Abstract
Background and aims: Protease activated receptors (PARs) have been postulated to play a role during intestinal inflammation. The presence and role played by PAR4 in gastrointestinal functions have not been fully clarified. The aims of this study were: (i) to examine expression of PAR4 in rat proximal colon; (ii) to determine the mechanical effects induced by PAR4 activation in longitudinal muscle; and (iii) to characterise the underlying mechanisms.
Methods: PAR4 expression was determined by reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry. Mechanical activity was recorded as changes in isometric tension.
Results: A PCR product corresponding to the predicted size of the PAR4 signal was amplified from RNA prepared from the colon of rats, showing the presence of PAR4 in those tissues. Immunohistochemistry revealed that PAR4 protein was expressed on epithelial surfaces and submucosa. PAR4 activating peptides, GYPGKF-NH2 and AYPGKG-NH2, produced concentration dependent contractile effects on longitudinal muscle. Tetrodotoxin (TTX) or atropine significantly reduced the contractile responses to AYPGKG-NH2, and atropine after TTX did not cause any further reduction. NK1 receptor antagonist, SR140333, or NK2 receptor antagonist, SR48968, alone or in combination, produced a reduction in PAR4 induced contractile effect, and when coadministered with TTX abolished it. Capsaicin markedly reduced the contractions evoked by AYPGKG-NH2.
Conclusions: The present results suggest that PAR4 is functionally expressed in rat colon and its activation induces contraction of the longitudinal muscle both through TTX sensitive release of acetylcholine and release of tachykinins, probably from sensory nerves. These actions may contribute to motility disturbances during intestinal trauma and inflammation.
Keywords: protease activated receptors, enteric nervous system, intestinal smooth muscle, PAR4, tachykinins
In addition to their established proteolytic roles, proteases such as trypsin, thrombin, or cathepsin G may also act as cell signalling molecules via protease activated receptors (PARs). This family of seven transmembrane G protein coupled receptors currently includes four receptor subtypes (PAR1, PAR2, PAR3, PAR4). PAR1, PAR3, and PAR4, but not PAR2, are activated by thrombin, whereas trypsin can activate PAR2 and PAR4.1,2 PAR4 can also be activated by the neutrophil granule protease cathepsin G.3 Activation of PARs is initiated by proteolytic cleavage of their extracellular N terminal domain resulting in the unmasking of an extracellular N terminal sequence that acts as a tethered receptor activating ligand. PAR1, PAR2, and PAR4 can also be activated by exogenously applied short synthetic peptides, based on the sequences of their tethered ligands.
Numerous studies have been performed to clarify the role of PARs in the physiology and pathophysiology of the gastrointestinal tract as these tissues, more than others, are exposed to proteinases and PARs are highly expressed throughout the gastrointestinal tract.4 However, most of the studies concerned the functions of PAR1 and PAR2. PAR1 and PAR2 most likely play a role during inflammation, allergy, and/or haemorrhage where thrombin and/or mast cell tryptase become available as endogenous agonists. PAR2 appears to be involved in exocrine secretion5,6 and in intestinal ion transport.7 PAR1 and PAR2 modulate smooth muscle motility8 and their activation can induce relaxant, contractile, or biphasic responses.9–20 Although northern blot analysis has shown that mRNA for PAR4 is present in human small and large intestine,21 the cell types that express PAR4 are still unknown and the physiological role played by PAR4 in the gastrointestinal tract has to be yet fully elucidated. Hollenberg and colleagues12 have reported contractile effects on the longitudinal muscle of rat stomach due to activation of PAR4 by the receptor activating peptide, GYPGQV-NH2, and lack of cross reactivity with PAR1 and PAR2. In addition, in rat oesophageal muscularis mucosae22 and isolated airways,23 PAR4 induces relaxation, a function opposed to PAR1. In contrast, PAR4 appears to mimic the actions of PAR1 in the murine airway smooth muscle.24
The present study was designed to investigate whether PAR4 is present in rat colon and if it affects gut motor functions. The purposes were to: (i) examine expression of PAR4 in rat proximal colon; (ii) determine the mechanical effects induced by PAR4 activation in longitudinal smooth muscle; and (iii) characterise the underlying mechanisms. Specifically, the possible involvement of neurogenic mechanisms and the relative contribution of different neurotransmitters were analysed.
METHODS
Animals
Male Wistar rats weighing 250–300 g were obtained from Morini (Italy) and from Charles River Laboratories (Quebec, Canada). All animals were housed in temperature controlled rooms and had free access to food and water. Local animal care committees approved all experimental protocols. Rats were sacrificed by cervical dislocation, and tissues were immediately removed for analysis.
RT-PCR
For PAR4 reverse transcription-polymerase chain reaction (RT-PCR), total RNA from rat colonic tissues was isolated using the Trizol method (Gibco, Canada). RNA (2 μg) was reverse transcribed and DNA was amplified according to the following cycle conditions: dissociation of nucleic strands at 94°C for one minute, annealing at 55°C for 30 seconds, and extension for one minute at 72°C. The primer sequences for rat PAR4 were 5′-GGA AGT CTT GAG AGA AAG GCA A and 3′-GAA CCA AGA GGC ATC ACC TAT C, and for glyceraldehyde-3-phosphate dehydrogenase (GADPH) 5′-CGG AGT CAA CGG ATT TGG TCG TAT and 3′-AGC CTT CTC CAT GGT GGT GAA GAC. Twenty seven cycles were performed for PAR4 and 23 cycles for GADPH. PCR products were then separated on a 1% agarose gel with ethidium bromide and the gel was scanned under UV light.
Immunohistochemistry
Rat colon tissues were harvested and fixed in Zamboni’s buffer overnight. Tissues were then washed three times in phosphate buffer saline (PBS) and placed in 20% sucrose with PBS. Tissues were embedded in OCT and frozen sections (10 μm) were placed on “superfrost plus” slides. Tissue sections were washed with PBS alone and then incubated for one hour at room temperature with PBS containing 10% donkey serum. Sections were then incubated with primary antibody (affinity purified goat polyclonal anti-PAR4 antibody; Santa Cruz Biotechnology, California, USA; 1/100 dilution) in PBS with 10% donkey serum overnight at 4°C. In control experiments, the anti-PAR4 antibody was preincubated (for 24–48 hours at 4°C with 1 or 10 µM of) with the blocking peptide used for immunisation (peptide mapping the C terminal domain of mouse PAR4; Santa Cruz Biotechnology). Slides were washed in PBS and incubated with secondary antibody (Cy3 conjugated AffiniPure donkey antigoat, 1/500 dilution in PBS plus 1.5% donkey serum) for one hour at room temperature. Tissue sections were then covered with Fluorsave reagent and examined using a Olympus IX50/FLA fluorescent microscope equipped with a coolsnap-fx camera.
Mechanical recording
Rat colon was removed distally to the caecum. The colonic lumen was cleaned with Krebs solution and segments of approximately 2 cm in length were cut. The preparation was then placed in a continuously perfused organ bath containing 5 ml of gassed (95% O2 and 5% CO2) and heated (37°C) Krebs solution with the following composition (mM): NaCl 119; KCl 4.5; MgSO4 2.5; NaHCO3 25; KH2PO4 1.2; CaCl2 2.5; and glucose 11.1. Preparations were then tied with silk thread to an isometric force transducer (DY2; Ugo Basile, Comerio, Varese, Italy) for recording of isometric tension of the longitudinal muscle on an ink writer recorder (Gemini; Ugo Basile). Segments were allowed to equilibrate for at least 30 minutes under a 1 g load before starting the experiment.
Experimental protocol
After the equilibration period, the preparation was challenged with carbachol at 10 μM which, in preliminary experiments, was demonstrated to induce a maximal effect. Then, responses of the preparations to cumulative concentrations of GYPGKF-NH2, the murine synthetic PAR4 activating peptide, AYPGKG-NH2, which is reported to be a stronger agonist for PAR4,25 or YAPGKG-NH2, the inactive control peptide, were examined. The peptides were added into the bath in volumes of 50 μl after switching off the perfusion to give the concentrations indicated. Each concentration was left in contact with the tissue for two minutes. In preliminary experiments, in order to assess the reproducibility of the responses, we found that concentration related responses obtained with the first concentration-response curve were not significantly different from the second curve.
The response to PAR4 activation with AYPGKG-NH2 was assessed in the absence and presence of the following inhibitors/antagonists: tetrodotoxin (TTX 1 μM), a Na+ channel blocker; atropine (1 μM), an antagonist of cholinergic muscarinic receptors; SR140333, a selective antagonist of NK1 receptors (1 μM); SR48968, a selective antagonist of NK2 receptors (1 μM); and capsaicin at a concentration of 10 μM, which is reported to cause depletion of tachykinins from sensory nerve fibres.26,27 Some antagonists were coadministered to clarify whether the effectors represented different steps of the same pathway. For each drug, the concentration used was known to be effective in our preparation.28,29 The antagonists were added to the perfusing solution at least 30 minutes before testing the PAR activating peptides, except capsaicin. To prove that TTX treatment was effective, the preparation was stimulated through a pair of platinum ring electrodes.
Data analysis and statistics
Because of spontaneous phasic contractions of the tissues, the contractile response of longitudinal muscle was defined as change in resting tone (the bottom level of the tension oscillations), and was expressed as a percentage of contraction caused by 10 μM carbachol. All data are expressed as means (SEM) where n indicates the number of animals from which intestinal segments were taken. The half maximal contractile concentration (EC50) was calculated from individual experiments by non-linear regression. Differences in peptide induced responses in the absence (control) and presence of inhibitors were analysed by the Student’s t test or analysis of variance (ANOVA) and Bonferroni’ correction, when required. A probability value of less than 0.05 was regarded as significant.
Drugs
The following drugs were used: carbamylcholine chloride (carbachol), capsaicin, tetrodotoxin (TTX) (Sigma, Chemical Corp, St Louis, Missouri, USA), ((S)-N-methyl-N [4-(4-acetyl-amino-4-phenylpiperi-dino)-2-(3, 4-dichloro-phenyl)butyl]benzamide (SR48968), (S)-1-[2-[3-(3, 4-dichlorphenyl)-1 (3-isopropoxy-phenylacetyl) piperidin-3yl] ethyl]-4-phenyl-1 azaniabicyclo [2.2.2] octane chloride (SR140333) (a kind gift from Sanofi Recherche, Montpellier Cédex, France), [Sar9, Met(O2)11]-SP, [β-Ala8]-NKA(4–10) (Calbiochem-Novabiochem AG, Laufelfingen, Switzerland). All drugs were dissolved in distilled water, except for the following: SR48968 and SR140333 dissolved in DMSO, capsaicin dissolved in absolute ethanol, [Sar9, Met(O2)11]-SP dissolved in diluted acetic acid, and [β-Ala8]-NKA(4–10) dissolved in diluted ammonia. Working solutions were prepared fresh on the day of the experiment by diluting the stock solutions. Control experiments using the different solvents alone showed that none had effects on the tissue responses studied.
GYPGKF-NH2, AYPGKG-NH2, and the inactive control peptide YAPGKF-NH2 were purchases from the Peptide Synthesis Core Facility at the University of Calgary (Canada), and prepared by standard solid phase synthesis procedures.
RESULTS
PAR4 expression
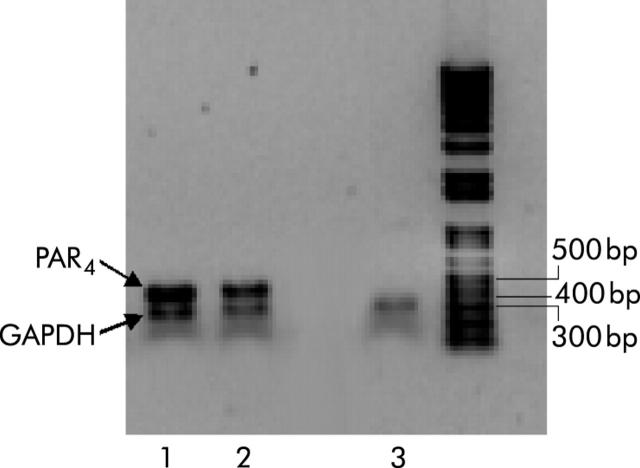
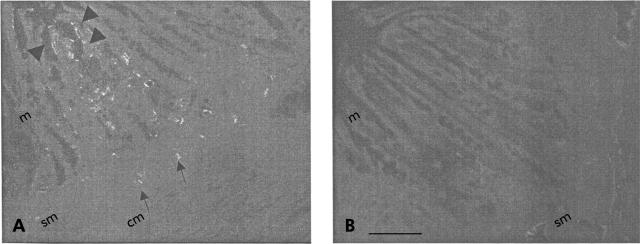
A PCR product of a predicted size of 463 bp was amplified from RNA prepared from the colon of rats (lanes 1 and 2; fig 1 ▶), showing PAR4 presence in those tissues. In the absence of PAR4 primer, only GAPDH amplified product (306 bp) was detected in those tissues (lane 3; fig 1 ▶). PAR4 protein immunoreactivity was also observed in rat colon (fig 2 ▶). PAR4 expression was prominently localised to the mucosal surface (colonocytes; arrowheads in fig 2A ▶), but staining was also observed in the submucosa (arrows in fig 2A ▶). Staining was abolished by preabsorption of antibody with the receptor fragment used to raise the anti-PAR4 antibody (fig 2B ▶).
Figure 1.
Detection of protease activated receptor 4 (PAR4) in rat colon by reverse transcription-polymerase chain reaction. Lanes 1 and 2, colon tissues where PAR4 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were amplified; a specific 463 bp fragment corresponds to PAR4 while GAPDH showed an expected amplified fragment of 306 bp. Lane 3 shows omission of PAR4 primer.
Figure 2.
Immunolocalisation of protease activated receptor 4 (PAR4) in rat colon. In (A), tissues were incubated with an anti-PAR4 primary antibody and then with a Cy3 conjugated secondary antibody. The control (B) shows incubation of primary anti-PAR4 antibody with blocking peptide, followed by Cy3 conjugated secondary antibody. m, mucosa, sm, submucosa, cm, circular muscle. Arrowheads shows colonocyte staining, arrows shows submucosa staining. Scale bar, 10 μm.
Functional studies
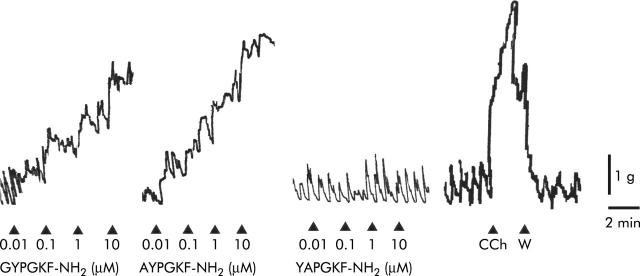
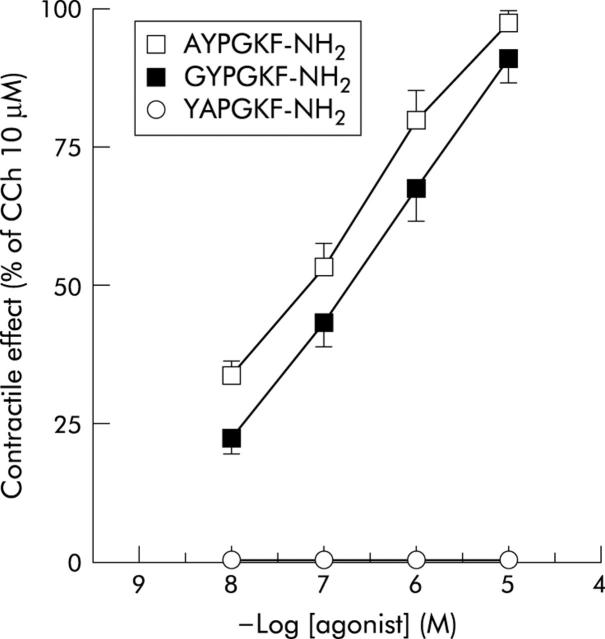
Under the present experimental conditions, rat colon smooth muscle showed spontaneous mechanical activity, consisting in rhythmic phasic changes of isometric tension (from 1 to 2.5 g). The PAR4 activating peptides, GYPGKF-NH2 (0.01–10 μM) and AYPGQV-NH2 (0.01–10 μM), caused a concentration dependent contractile response in the longitudinal muscle of rat colon, consisting of an increase in basal tension with maintenance of the phasic contraction (fig 3 ▶). AYPGQV-NH2 (EC50 = 0.08 (0.06) μM; n = 6) was more potent than GYPGKF-NH2 (EC50 = 0.3 (0.1) μM; n = 8) but efficacy was similar, both peptides being able to reach approximately 90% of the contraction induced by carbachol (10 μM) at the maximal concentration tested (fig 4 ▶). In order to verify the specificity of the response, we applied to the preparations the inactive control peptide YAPGQV-NH2 (0.01–10 μM) and this had no effect (figs 3 ▶, 4 ▶).
Figure 3.
Representative recordings of the contractile effects induced by the protease activated receptor 4 (PAR4) activating peptides, GYPGKF-NH2 and AYPGKF-NH2, by the inactive control peptide, YAPGKF-NH2, and by carbachol (CCh 10 μM) on the longitudinal muscle of rat colon. Arrows indicate application of the agonist. W, washout.
Figure 4.
Concentration-response curves for the effects evoked by the protease activated receptor 4 (PAR4) activating peptides, GYPGKF-NH2 and AYPGKF-NH2, and the inactive control peptide, YAPGKF-NH2, on the longitudinal muscle of rat colon. The contractile effects are expressed as a percentage of the contraction to carbachol (CCh 10 μM). Each value is the mean (SEM) of 4–8 experiments.
To clarify the mechanism underlying the contractile effect produced by PAR4 activation, we evaluated the responses evoked by AYPGQV-NH2 in the presence of TTX. TTX (1 μM), which per se did not modify spontaneous contractions, significantly reduced the responses induced by the PAR4 activating peptide, but did not abolish them, indicating partial involvement of neural mechanism (fig 5A ▶).
Figure 5.
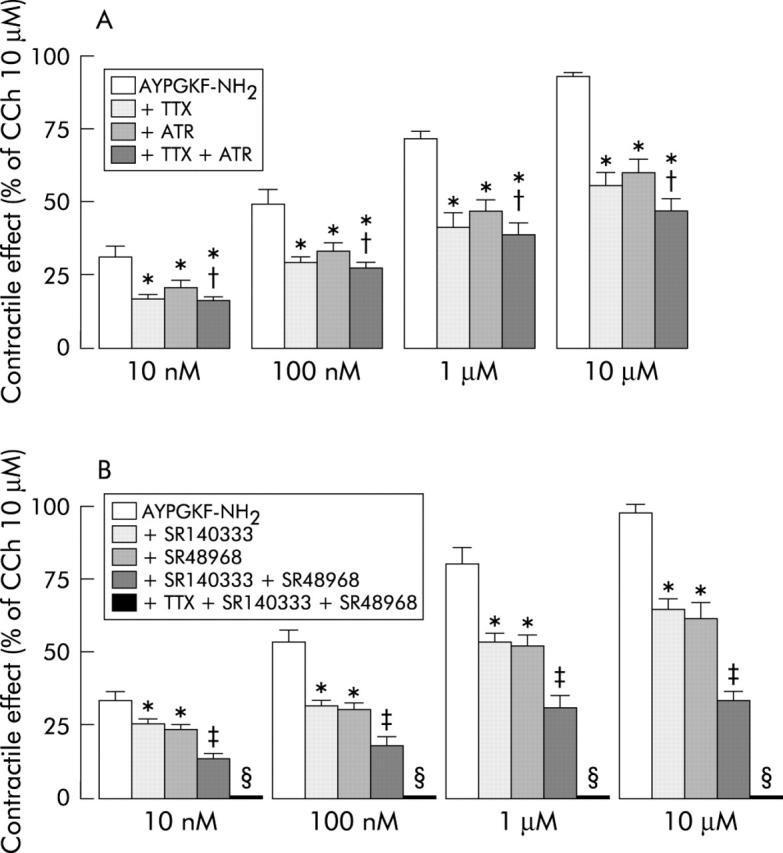
(A) Effects of tetrodotoxin (TTX) and atropine, alone or in combination, on the contractile responses evoked by protease activated receptor 4 (PAR4) activating peptide, AYPGKF-NH2, on the longitudinal muscle of rat colon. The contractile responses to AYPGKF-NH2 were significantly reduced by TTX (1 μM) or atropine (1 μM). The combination of atropine (1 μM) and TTX (1 μM) did not cause any further reduction. (B) Effects of SR140333 and SR48968, alone, in combination, or after treatment with TTX, on the contractile responses evoked by PAR4 activating peptide, AYPGKF-NH2, on the longitudinal muscle of rat colon. The contractile responses to AYPGKF-NH2 were significantly reduced by SR140333 (1 μM), an antagonist of NK1 receptors, by SR48968 (1 μM), an antagonist of NK2 receptors, and even more by a combination of SR48968 (1 μM) and SR140333 (1 μM). AYPGKF-NH2 did not induce any response in the presence of a combination of SR48968 (1 μM), SR140333 (1 μM), and TTX (1 μM). The contractile effects are expressed as a percentage of the contraction to carbachol (CCh 10 μM). Each value is mean (SEM) of five experiments. *p<0.05 compared with control value; †p>0.05 compared with TTX or atropine alone; ‡p<0.05 compared with SR140333 and SR48968 alone; §p<0.05 compared with the combination of SR140333 and SR48968.
As tachykinins and acetylcholine are among the major excitatory neurotransmitters to intestinal smooth muscle, we evaluated if these mediators could be involved in the PAR4 agonist induced contractile response. Therefore, we tested the effects of their antagonists on responses evoked by PAR4 activation both in the absence and presence of TTX. Pretreatment of tissues with the muscarinic antagonist atropine (1 μM) significantly decreased the contractile responses induced by AYPGKF-NH2 (fig 5A ▶). Contractile responses to 10 μM carbachol were absent in these tissues (data not shown). It is interesting that the reduction in the PAR4 contractile effect induced by atropine was of the same degree as that induced by TTX; furthermore, subsequent addition of atropine (1 μM) after TTX caused no further reduction of the PAR4 induced contractile effect (fig 5A ▶).
Both NK1 and NK2 receptor antagonists, SR140333 (1 μM) and SR48968 (1 μM), respectively, reduced independently the contractile effects induced by PAR4 activating peptide (fig 5B ▶). The concentration of the NK1 receptor selective antagonist SR140333 that we used (1 μM) antagonised the contractile response to the NK1 receptor agonist [SAR9, Met(O2)11]-SP but not to the NK2 receptor agonist [β-ala8]-neurokinin A (4–10). The concentration of the NK2 receptor selective antagonist SR48968 that we used (1 μM) antagonised the contractile response to the NK2 receptor agonist [β-ala8]-neurokinin A (4–10) but not to the NK1 receptor agonist [SAR9, Met(O2)11]-SP (data not shown). The suppressive effects of the NK1 receptor selective antagonist SR140333 (1 μM) were additive to those induced by the NK2 receptor selective antagonist SR48968 (1 μM) (fig 5B ▶).When both antagonists were coadministered with TTX (1 μM), the contractile effects evoked by PAR4 activating peptide were abolished (fig 5B ▶).
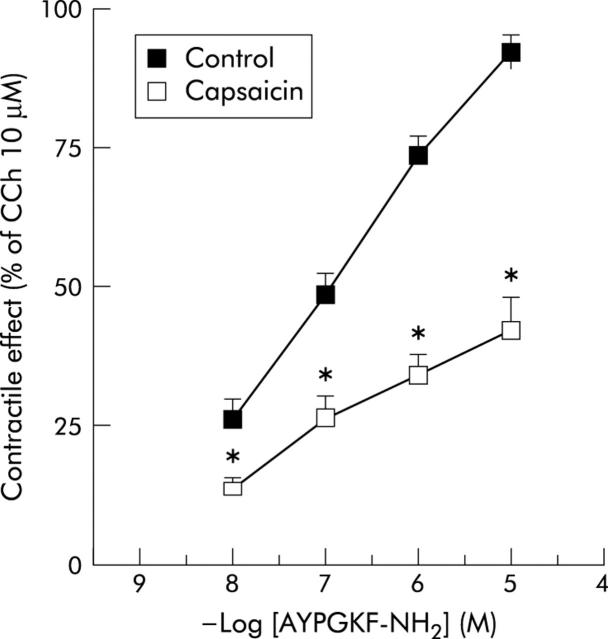
Lastly, pretreatment of the preparation with capsaicin (10 μM) for 90 minutes significantly reduced the contractile effects induced by PAR4 activating peptide (fig 6 ▶).
Figure 6.
Concentration-response curves for the effects evoked by the protease activated receptor 4 (PAR4) activating peptide AYPGKF-NH2 on the longitudinal muscle of rat colon in the absence or presence of capsaicin. Capsaicin (10 μM) significantly antagonised the contractile response to the PAR4 agonist. The contractile effects are expressed as a percentage of the contraction to carbachol (CCh 10 μM). Each value is the mean (SEM) of five experiments. *p<0.05 compared with control values.
DISCUSSION
Possible physiological/pathophysiological roles for PAR4 in tissues or cells other than platelets are poorly understood, in spite of their wide distribution in varying tissues.12,21,30–32 Considering the effects of PAR4 agonists on smooth muscles, some evidence indicates the PAR4 is not involved in modulation of the motility of rat duodenum16 or guinea pig gall bladder.18 However, Hollenberg and colleagues12 have reported that PAR4 agonists induce contraction of gastric longitudinal smooth muscle and nitric oxide dependent relaxation of vascular smooth muscle. It is not yet clear whether PAR4 activation plays a role similar or opposite to PAR1. In fact, PAR4 activation appears to mimic the effects of PAR1 activation in gastric and airway smooth muscle,12,24 whereas in the rat oesophageal muscularis mucosae, PAR4 appears to mediate relaxation, in contrast to PAR1.22
The present study demonstrates that PAR4 is present in rat colon and its activation evokes contraction of intestinal longitudinal smooth muscle. Both GYPGKF-NH2, corresponding to the murine PAR4 tethered ligand, and AYPGKF-NH2, reported to be a more potent peptide analogue for PAR4,25 induced a concentration dependent contractile response. The control inactive peptide YAPGKF-NH2 had no contractile effect on longitudinal smooth muscle preparations, thus confirming the specificity of action of the two PAR4 peptide agonists used. AYPGQV-NH2 was more potent than GYPGKF-NH2, which is in accordance with data from the literature.25 The effective concentration range of GYPGKF-NH2 and AYPGQV-NH2 for induction of the contractile responses was lower than that required for production of contraction in other preparations.12 One possible explanation for this observation is that in our preparation the PAR4 activating peptides were not susceptible to rapid protease degradation, or alternatively the high potency of agonist may reflect greater expression of this type of receptor in the colon than in the stomach, as reported by Xu and colleagues.21
However, the contractile effects we demonstrated for PAR4 agonists in rat colonic smooth muscle preparations suggest a role for this receptor in motility functions of the gut. Whether or not this role is associated with pathophysiological circumstances may depend on the availability of endogenous proteases in the vicinity of the receptor. Which endogenous protease activates PAR4 in intestinal tissues remains to be established. Both thrombin and trypsin have been reported to activate PAR4.21 In a previous study, we demonstrated that thrombin and trypsin induced contractile effects in rat colonic longitudinal muscle preparations, showing biphasic responses: relaxation followed by contraction for the highest concentrations.19 These effects were mimicked by PAR1 and PAR2 activating peptides, suggesting that thrombin and trypsin could be responsible for endogenous activation of PAR1 and PAR2, respectively. However, the present results highlight PAR4 as another potential effector of both thrombin and trypsin. The similar effects of PAR1, PAR2, and PAR4 could make redundant the effects of thrombin and trypsin in rat intestinal longitudinal muscle as was suggested for platelets and smooth muscles from gastric, vascular, and airway tissues.12,24,30,31,33 Recently, the neutrophil granule protease cathepsin G3 has been found to stimulate PAR4. This adds cathepsin G to the list of potential endogenous activators of PAR4 in intestinal tissues. Moreover, it links PAR4 activation in those tissues to the presence of granulocytes, suggesting that inflammatory pathophysiological circumstances, such as inflammatory bowel diseases, may be associated with PAR4 activation.
We examined the mechanism by which PAR4 activation induces contractile effects in rat colonic longitudinal muscle by treating the preparation with TTX, a neuronal conduction blocker, to assess the role of neurotransmission in the evoked effect. The finding that the contractile responses to PAR4 activating peptides were antagonised, although not completely, by TTX, suggests that a neurogenic component is involved in the PAR4 agonist evoked effect. Previous studies have reported that PAR1 and PAR2 are expressed by a large proportion of neurones of the myenteric plexus of the guinea pig intestine34 and PAR2 receptors are expressed on cholinergic nerves in porcine ileum.35 However, information on the cellular localisation of PAR4 in intestinal tissue is lacking. In mouse, PAR4 expression on peripheral nerves of bladder has been shown.36 We observed immunostainning for PAR4 in submucosal structures which could be submucosal neurones (fig 2A ▶), but double labelling with a neuronal marker would be necessary to ascertain that PAR4 is expressed on neurones in the rat colon. We have evaluated if acetylcholine or tachykinins could be responsible for the PAR4 neurally mediated effect because these chemical mediators are considered the major excitatory neurotransmitters of intestinal smooth muscle. Atropine reduced the contractile response to PAR4 activation to the same extent as TTX but failed to decrease it further after TTX treatment. This indicates that PAR4 activation can induce acetylcholine release through an action potential dependent neural mechanism. In addition, NK1 and NK2 receptor antagonists, when administered separately or in combination, reduced contraction due to PAR4 activation, suggesting involvement of these receptors in the effects evoked by AYPGKF-NH2. The presence of these receptors, which mediate contraction in the rat colon, has been demonstrated previously by histochemical and functional studies.26,37,38 On the other hand, the action of both SR140333 and SR48968 has to be considered as specifically linked to occupancy of NK1 or NK2 receptors, respectively, as these drugs antagonised exclusively contraction induced by selective agonists of the respective receptors. Because the suppressive effects of NK1 and NK2 receptor antagonists were additive to the effect of TTX, it could be hypothesised that PAR4 activation can induce tachykinin release without the contribution of propagated action potential (TTX resistant release). In fact, TTX may not inhibit release of neurotransmitters following direct stimulation of receptors present in nerve terminals. Therefore, we hypothesise that prejunctional PAR4 may directly induce the release of tachykinins, which are mostly released by neurones in the gut.26
In addition, capsaicin significantly reduced the response induced by PAR4 activating peptide. Although capsaicin can cause different effects, such as VR1 receptor desensitisation or blockade of voltage gated calcium channels, it is likely that the effect of 10 μM capsaicin for 90 minutes can be attributed to an inhibitory effect on the release of biologically active substances, such as tachykinins, by sensory nerve fibres.26,27 Depletion of tachykinins from sensory nerves could account for the reduction in contractile effect to PAR4 activation. It has been reported that PAR2 activation stimulates the release of substance P from terminals of capsaicin sensitive primary sensory neurones, thus causing neurogenic inflammatory responses in peripheral tissue.39 Interestingly, a similar system has also been described for trypsin induced contraction of the guinea pig bronchus, with release of tachykinins from sensory nerves being responsible for the final contractile response.40,41 In addition, our recent data have suggested that PAR1 and PAR2 activation can induce neural tachykinin release.29
In conclusion, this study has demonstrated for the first time that PAR4 is functionally expressed in rat colon. It can mediate a contractile response of longitudinal muscle through TTX sensitive release of acetylcholine and through action potential independent release of tachykinins, probably from sensory nerves. This mechanism of action may support a proinflammatory role for PAR4, which could contribute to motility disturbances observed during intestinal trauma and inflammation.
Acknowledgments
This work was supported by a grant from Ministero dell’Università e della Ricerca scientifica, Italy (FM), the Canadian Institute for Health Research (NV), and the Canadian Association of Gastroenterology (NV).
Abbreviations
PARs, protease activated receptors
PBS, phosphate buffer saline
TTX, tetrodotoxin
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
EC50, half maximal contractile concentration
RT-PCR, reverse transcription-polymerase chain reaction
REFERENCES
- 1.Dery O, Corvera CU, Steinhoff M, et al. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol 1998;274:C1429–52. [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane SR, Seatter MJ, Kanke T, et al. Proteinase-activated receptors. Pharmacol Rev 2001;53:245–82. [PubMed] [Google Scholar]
- 3.Sambrano GR, Huang W, Faruqi T, et al. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem 2000;275:6818–23. [DOI] [PubMed] [Google Scholar]
- 4.Vergnolle N. Proteinase-activated receptors—novel signals for gastrointestinal pathophysiology. Aliment Pharmacol Ther 2000;14:257–66. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TD, Moody MW, Steinhoff M, et al. Trypsin activates pancreatic duct epithelial cell ion channels. J Clin Invest 1999;103:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawabata A, Nishikawa H, Kuroda R, et al. Proteinase-activated receptor-2 (PAR-2): regulation of salivary and pancreatic exocrine secretion in vivo in rats and mice. Br J Pharmacol 2000;129:1808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergnolle N, Macnaughton WK, Al-Ani B, et al. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc Natl Acad Sci U S A 1998;95:7766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawabata A, Kuroda R, Nagata N, et al. In vivo evidence that protease-activated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br J Pharmacol 2001;133:1213–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Ani B, Saifeddine M, Hollenberg MD. Detection of functional receptors for the proteinase-activated-receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can J Physiol Pharmacol 1995;73:1203–7. [DOI] [PubMed] [Google Scholar]
- 10.Saifeddine M, Al-Ani B, Cheng CH, et al. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br J Pharmacol 1996;118:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corvera CU, Déry O, Mcconalogue K, et al. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest 1997;100:1383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollenberg MD, Saifeddine M, Al-Ani B, et al. Proteinase-activated receptor 4 (PAR4): action of PAR4-activating peptides in vascular and gastric tissue and lack of cross-reactivity with PAR1 and PAR2. Can J Physiol Pharmacol 1999;77:458–64. [PubMed] [Google Scholar]
- 13.Hollenberg MD, Saifeddine M, Al-Ani B, et al. Proteinase activated receptor: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can J Physiol Pharmacol 1997;75:832–41. [PubMed] [Google Scholar]
- 14.Zheng XL, Renaux B, Hollenberg MD. Parallel contractile signal transduction pathways activated by receptors for thrombin and epidermal growth factor-urogastrone in guinea pig gastric smooth muscle: blockade by inhibitors of mitogen-activated protein kinase-kinase and phosphatidyl inositol 3’-kinase. J Pharmacol Exp Ther 1998;285:325–34. [PubMed] [Google Scholar]
- 15.Cocks TM, Sozzi V, Moffatt JD, et al. Protease-activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology 1999;116:586–92. [DOI] [PubMed] [Google Scholar]
- 16.Kawabata A, Kuroda R, Nishikawa H, et al. Modulation by protease-activated receptors of the rat duodenal motility in vitro: possible mechanisms underlying the evoked contraction and relaxation. Br J Pharmacol 1999;128:865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawabata A, Kuroda R, Kuroki N, et al. Characterization of protease-activated receptor-1-mediated contraction and relaxation in the rat duodenal smooth muscle. Life Sci 2000;67:2521–30. [DOI] [PubMed] [Google Scholar]
- 18.Tognetto M, Trevisani M, Maggiore B, et al. Evidence that PAR-1 and PAR-2 mediate prostanoid-dependent contraction in isolated guinea-pig gallbladder. Br J Pharmacol 2000;131:689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulè F, Baffi MC, Cerra MC. Dual effect mediated by protease-activated receptors on the mechanical activity of rat colon. Br J Pharmacol 2002;136:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulè F, Baffi MC, Falzone M, et al. Signal transduction pathways involved in the mechanical responses to the protease-activated receptors in rat colon. J Pharmacol Exp Ther 2002;303:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Xu WF, Andersen H, Whitmore TE, et al. Cloning and characterization of human protease-activated receptor 4. Proc Nat Acad Sci U S A 1998;95:6642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawabata A, Kuroda R, Kuroki N, et al. Dual modulation by thrombin of the motility of rat oesophageal muscularis mucosae via two distinct protease-activated receptors (PARs): a novel role for PAR-4 as opposed to PAR-1. Br J Pharmacol 2000;131:578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow JM, Moffatt JD, Cocks TM. Effect of protease-activated receptor (PAR)-1, -2 and -4-activating peptides, thrombin and trypsin in rat isolated airways. Br J Pharmacol 2000;131:1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan RS, Stewart GA, Henry PJ. Modulation of airway smooth muscle tone by protease activated receptor-1, -2, -3 and -4 in trachea isolated from influenza A virus-infected mice. Br J Pharmacol 2000;129:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faruqi TR, Weiss EJ, Shapiro MJ, et al. Structure-function analysis of protease-activated receptor 4 tethered ligand peptides. J Biol Chem 2000;275:19728–34. [DOI] [PubMed] [Google Scholar]
- 26.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as cotransmitters released from peripheral endings of sensory nerves. Prog Neurobiol 1995;45:1–98. [DOI] [PubMed] [Google Scholar]
- 27.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 1991;43:143–201. [PubMed] [Google Scholar]
- 28.Mulè F, D’angelo S, Tabacchi G, et al. Involvement of tachykinin NK2 receptors in the modulation of spontaneous motility in rat proximal colon. Neurogastroenterol Mot 2000;12:459–66. [DOI] [PubMed] [Google Scholar]
- 29.Mulè F, Baffi MC, Capparelli A, et al. Involvement of nitric oxide and tachykinins in the effects induced by protease-activated receptors in rat colon longitudinal muscle. Br J Pharmacol 2003;139:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahn ML, Zheng YW, Huang W, et al. A dual thrombin receptor system for platelet activation. Nature 1998;394:690–4. [DOI] [PubMed] [Google Scholar]
- 31.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, et al. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest 1999;103:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bretschneider E, Kaufmann R, Braun M, et al. Evidence for functionally active protease-activated receptor-4 (PAR-4) in human vascular smooth muscle cells. Br J Pharmacol 2000;132:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coughlin SR. Protease-ativated receptors and platelet function. Thomb Haemost 1999;82:353–6. [PubMed] [Google Scholar]
- 34.Corvera CU, Déry O, Mcconalogue K, et al. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J Physiol 1999;517:741–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green BT, Bunnett NW, Kulkarni-Narla A, et al. Intestinal type 2 proteinase-activated receptors: expression in opioid-sensitive secretomotor neural circuits that mediate epithelial ion transport. J Pharmacol Exp Therap 2000;295:410–16. [PubMed] [Google Scholar]
- 36.D’Andrea MR, Saban MR, Nguyen NB, et al. Expression of protease-activated receptor-1, -2, -3, and -4 in control and experimentally inflamed mouse bladder. Am J Pathol 2003;162:907–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grady EF, Baluk P, Böhm S, et al. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J Neurosci 1996;16:6975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serio R, Mulè F, Bonvissuto F, et al. Tachykinins mediate noncholinergic excitatory neural responses in the circular muscle of rat proximal colon. Can J Physiol Pharmacol 1998;76:684–9. [DOI] [PubMed] [Google Scholar]
- 39.Steinhoff M, Vergnolle N, Young SH, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med 2000;6:151–8. [DOI] [PubMed] [Google Scholar]
- 40.Carr MJ, Schechter M, Undem BJ. Trypsin-induced, neurokinin-mediated contraction of guinea-pig bronchus. Am J Resp Crit Care Med 2000;162:1662–7. [DOI] [PubMed] [Google Scholar]
- 41.Ricciardolo FL, Steinhoff M, Amadesi S, et al. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Resp Crit Care Med 2000;161:1672–80. [DOI] [PubMed] [Google Scholar]