks of their surgery. However, it should not be a barrier to undertake major liver resection when the chance for cure and the patient’s good condition outweighs the risk. Only half of patients who underwent liver resection leaving less than the critical %RLV developed postoperative severe hepatic dysfunction. However, in all of these patients additional predictive factors, such as high BMI, long operating time, and significant intraoperative blood loss, were evident. BMI was found as a reliable surrogate marker of hepatic steatosis and steatosis is known as a potential risk factor for major liver resection.38,39 We found a good correlation between the number of liver specimens showing moderate to severe steatosis and BMI, supporting the assumption that steatosis is an important factor for the development of hepatic dysfunction in patients who underwent liver resection leaving only a small %RLV. This observation is of particular interest as BMI can easily be calculated before surgery and included in the preoperative risk assessment.
Infections are seen frequently after liver resection and in the present study caused a significant proportion of postoperative complications. Several studies suggest an important role for the liver in postoperative innate immune response40,41 but no single study has investigated the relationship between RLV and the incidence of postoperative infection. Significant loss of hepatic phagocytes (Kupffer cells) together with decreased synthesis of hepatic proteins involved in antigen recognition, opsonisation, and phagocytosis are considered likely to be responsible for the impaired innate immune function following major liver resection and consequently render the patient more susceptible to infection.13,17,42–44 We found a significant relation between the extent of liver resection, %RLV, and the incidence of postoperative infection. However, a precise %RLV to predict infection with high sensitivity and specificity could not be defined. Postoperative infections are a heterogeneous group of diagnoses.45 Some may be dependent on the condition of the patient, the extent of liver resection performed, and liver function, but others will be determined by many other factors. Thus lack of a definitive cut off value for %RLV in predicting infection following liver resection might be explained by the study being under powered to account for such heterogeneity rather than there being no true relationship. Analysis of a subgroup of patients with small residual liver volume showed a significant relation between severe hepatic dysfunction and infection, supporting the proposed relationship between liver function, innate immunity, and susceptibility to infection. Studies assessing the reticuloendothelial cell clearance capacity of healthy and diseased liver after major liver resection would be useful in further evaluating the relation between residual liver volume, liver function, and innate immunity.
Estimating %RLV from three dimensional hepatic volumetry and virtual resection in patients undergoing liver resection provides important information in assessing the individual risk for postoperative severe hepatic dysfunction. A critical %RLV of 26.6% was identified below which the risk of developing severe hepatic dysfunction increased significantly. Additionally, obesity renders patients even more likely to develop severe hepatic dysfunction following resection. Although we found an association between small %RLV and postoperative infection, it was not possible to define this risk in terms of a critical %RLV with precision. Studies assessing the various aspects of liver function, including its contribution to innate immunity, and relating these results to actual preoperative and estimated residual liver volume would be of value in understanding the relationship between liver volume and function in further detail. This would also help in developing novel strategies to reduce the incidence of complications related to hepatic dysfunction following major liver resection.
Acknowledgments
The study was performed in collaboration with the Image Analysis Core, Wellcome Trust Clinical Research Facility (Edinburgh), and was supported by a grant from Tenovus Scotland. MS was funded by a Austrian Science Fund Grant No J2147 and by the Austrian Surgical Society. SJW was funded by a Wellcome Trust Grant No 065029. The authors thank all participants of the eLISTER research group for their clinical and scientific contributions to the study.
Abbreviations
CT, computed tomography
CTAP, CT angioportography
BMI, body mass index
ROC, receiver operator characteristic
TLV, total liver volume
TuV, tumour volume
TFLV, total functional liver volume
RLV, residual liver volume
%RLV, relative residual liver volume
APPENDIX
PARTICIPANTS IN THE EDINBURGH LIVER SURGERY AND TRANSPLANTATION EXPERIMENTAL RESEARCH GROUP (ELISTER)
Mr Stephen J Wigmore, Professor Kenneth C H Fearon, Professor O James Garden, Mr Rowan W Parks, Department of Surgery, Royal Infirmary Edinburgh, UK; Professor Peter C Hayes, Hepatology, Royal Infirmary Edinburgh, UK; Dr Kees Dejong, Department of Surgery, Maastricht, the Netherlands; Dr James A Ross, Centre of Inflammation Research, University of Edinburgh, UK; Dr Doris Redhead, Department of Radiology, Royal Infirmary Edinburgh, UK; Dr Alistair Millar, Radiopharmacy, Royal Infirmary Edinburgh, UK; Dr Tom Preston, Scottish Universities Research and Reactor Centre, East Kilbride, Glasgow, UK; Dr Martin Schindl, Department of Surgery, University of Vienna, Austria.
Conflict of interest: None declared.
REFERENCES
- 1.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y , Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaeck D , Bachellier P, Guiguet M, et al. Long-term survival following resection of colorectal hepatic metastases. Association Francaise de Chirurgie. Br J Surg 1997;84:977–80. [DOI] [PubMed] [Google Scholar]
- 4.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg 1997;132:505–10. [DOI] [PubMed] [Google Scholar]
- 5.Rees M , Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg 1997;84:1136–40. [PubMed] [Google Scholar]
- 6.Moroz P , Salama PR, Gray BN. Resecting large numbers of hepatic colorectal metastases. Aust NZ J Surg 2002;72:5–10. [DOI] [PubMed] [Google Scholar]
- 7.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minagawa M , Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg 2000;231:487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurent C , Sa Cunha A, Couderc P, et al. Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br J Surg 2003;90:1131–6. [DOI] [PubMed] [Google Scholar]
- 10.Cohnert TU, Rau HG, Buttler E, et al. Preoperative risk assessment of hepatic resection for malignant disease. World J Surg 1997;21:396–400. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R , Janeway C Jr. Innate immunity. N Engl J Med 2000;343:338–44. [DOI] [PubMed] [Google Scholar]
- 12.Shiomi S , Kuroki T, Ueda T, et al. Diagnosis by routine scintigraphy of hepatic reticuloendothelial failure before severe liver dysfunction. Am J Gastroenterol 1996;91:140–2. [PubMed] [Google Scholar]
- 13.Wiezer MJ, Meijer C, Wallast-Groenewoud HP, et al. Impaired leukocyte phagocytosis in patients undergoing hemihepatectomy for liver metastases. Liver Transpl Surg 1999;5:238–45. [DOI] [PubMed] [Google Scholar]
- 14.Nakatani Y , Fukui H, Kitano H, et al. Endotoxin clearance and its relation to hepatic and renal disturbances in rats with liver cirrhosis. Liver 2001;21:64–70. [DOI] [PubMed] [Google Scholar]
- 15.Navasa M . Bacterial infections in patients with cirrhosis: reasons, comments and suggestions. Dig Liver Dis 2001;33:9–12. [DOI] [PubMed] [Google Scholar]
- 16.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol 2002;8:961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan AK, Luk HN, Goto S, et al. Stress response to hepatectomy in patients with a healthy or a diseased liver. World J Surg 2003;27:761–4. [DOI] [PubMed] [Google Scholar]
- 18.Hiroshige S , Shimada M, Harada N, et al. Accurate preoperative estimation of liver-graft volumetry using three-dimensional computed tomography. Transplantation 2003;75:1561–4. [DOI] [PubMed] [Google Scholar]
- 19.Leelaudomlipi S , Sugawara Y, Kaneko J, et al. Volumetric analysis of liver segments in 155 living donors. Liver Transpl 2002;8:612–14. [DOI] [PubMed] [Google Scholar]
- 20.Ishifuro M , Horiguchi J, Nakashige A, et al. Use of multidetector row CT with volume renderings in right lobe living liver transplantation. Eur Radiol 2002;12:2477–83. [DOI] [PubMed] [Google Scholar]
- 21.Shoup M , Gonen M, D’Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7:325–30. [DOI] [PubMed] [Google Scholar]
- 22.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512–19. [DOI] [PubMed] [Google Scholar]
- 23.Shirabe K , Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999;188:304–9. [DOI] [PubMed] [Google Scholar]
- 24.Kubota K , Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 1997;26:1176–81. [DOI] [PubMed] [Google Scholar]
- 25.Yanaga K , Honda H, Ikeda Y, et al. Significance of liver size in hepatic surgery. HPB Surg 1997;10:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogasawara K , Une Y, Nakajima Y, et al. The significance of measuring liver volume using computed tomographic images before and after hepatectomy. Surg Today 1995;25:43–8. [DOI] [PubMed] [Google Scholar]
- 27.Wigmore SJ, Redhead DN, Yan XJ, et al. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg 2001;233:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wigmore SJ, Madhavan K, Currie EJ, et al. Does the subspecialty of the surgeon performing primary colonic resection influence the outcome of patients with hepatic metastases referred for resection? Ann Surg 1999;230:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IHPBA. The Brisbane 2000 terminology of hepatic anatomy and resections. HPB Surg 2000;2:333–9. [Google Scholar]
- 30.Farges O , Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferenci P , Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35:716–21. [DOI] [PubMed] [Google Scholar]
- 32.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–6. [DOI] [PubMed] [Google Scholar]
- 33.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–55. [DOI] [PubMed] [Google Scholar]
- 34.Marshall JC, Vincent JL, Fink MP, et al. Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25–26 2000. Crit Care Med 2003;31:1560–7. [DOI] [PubMed] [Google Scholar]
- 35.Belghiti J , Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38–46. [DOI] [PubMed] [Google Scholar]
- 36.Melendez J , Ferri E, Zwillman M, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg 2001;192:47–53. [DOI] [PubMed] [Google Scholar]
- 37.Didolkar MS, Fitzpatrick JL, Elias EG, et al. Risk factors before hepatectomy, hepatic function after hepatectomy and computed tomographic changes as indicators of mortality from hepatic failure. Surg Gynecol Obstet 1989;169:17–26. [PubMed] [Google Scholar]
- 38.Rinella ME, Alonso E, Rao S, et al. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl 2001;7:409–14. [DOI] [PubMed] [Google Scholar]
- 39.Behrns KE, Tsiotos GG, DeSouza NF, et al. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg 1998;2:292–8. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki S , Nakamura S, Sakaguchi T, et al. Alteration of reticuloendothelial phagocytic function and tumor necrosis factor-alpha production after total hepatic ischemia. Transplantation 1997;64:821–7. [DOI] [PubMed] [Google Scholar]
- 41.Suttner SW, Surder C, Lang K, et al. Does age affect liver function and the hepatic acute phase response after major abdominal surgery? Intensive Care Med 2001;27:1762–9. [DOI] [PubMed] [Google Scholar]
- 42.Arii S , Shibagaki M, Takahashi S, et al. Changes in the reticuloendothelial phagocytic function after partial hepatectomy. J Lab Clin Med 1985;105:668–72. [PubMed] [Google Scholar]
- 43.Kimura F , Miyazaki M, Suwa T, et al. Reduction of hepatic acute phase response after partial hepatectomy in elderly patients. Res Exp Med (Berl) 1996;196:281–90. [DOI] [PubMed] [Google Scholar]
- 44.Shirabe K , Takenaka K, Yamatomto K, et al. Impaired systemic immunity and frequent infection in patients with Candida antigen after hepatectomy. Hepatogastroenterology 1997;44:199–204. [PubMed] [Google Scholar]
- 45.Pessaux P , Msika S, Atalla D, et al. Risk factors for postoperative infectious complications in noncolorectal abdominal surgery: a multivariate analysis based on a prospective multicenter study of 4718 patients. Arch Surg 2003;138:314–24. [DOI] [PubMed] [Google Scholar]