Abstract
Background and aims: There are no available effective therapies for fatigue associated with chronic hepatitis C (CHC). The serotonin antagonist ondansetron has been shown to be effective in the chronic fatigue syndrome. In this randomised, placebo controlled, double blind trial, we investigated the effect of orally administered ondansetron on fatigue in CHC.
Methods: Thirty six patients with CHC were included if fatigue was their predominant symptom and they scored more than 4 on a visual analogue scale (0–10). During the study, fatigue and depression were measured on days 0, 15, 30, and 60 using a validated self report questionnaire (fatigue impact scale and Beck depression inventory). Patients were randomised to receive ondansetron tablets 4 mg twice daily or placebo for one month followed by an additional four weeks of observation.
Results: Fatigue score was 85.4 (28.2) and 98.2 (26.9) in the ondansetron and placebo groups, respectively (NS). Ondansetron significantly reduced the fatigue score with more than 30% improvement on day 15 (57.1 (38.9); p<0.01), day 30 (54.5 (37.6); p<0.01), and day 60 (60.8 (37.3); p<0.01) whereas placebo did not. Overall, the reduction in fatigue was significantly higher with ondansetron compared with placebo (ANOVA for repeated measurements) for the whole follow up period (p = 0.03) or for the treatment period only (p = 0.04). Ondansetron also significantly reduced depression scores.
Conclusions: The 5-hydroxytryptamine receptor type 3 antagonist ondansetron had a significant positive effect on fatigue in CHC. These observations support the concept that fatigue involves serotoninergic pathways and may encourage further evaluations of the efficacy of ondansetron on fatigue in chronic liver diseases.
Keywords: fatigue, chronic hepatitis C, ondansetron, randomised controlled trial
Fatigue is the seventh most common symptom in primary care.1 This persistent feeling of exhaustion, inability to perform usual daily chores, and decreased capacity for physical and mental work is frequently associated with chronic hepatitis C (CHC)2,3 and is an important determinant of the quality of life of infected patients.4,5 The prevalence of psychiatric disorders, including depression, may also be greater in such patients than in the general population.6 Accordingly, elucidation of the pathogenesis of fatigue occurring with CHC and the development of effective therapies for its relief are important goals for hepatologists. Despite its significance as a crucial issue for health care in CHC, little progress has been made to achieve these goals. This important topic in hepatology has been woefully under investigated because of the lack of an objective measurement of the subjective experience of fatigue. The fatigue impact scale (FIS) questionnaire was developed by Fisk and colleagues to quantify the specific impact of fatigue on quality of life.7 To date, this tool has been used to confirm that fatigue is a major complaint in patients with CHC2,8 or primary biliary cirrhosis.9,10 Hence the FIS questionnaire should be considered as a relevant tool for therapeutic trials aimed at reducing fatigue.
The pathogenesis of fatigue remains unclear, in the presence or absence of chronic liver diseases. In animal models, three major central changes are involved: (i) neuroendocrine causes, with abnormal function of the hypothalamic-pituitary-adrenal axis; (ii) abnormalities in neurotransmission, particularly in the central serotonin pathway; and (iii) alterations in immune activation and cytokine release.11 Subsequent studies in humans have documented that fatigue occurred through changes within the central nervous system in the chronic fatigue syndrome12,13 and also in cholestatic liver diseases.14 Addressing the problem in patients with CHC, there is emerging evidence that fatigue may be associated with central dysfunction. The recent detection of hepatitis C virus (HCV) genetic sequences in post mortem brain tissue raises the intriguing possibility that HCV infection of the central nervous system may be related to the reported neuropsychological symptoms (that is, fatigue) and cognitive impairment.15
In terms of altered neurotransmission, there is growing acceptance that cholestasis is associated with altered opioid and serotonin mediated neuromodulation.14 It is noteworthy that serotonin (5-HT), like opioid agonists, modulates nociception in normal rats.16 This concept is supported by the fact that intravenous administration of the selective 5-HT3 receptor antagonist ondansetron to patients with cholestatic liver diseases has been associated with acute improvement of pruritus.17 Wilson and Maughan18 have also shown that exercise endurance of athletes was significantly reduced after administration of the 5-HT reuptake inhibitor paroxetine (for example, a drug acting centrally in the treatment of depression). Conversely, oral administration of the 5-HT3 receptor antagonist ondansetron has been shown to relieve fatigue in patients with the chronic fatigue syndrome19 and also in the case of a woman with CHC and profound fatigue who became symptom free when treated with long term ondansetron 4 mg twice daily.20 Therefore, it is pertinent to consider that there is a central component to fatigue, mediated by the activity of serotoninergic neurones in chronic liver diseases and that there is a theoretical rationale to evaluate selective 5-HT3 receptor antagonists in the treatment of fatigue associated with CHC.
The primary end point of the study was to evaluate the effect of ondansetron on the severity of fatigue in CHC. The secondary outcome was to assess the efficacy of ondansetron on depression in these patients. We performed a randomised, placebo controlled, double blind trial testing orally administered ondansetron 4 mg twice daily for four weeks followed by a four week follow up period.
PATIENTS AND METHODS
Participants
Adult patients, aged 18–70 years, were eligible to enrol in the trial if they had compensated CHC associated with a fatigue score of more than 4 according to the visual analogue scale (VAS) of the FIS. This cut off score was chosen as it correlated well with verbally reported significant fatigue in CHC patients.2 The diagnosis of CHC was based on the association of: (a) elevated serum alanine aminotransferase to >40 U/l (upper normal limit) for ⩾6 months; (b) presence of anti-HCV antibodies; (c) detectable HCV viraemia; and (d) exclusion of other causes of chronic liver disease (alcoholism, chronic hepatitis B, Wilson’s disease, hepatotoxic drugs, haemochromatosis, α1 antitrypsin deficiency, autoimmune chronic active hepatitis). Liver biopsy was performed in all patients; none was cirrhotic. Patients had no evidence of acute or chronic disease likely to cause fatigue such as detectable human immunodeficiency virus viraemia, renal failure, thyroid disease, diabetes, constipation, irritable bowel syndrome, or severe clinical depression evaluated by a psychophysician (FC). None was treated with medications that could interfere with fatigue (for example, beta blockers, antidepressants, sedatives, steroids) or had received any antiviral therapy. These patients were considered for antiviral therapy after the study. Women were not included if pregnant or were not using effective contraception.
Participants gave written informed consent and the protocol was approved by the local research ethics committee (Comité Consultatif pour la Protection des Personnes dans la Recherche Biomédicale du Chu de Nice).
Virological evaluation
Anti-HCV antibodies were detected by a third generation enzyme immunoassay (HCV EIA 3.0; Abbott Laboratories, North Chicago, Illinois, USA). Serum HCV-RNA was detected by a reverse transcription-polymerase chain reaction method (Amplicor; Roche Diagnostic Systems, Branchburg, New Jersey, USA). Levels of viraemia were determined by a branched DNA technique (Quantiplex HCV RNA 2.0 Assay bDNA Chiron). Liver biopsy specimens were fixed in Bouin’s solution and embedded in paraffin for routine staining with haematoxylin-eosin-saffron, and were examined by the same pathologist and evaluated using a validated scoring system, the METAVIR score.21
Assessment of fatigue and depression
The impact of fatigue on quality of life was measured by the FIS questionnaire.7 The questionnaire was translated into French by one of the authors (PMH) and validated in healthy blood donors in Montreal.9 In addition, we have reported previously that the French version of this questionnaire is applicable to a local cohort of patients with CHC.2 FIS is a self report questionnaire consisting of 40 statements that describe possible manifestations of fatigue. These statements are divided in three categories: cognitive (n = 10), physical (n = 10), and psychosocial (n = 20). Each item is rated on a five point scale of distress, ranging from 0 (“no problem”) to 4 (“extreme problem”) with a maximum of 160 points. In addition to the 40 items, the FIS asks for the frequency of fatigue in terms of days per month and the usual duration of fatigue per day.
Depression was assessed using the short version of the Beck depression inventory (BDI), a well standardised 13 item measure of cognitive, affective, and somatic symptoms of depression22,23 available in French.24 The short 13 item BDI contains only pertinent items that correlate significantly with both the global score of the original 21 items form and the clinical evaluation of depression by physicians.25 This questionnaire is generally well accepted by patients and routinely used for psychopharmacological studies. Four sentences are included in each item with a rating from 0 to 3 in function for the severity of a symptom. Addition of each item gives a global score, allowing an evaluation of the severity of depression: 0–4 absent, 5–7 minor, 8–15 moderate, and ⩾16 severe depression.
Study design
Participants were recruited between November 2002 and December 2003. Eligible CHC patients experiencing fatigue (VAS >4) were randomised in a double blind manner to receive ondansetron orally 4 mg twice daily or placebo for four weeks. Constipation is an expected side effect of ondansetron that may interfere with fatigue or quality of life. Constipation was treated with laxatives (polyethylene glycol (Transipeg 5.9), 1 sachet per day) if necessary. The study drug and matching placebo as well as the randomisation code were provided by GlaxoSmithKline Research and Development Ltd (Greenford, Middlesex, UK). Compliance of patients was assessed by pill count at each visit.
All participants were evaluated under the same conditions in the morning at the research centre of the Department of Hepatogastroenterology at the initiation visit (day 0), on day 15, and on day 30. They were then seen one month after the treatment was stopped (day 60). At each visit, patients were instructed to complete the FIS and BDI questionnaires before blood samples were obtained for routine haematological and renal examinations. Patients were discharged from the centre following one hour of observation and were asked to report any adverse events (nature, intensity, duration) that occurred by telephone.
Statistical analysis
The sample size was calculated at 17 evaluable patients in each group, for an expected reduction in total fatigue score of 30% with ondansetron versus placebo, and with a risk alpha of 5% and beta of 20%. The maximum attrition rate was assumed to be 10%. Thirty six patients were enrolled to achieve statistical power. Quantitative data are expressed as mean (SD). Fatigue scores at different time points in each group were compared with baseline using the paired t test. Comparisons between groups required ANOVA for repeated measurements and the unpaired t test. The χ2 test was used to compare qualitative data. A p value <0.05 was considered significant. Results are presented as an intent to treat analysis.
RESULTS
Baseline characteristics of the study population (table 1 ▶)
Table 1.
Characteristic of the study population
| Ondansetron (n = 18) | Placebo (n = 18) | |
| Sex | 10F/8M | 15F/3M |
| Age (y) | 48.2 (8.3) | 52.5 (11.4) |
| Body weight (kg) | 63.5 (10.5) | 62.8 (10.2) |
| Height (cm) | 160 (20.6) | 162 (7.3) |
| ALT (U/l) | 72.8 (58.0) | 64.5 (50.9) |
| Hep C viral load (106 copies/ml) | 11.1 (10.6) | 7.0 (7.5) |
| METAVIR score | ||
| Activity index | 1.2 (0.4) | 1.2 (0.9) |
| Fibrosis index | 1.2 (0.5) | 1.1 (0.4) |
Hep C, hepatitis C virus; ALT, alanine aminotransferase.
There were no significant differences between the groups.
There were no significant differences at baseline between the ondansetron and placebo groups with respect to sex distribution, age, weight, height, or liver function tests (table 1 ▶). Thirty one of 36 patients (86%) considered fatigue as their initial symptom and 22 (60%) as the worst symptom of their disease. Seventy seven per cent of patients suffered from fatigue between 10 and 19 days per month and, for the majority (86%), duration of fatigue was more than six hours per day. One patient in the ondansetron group withdrew from the study after 15 days. Pill count at the end of each treatment period showed >97% compliance for all subjects for both ondansetron and placebo.
Evaluation of fatigue
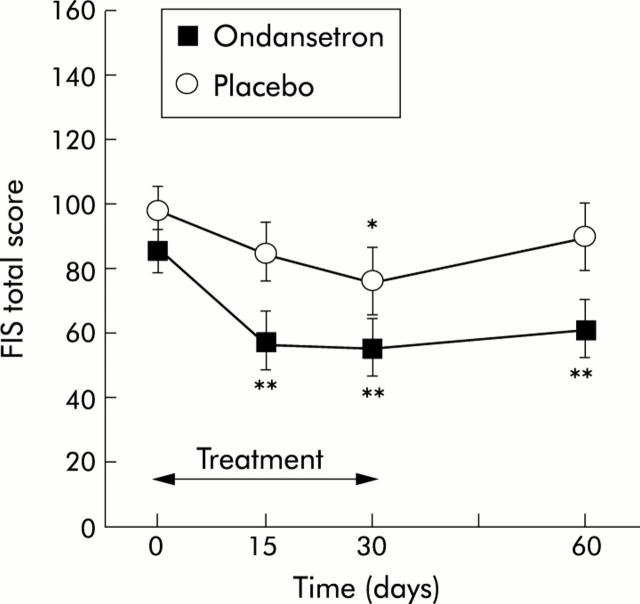
On day 0, the fatigue score was 85.4 (28.2) and 98.2 (26.9) in the ondansetron and placebo groups, respectively (NS). Treatment with ondansetron significantly reduced the fatigue score with 32.2% improvement by day 15 (57.1 (38.9); p<0.01), 37.8% by day 30 (54.5 (37.6); p<0.01), and 31.5% by day 60 (60.8 (37.3); p<0.01). In contrast, the reduction in fatigue in patients treated with placebo always remained less than 30% (13.6%, 20.4%, and 5.8% on days 15, 30, and 60, respectively) and the difference reached statistical significance only on day 30 (98.2 (26.9) v 76.0 (43.2); p = 0.03). Overall, the reduction in fatigue was significantly more pronounced with ondansetron compared with placebo for the whole follow up period (p = 0.03) or for the treatment period only (p = 0.04). When comparing directly both groups at different times, treatment with ondansetron significantly reduced fatigue by day 15 (p = 0.03) and day 60 (p = 0.04) whereas there was a statistical trend in favour of ondansetron by day 30 (p = 0.1) (fig 1 ▶). Each subscale of the FIS was improved at the end of follow up (day 60) but the findings were significant only for the cognitive (p = 0.02) and physical (p = 0.04) domains.
Figure 1.
Effect of ondansetron and placebo on the fatigue total score before (day 0), during (days 15 and 30), and one month after treatment (day 60). FIS, fatigue impact scale. *p<0.05, **p<0.01 versus day 0. Values are mean (SEM).
Evaluation of depression
According to the BDI, 56% of patients were classified as depressed (BDI score >4). Fatigue scores were significantly higher in depressed compared with non-depressed patients (FIS 100.6 (23.5) v 80.8 (29.9); p = 0.03). At day 0, the BDI score was 9.3 (5.8) and 12.2 (5.9) in the ondansetron and placebo groups, respectively (NS). Treatment with ondansetron significantly reduced the BDI score, with 37.3% improvement by day 15 (9.3 (5.8) v 5.8 (4.7); p<0.01), 35.2% by day 30 (9.3 (5.8) v 5.7 (4.2); p<0.01), and 41.9% by day 60 (9.3 (5.8) v 5.4 (4.7); p<0.01). In contrast, the improvement in BDI score in patients treated with placebo did not achieved 30% during the study (17.3%, 20.4%, and 5.8% on days 15, 30, and 60 respectively) and reached statistical significance only on day 15 (12.2 (5.9) v 10.1 (5.7); p = 0.04) (fig 2 ▶). Overall, the reduction in depression was significantly more pronounced with ondansetron compared with placebo for the whole follow up period (p<0.05) or the treatment period only (p<0.05). When comparing directly both groups at different times, treatment with ondansetron significantly reduced BDI scores on day 15 (p = 0.02), day 30 (p = 0.01), and day 60 (p = 0.04) (fig 2 ▶).
Figure 2.
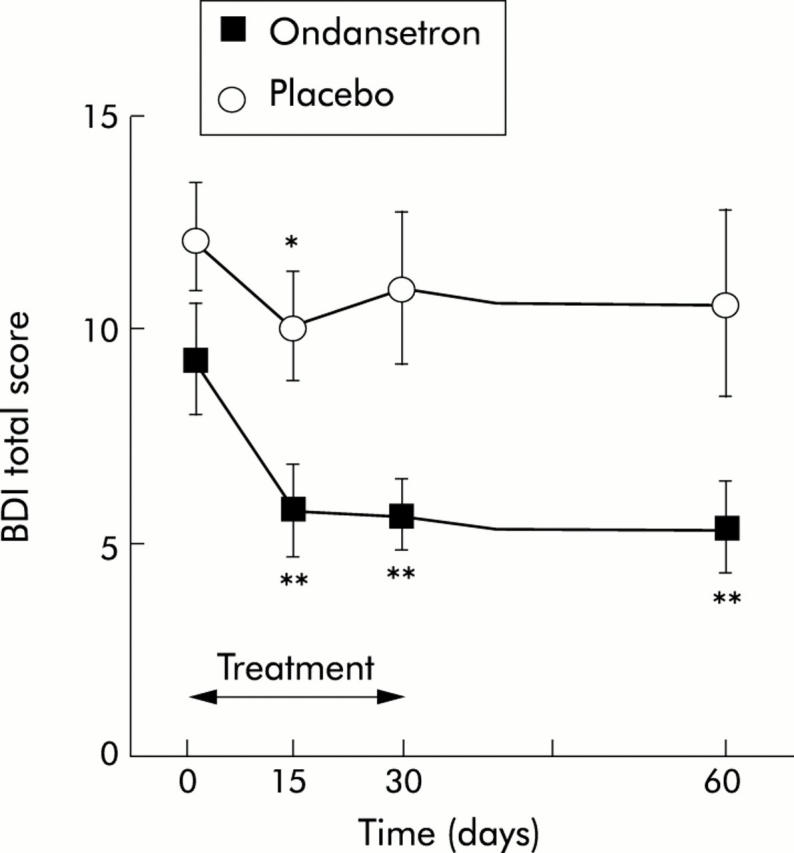
Effect of ondansetron and placebo on depression score before (day 0), during (days 15 and 30), and one month after treatment (day 60). BDI, Beck depression inventory. *p<0.05, **p<0.01 versus day 0. Values are mean (SEM).
Safety and tolerance
Adverse events reported are summarised in table 2 ▶. Constipation occurred in 38% and 33% of patients treated with ondansetron and placebo, respectively (NS). Laxatives were used by three patients in the ondansetron group and by one patient in the placebo group. Duration of “on demand” laxative treatment was less than 10 days in each group. Only one patient treated with ondansetron required a reduction in the daily dose to 4 mg because of constipation; however, ondansetron remained effective against fatigue.
Table 2.
Adverse events
| Ondansetron (n = 18) | Placebo (n = 18) | |
| Constipation | 7 | 6 |
| Required laxative | 3 | 1 |
| Headache | 1 | 1 |
| ALT (d0/d60) | 72.8 (58.0)/61.6 (56.3) | 64.5 (50.9)/68.0 (64.0) |
There were no significant changes in laboratory parameters during the study, and in particular no increases in liver enzymes (alanine aminotransferase (ALT)).
DISCUSSION
This randomised, placebo controlled, double blind trial showed a positive effect of the 5-HT3 (serotonin) receptor antagonist ondansetron, given orally for one month at a daily dose of 4 mg twice daily, on fatigue in patients with CHC.
Our main result was the significant reduction in fatigue in CHC patients treated with ondansetron and the contribution of the serotonin pathway in the pathogenesis of fatigue. In the present study in CHC patients, fatigue was more severe than previously reported (91.8 (27.9) v 53.3 (40.1)),2 which can be explained by prior selection of more fatigued patients based on the VAS. The clinical relevance of a reduced fatigue score with ondansetron must be debated. Undoubtedly, the major issue when treating fatigue is to ensure that verbal expression of fatigue can be evaluated objectively. We therefore enrolled CHC patients with a fatigue score of more than 4 on a VAS which was considered a reasonable threshold for verbal expression of fatigue.9 Accordingly, we postulated that a 30% improvement in fatigue may be of clinical interest based on the data of Spath et al who found a remarkable improvement (>35%) in fatigue in patients treated with ondansetron.19 In our study, ondansetron reduced the severity of fatigue by more than 30% on days 15, 30, and 60 whereas placebo did not. Although fatigue scores evaluated on day 30 were not statistically different between the ondansetron and placebo groups, a statistical trend in favour of ondansetron was observed and the difference was significant by day 15 and also one month after treatment was stopped. Given the large overlap in FIS scores, we are conscious that the study was underpowered to detect meaningful group differences but it was not our major goal. We do not believe that the significant improvement in fatigue was confused by the inherent variability of self reported questionnaires. Indeed, a recent study performed in untreated primary biliary cirrhosis patients confirmed that the score variability of the FIS was very limited (mean of 0.06).26 Although a placebo effect on fatigue was observed on day 30, this effect disappeared after the treatment was stopped, contrary to ondansetron. We do not believe that a potential improvement in self reported scores between the groups might be associated with trial participation, more frequent clinical visits, or enhanced attention, because the FIS score of patients treated with placebo was not different between day 0 and day 60. Taken together, these findings support the clinical relevance of the observed reduction in fatigue score with ondansetron.
The longevity of the effect beyond the ondansetron period is also of concern. Ondansetron is primarily eliminated via hepatic oxidative metabolism of the indole moiety; thus liver disease may affect its clearance and it has been shown that a progressive decline in systemic clearance of ondansetron occurred with increased severity of liver disease.27 Although a pharmacological survey was not performed in this study, it is uncertain whether the durable effect of ondansetron on fatigue was related to delayed hepatic clearance of the drug. Indeed, the half life of the drug is approximately three hours, all of our patients had compensated liver disease without cirrhosis (see table 1 ▶), and the daily doses of ondansetron (that is, 8 mg orally) administered in the present study were safe in such conditions.27 Further studies are required to explore the underlying mechanism of the 5-HT3 receptor antagonist on fatigue in humans. Thus an important question is whether ondansetron mediates this behavioural effect. The longevity of potential effects could be discussed in terms of regression to the mean phenomenon or possibly related to sustained neuronal mechanisms: hippocampal neurogenesis as a possible mode of antidepressant action might represent a potential mechanism for the sustained effects of 5-HT3 antagonists.28 In a previous study, we partly related the elevated fatigue score of CHC patients to elevated circulating leptin2 which may interact with serotonin neurotransmission.29 Obviously, the underlying mechanisms of fatigue are multifactorial and neuroendocrine causes with abnormal function of the hypothalamic-pituitary-adrenal axis and alterations in immune activation and cytokine release have also been implicated in behavioural changes.11 However, the serotoninergic pathway may have an important role, as noted by the great improvement in fatigue observed in patients with CHC.
It is well documented that CHC patients may have a higher prevalence of psychiatric disorders, including depression.6 The causes of this high rate of depression are unclear. It has been suggested that infected patients are relatively young and may suffer from reactive depression related to concerns about their own long term prognosis.30 It is also well documented that fatigue and depression are related in such patients.9 CHC patients also tend to have additional risk factors for mood disorders, such as concurrent substance abuse.31 In the present study, the few drug users had not taken any substance for at least six months and were equally distributed in both groups. Although we did not observe a significant positive correlation between the FIS and BDI, the significant reduction in fatigue and depression was temporally associated, which suggests that the inter-relationship between depression and fatigue in the context of CHC is complex. In the present study, we deliberately used the BDI rather than the hospital anxiety and depression scale (HADS) questionnaire to evaluate depression. Indeed, we have previously shown that major depression was not clinically apparent in fatigued patients with CHC2 and that FIS and BDI were complementary in evaluating fatigue in patients with primary biliary cirrhosis.9 It is also known that a cognitive based approach is justified in screening depression in CHC and that the two scales (HADS and BDI) perform similarly in identifying major depression whereas the BDI seems to be more effective in screening minor depression.32 It is noteworthy that the placebo group had a greater influence on fatigue than on depression. These apparent discrepancies can be the consequence of patient information of an expected benefit of the drug on fatigue and not depression.
In the present study, we observed a higher rate of constipation than previously reported in studies on the safety of ondansetron.33 We believe that knowledge of an expected side effect might have been suggestive and increased the complaint. It is doubtful that constipation influences fatigue because the impact of constipation on laxative use remained small, constipation was equally observed in both arms, and fatigue scores were not significantly different in constipated patients or those with normal bowel habits (data not shown). Considering that constipation may interfere with fatigue and/or quality of life, we decided to treat it “on demand” if necessary. In the present study, a dose of 8 mg daily was chosen with reference to previous work in which the same dose given orally did not induce constipation34; most patients experienced constipation when treated with 12 mg daily.26 We were conscious that patient knowledge of possible constipation may have biased the double blind nature of the study and amplified the effect of the ondansetron arm. In fact, the occurrence of constipation was not only similar in the two groups but also in both responders (>30% reduction of fatigue) and non-responders in each group, necessitating laxatives in only four of 13 constipated patients and with no significant influence on fatigue. Therefore, we believe that patient knowledge of possible constipation may have amplified the unusual high rate of constipation that we observed in both groups. Finally, there were no changes in laboratory parameters during the study, in particular liver enzymes.
In conclusion, this is the first randomised placebo controlled study on fatigue in CHC to show a positive effect of the specific 5-HT3 receptor antagonist ondansetron. Considering the negative impact of fatigue on quality of life and reduced adherence to antiviral therapy in infected patients, it is crucial to further confirm these data in large multicentre trials. Duration of treatment must also be determined as we observed prolonged benefit after ondansetron was stopped.
Acknowledgments
The authors gratefully acknowledge GSK France for supporting the study. This trial was sponsored by the “Société Nationale Française de Gastro-Entérologie (SNFGE)”. We thank our patients for their participation.
Abbreviations
CHC, chronic hepatitis C
5-HT, 5-hydroxytryptamine (serotonin)
FIS, fatigue impact scale
HCV, hepatitis C virus
VAS, visual analogue scale
BDI, Beck depression inventory
HADS, hospital anxiety and depression scale
Conflict of interest: None declared.
REFERENCES
- 1.Kroenke MD, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care. JAMA 1988;260:929–34. [PubMed] [Google Scholar]
- 2.Piche T, Gelsi E, Schneider SM, et al. Fatigue is associated with high circulating leptin levels in chronic hepatitis C. Gut 2002;51:434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumber SC, Chopra S. Hepatitis C. A multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med 1995;123:615–20. [DOI] [PubMed] [Google Scholar]
- 4.Cauch-Dudek K, Abbey S, Stewart DE, et al. Fatigue in primary biliary cirrhosis. Gut 1998;43:705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 1998;27:209–12. [DOI] [PubMed] [Google Scholar]
- 6.Zdilar D, Franco-Bronson K, Buchler N, et al. Hepatitis C, interferon alfa, and depression. Hepatology 2000;31:1207–11. [DOI] [PubMed] [Google Scholar]
- 7.Fisk JD, Pontrefact A, Ritvo PF, et al. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Can J Neurol Sci 1994;21:9–14. [DOI] [PubMed] [Google Scholar]
- 8.Hassoun Z, Willems B, Deslauriers J, et al. Assessment of fatigue in patients with chronic hepatitis C using the fatigue impact scale (FIS). Dig Dis Sci 2002;47:2674–81. [DOI] [PubMed] [Google Scholar]
- 9.Huet PM, Deslauriers J, Tran A, et al. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterol 2000;95:760–7. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt J, Taylor PJS, Lipman T, et al. The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology 2002;122:1235–41. [DOI] [PubMed] [Google Scholar]
- 11.Swain MG, Maric M. Defective corticotropin-releasing hormone mediated neuroendocrine and behavioral responses in cholestatic rats: implications for cholestatic liver disease-related sickness behaviors. Hepatology 1995;22:1560–4. [PubMed] [Google Scholar]
- 12.de Lange FP, Kalkman JS, Bleijenberg G, et al. Neural correlates of the chronic fatigue syndrome—an fMRI study. Brain 2004;127:1948–57. [DOI] [PubMed] [Google Scholar]
- 13.Siemionow V, Fang Y, Calabrese L, et al. Altered central nervous system signal during motor performance in chronic fatigue syndrome. Clin Neurophysiol 2004;115:2372–81. [DOI] [PubMed] [Google Scholar]
- 14.Bergasa NV, Jones EA. The pruritus of cholestasis. Potential pathogenic and therapeutic implications of opioids. Gastroenterology 1995;108:1582–8. [DOI] [PubMed] [Google Scholar]
- 15.Forton D, Karayiannis P, Mahmud N, et al. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J Virol 2004;78:5170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson BP. Serotonin and nociception. Ann N Y Acad Sci 1990;600:511–20. [DOI] [PubMed] [Google Scholar]
- 17.Schworer H, Hartmann H, Ramadori G. Relief of cholestatic pruritus by a novel class of drugs: 5-hydroxytryptamine type 3 (5HT3) receptor antagonists: effectiveness of ondansetron. Pain 1995;61:33–7. [DOI] [PubMed] [Google Scholar]
- 18.Wilson WM, Maughan RJ. Evidence for a possible role of 5-hydroxytryptamine in the genesis of fatigue in man: administration of paroxetine, a 5-HT re-uptake inhibitor, reduces the capacity to perform prolonged exercise. Exp Physiol 1992;77:921–4. [DOI] [PubMed] [Google Scholar]
- 19.Spath M, Welzel D, Farber L. Treatment of chronic fatigue syndrome with 5-HT3 receptor antagonists—preliminary results. Scand J Rheumatol Suppl 2000;113:72–7. [PubMed] [Google Scholar]
- 20.Jones EA. Relief from profound fatigue associated with chronic liver disease by long-term ondansetron therapy. Lancet 1999;354:397. [DOI] [PubMed] [Google Scholar]
- 21.Group TFMCS. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15–20. [PubMed] [Google Scholar]
- 22.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. In: Pichot P, ed. Psychological measurements in psychopharmacology. Basel: Karger, 1974:151–9.
- 23.Beck AT, Steer R, Garbin M. Psychometric properties of the Beck depression inventory: twenty five years of research. Clin Psychol Rev 1988;8:77–100. [Google Scholar]
- 24.Lemperiere T, Lepine JP, Rouillon F, et al. Comparaison de différents instruments d’ évaluation de la dépression à l’occasion d’une étude sur l’Athymil 30 mg. Ann Méd Psychol (Paris) 1984;142:1206–12. [PubMed] [Google Scholar]
- 25.Collet L, Cottraux J. Inventaire abrégé de la dépression de Beck (13 items). Etude de la validité concurrente avec les échelles de Hamilton et de ralentissement de Widlcher. Encephale 1986;12:77–9. [PubMed] [Google Scholar]
- 26.Theal JJ, Toosi MN, Girlan LM, et al. Ondansetron ameliorates fatigue in patients with primary biliary cirrhosis (PBC). Hepatology 2002;36:296A. [DOI] [PubMed] [Google Scholar]
- 27.Figg WD, Dukes GE, Pritchard JF, et al. Pharmacokinetics of ondansetron in patients with hepatic insufficiency. J Clin Pharmacol 1996;36:206–15. [DOI] [PubMed] [Google Scholar]
- 28.Khawaja X, Xu J, Liang JJ, et al. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: implications for depressive disorders and future therapies. J Neurosci Res 2004;75:451–60. [DOI] [PubMed] [Google Scholar]
- 29.Calapai G, Corica F, Corsonello A, et al. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest 1999;104:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dsingh N, Gayowski T, Wagener MM, et al. Vulnerability tto psychological distress and depression in patients with end-stage liver disease due to hepatitis C. Clin Transplant 1997;11:406–11. [PubMed] [Google Scholar]
- 31.Jonhson ME, Fisher DG, Fenaughty A, et al. Hepatitis C virus and depression in drug users. Am J Gastroenterol 1998;93:785–9. [DOI] [PubMed] [Google Scholar]
- 32.Love AW, Grabsch B, Clarcke DM, et al. Screening for depression in women with metastatic breast cancer: a comparison of the Beck Depression Inventory Short Form and the Hospital Anxiety and Depression Scale. Aust N Z J Psychiatry 2004;38:526–31. [DOI] [PubMed] [Google Scholar]
- 33.Bryson J. Clinical safety of ondansetron. Semin Oncol 1992;19:26–32. [PubMed] [Google Scholar]
- 34.Müller C, Pongratz S, Pidlich J, et al. Treatment of pruritus in chronic liver disease with the 5-hydroxytryptamine receptor type 3 antagonist ondansetron: a randomized, placebo-controlled, double-blind cross-over trial. Eur J Gastroenterol Hepatol 1998;10:865–70. [DOI] [PubMed] [Google Scholar]