Abstract
Background and aims: Oesophageal pH is conventionally recorded from a point 5 cm above the lower oesophageal sphincter. However, the mucosal changes of reflux oesophagitis and intestinal metaplasia tend to affect the segment of oesophagus distal to this and close to the squamocolumnar junction. This study set out to investigate oesophageal acid exposure of squamous mucosa close to the squamocolumnar junction.
Methods: Dual channel 24 hour pH monitoring was carried out in 11 patients with endoscopy negative dyspepsia and no evidence of gastro-oesophageal reflux by conventional oesophageal pH metry. Oesophageal pH was recorded from electrodes positioned 5 mm and 55 mm proximal to the squamocolumnar junction. A novel technique was developed using metal clips to secure the pH catheter to the oesophageal mucosa and maintain these electrode positions. Oesophageal manometry indicated that the distal electrode was within the high pressure zone of the lower oesophageal sphincter.
Results: We found that 24 hour oesophageal acid exposure (per cent time pH <4) was greater 5 mm above the squamocolumnar junction compared with the conventional position 5 cm more proximal (11.7% v 1.8%; p<0.001). The greater acid exposure at the distal versus the conventional site was apparent in both the upright (12.7% v 2.3%) and supine (10.5% v 1.3%) positions, as well as during preprandial (14.2% v 1.6%) and postprandial (21.8% v 2.8%) periods (p<0.001 for each). The number of reflux events recorded close to the squamocolumnar junction was also higher than at the conventional position (168 v 33; p<0.001). There was no correlation between acid exposure at the two sites.
Conclusions: The squamous mucosa of the most distal oesophagus is exposed to substantial acidic reflux, even in patients without evidence of conventional reflux disease. This short segment reflux may explain the high incidence of metaplasia and neoplasia at the gastro-oesophageal junction.
Keywords: lower oesophageal sphincter, acid exposure, oesophageal squamocolumnar junction, reflux oesophagitis
Gastro-oesophageal reflux is probably the commonest chronic disease of the population of the Western world.1 The mechanisms involved in the aetiology of the disorder are complex and incompletely understood. In recent years there has been considerable interest in transient lower oesophageal sphincter relaxations (TLOSRs) and their role in allowing gastric contents to reflux into the oesophagus.2,3 There has also been a resurgence of interest in the role of hiatus hernia and the accompanying loss of the extrinsic sphincter mechanisms.4,5 Gastro-oesophageal reflux from both of the above mechanisms arises from temporary or more prolonged loss of function of the full length of the lower oesophageal sphincter (LOS), allowing gastric contents to pass into the body of the oesophagus.
We recently reported that acid in the most proximal cardia region of the stomach escapes the buffering effects of food and remains highly acidic during the postprandial period.6 In addition, we observed that this unbuffered pocket of acid may traverse the squamocolumnar junction and extend 1–2 cm into the distal oesophagus.6 This suggested the existence of short segment reflux with acid reaching the most distal intrasphincteric segment of the oesophagus but without traversing the sphincter.
In studies of gastro-oesophageal reflux, the oesophageal pH electrode is traditionally positioned 5 cm above the proximal limit of the LOS. This convention was adopted early on to avoid the electrode slipping into the stomach during swallowing when the oesophagus shortens by 2–3 cm.7 As a consequence, a conventionally placed pH electrode will only detect acid refluxing into the distal oesophagus if it reaches this point 5 cm above the LOS.
If acid exposure of the most distal oesophagus is greater than that of the conventional measuring point proximal to the LOS, it could have significance clinically. In particular, it could explain the fact that metaplasia of the distal oesophagus is most prevalent at and immediately proximal to the gastro-oesophageal junction. Both short segment Barrett’s oesophagus and specialised intestinal metaplasia of the gastro-oesophageal junction are 3.5 times more prevalent than long segment Barrett’s.8
Our earlier study suggesting the existence of short segment reflux involved monitoring luminal pH while the electrode was withdrawn from the stomach into the oesophagus at 1 cm increments every one minute.6 The methodology provided little information about the frequency or duration of short segment reflux or its relationship to the traditionally recognised long segment reflux. We have extended our studies by endoscopically securing a pH electrode in the most distal oesophagus and comparing acid exposure at this site with that of a conventionally placed electrode.
The aim of this study was to compare acid exposure in the most distal oesophagus with that at the conventional oesophageal position, 5 cm above the LOS.
SUBJECTS AND METHODS
Patients
We enrolled 14 patients with chronic dyspepsia and normal upper gastrointestinal endoscopy with no evidence of oesophagitis or a hiatus hernia. The CLO test and antral histology showed no evidence of Helicobacter pylori infection in any patient. All patients had normal 24 hour oesophageal pH monitoring (total per cent time < pH 4: mean 2.7 (range 0.6–4.8)). Mean age was 43 years (range 28–56), and there were six males and five females. All antisecretory therapy was discontinued for three weeks prior to the study. The character of their dyspepsia encompassed a range of upper gastrointestinal symptoms: retrosternal discomfort (n = 5), epigastric discomfort (n = 4), and bloating (n = 2).
Placement of pH catheter
pH monitoring was undertaken with a modified custom made two channel pH catheter (Synectics, Enfield, UK). This catheter incorporated two unipolar antimony electrodes 105 mm and 155 mm from the catheter tip. Three small prolene loops were tied to the catheter, two at 95 mm from the catheter tip and one 200 mm from the tip.
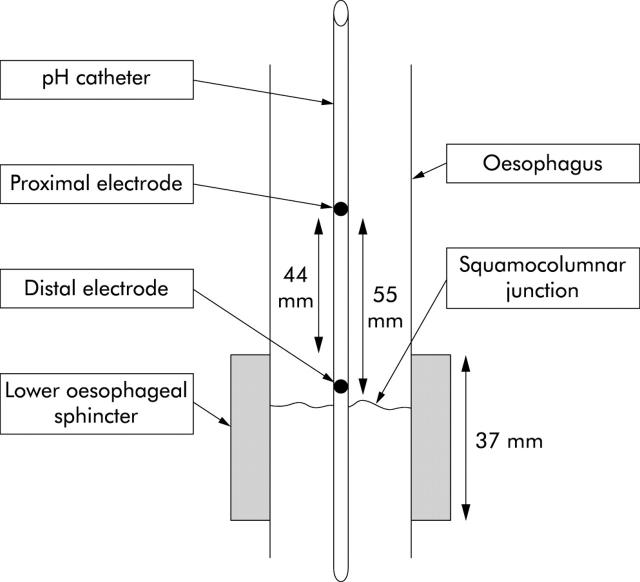
Patients attended the endoscopy unit at 09:00 having fasted, and were given intravenous midazolam to produce conscious sedation. The pH catheter was passed into the oesophagus via the nose. Occasionally grasping forceps were used to position the pH catheter under endoscopic vision. Once the pH catheter was in the stomach it was gently withdrawn until the distal oesophageal electrode was 5 mm proximal to the squamocolumnar junction. An endoscopic clip fixing device (Olympus, Middlesex, UK) was then used to clip one of the prolene loops on the probe to the squamocolumnar junction (fig 1 ▶). A second clip was applied to attach the proximal prolene loop in the oesophageal body at approximately 30 cm from the incisors. The prolene loops were small so that after clipping, the pH probe was unable to move by more than 1 or 2 mm around these anchor points.
Figure 1.
The pH catheter attached to the squamocolumnar junction. Two metal clips have fixed a blue prolene loop to the mucosa at this point. The distal oesophageal electrode is visible 5 mm proximal to the squamocolumnar junction.
During the procedure, particular attention was paid to the appearance of the gastro-oesophageal junction. Distances from the incisors to the squamocolumnar junction, proximal end of the gastric folds, and diaphragmatic pinch were measured. At the end of the procedure there were two electrodes in the oesophagus (electrodes 1 and 2). Electrode 1 was positioned 5.5 cm proximal to the squamocolumnar junction and electrode 2 was positioned 5 mm proximal to the squamocolumnar junction. All patients had LOS measured by the station pull through technique with a water perfused manometry catheter. The length of the LOS high pressure zone was measured and the pressure inversion point (PIP) identified. The thoracic/abdominal ratio of the LOS was derived from these measurements. The pinch of the diaphragm at endoscopy was assumed to correspond with the manometric PIP. The distance from the oesophageal pH electrodes to the pinch of the diaphragm was measured and assumed to be equal to the distance to the PIP. From the manometry measurements it was then possible to estimate the distance between the pH electrode and the upper border of the LOS.
On recovery, the patient was monitored for 24 hour pH in the research unit. A standard lunch (soup, sandwich, and rice pudding snack) and dinner (battered cod and French fries) were provided. During the day subjects were seated for most of the time.
Six hours after the probe had been clipped in place, a plain radiograph of the left upper quadrant was obtained to determine the probe alignment in the stomach and confirm that the clips were still in place. The following morning a second endoscopy was undertaken. The probe position was examined to assess the position of the two electrodes and to check any movement of the clips. After the second endoscopy the pH probe was removed. The endoscopic clips were painlessly detached from the oesophageal mucosa with firm traction.
Analyses
pH data were recorded on a digital data logger (Diggitrapper MK III; Synectics), downloaded onto a PC, and analysed by oesophogram 5.4 software (Synectics). The postprandial period was defined as the three hours after the evening meal. The preprandial period was defined as the three hours prior to the evening meal. Oesophageal acid exposure was measured as per cent time pH <4 for total, upright, supine, preprandial, and postprandial time periods. The DeMeester score was also calculated. This is a scoring system which is derived from six pH parameters defining the number of reflux events, duration of reflux events, and per cent time pH <4.9
The minimum pH recorded during a reflux episode was measured manually in each subject. The median value of these measurements was determined and termed the median pH nadir. A reflux episode was defined as starting when the pH fell below 4 and ending when the pH rose above 4.
Statistical analyses
Values are given as mean and ranges and were compared using the paired Student’s t test.
Ethics
The study was approved by the North Glasgow University NHS Trust and all subjects gave written informed consent.
RESULTS
Three of the recruited patients did not provide results for this study. Two of these were unable to tolerate passage of the pH probe at endoscopy and in a further patient the pH probe dislodged, advancing the oesophageal electrodes into the stomach. Analysis was carried out in the remaining 11 patients.
The proximal oesophageal pH electrode was positioned a mean of 4.4 cm (range 3–6) above the upper border of the LOS and 5.5 cm above the squamocolumnar junction. The distal oesophageal pH electrode was positioned a mean of 5 mm above the squamocolumnar junction and 6 mm below the upper border of the LOS (range 1 to −1). Positioning of the electrodes relative to the squamocolumnar junction and LOS is shown in fig 2 ▶.
Figure 2.
Schematic diagram showing the location of the pH electrodes relative to the squamocolumnar junction and the high pressure zone of the lower oesophageal sphincter.
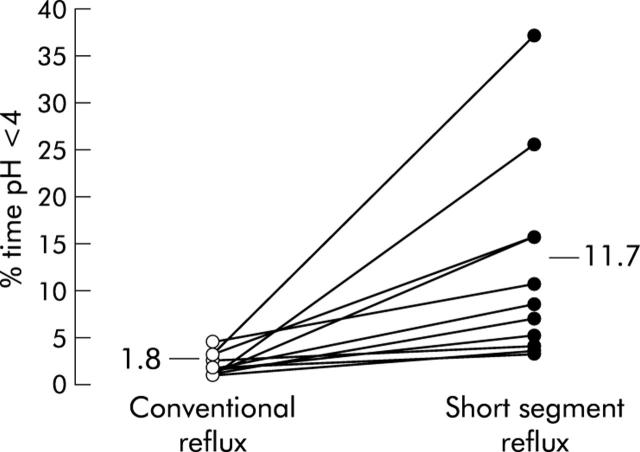
pH measurements revealed that close to the squamocolumnar junction, oesophageal acid exposure was significantly greater than at the electrode position 5.5 cm more proximal. Median total per cent time pH <4 was 11.7% (range 2.4–36.3) versus 1.8% (range 0.8–4.0) (fig 3 ▶). This greater acid exposure of the most distal oesophagus was apparent both in the upright recording period (per cent time pH <4: 12.7% (range 2.3–32.1) v 2.3% (range 0.9–4.3)) and during the supine period (per cent time pH <4: 10.5% (range 1.2–42.9) v 1.3% (range 0–6.1)) (p<0.001) (table 1 ▶). This increased acid exposure in the distal oesophagus was also apparent in the preprandial (per cent time pH <4: 14.2% (range 1.5–57.6) v 1.6% (range 0–6.7)) and postprandial (per cent time pH <4: 21.8% (range 1.0–55.3) v 2.8% (range 0–9.3)) periods (table 2 ▶).
Figure 3.
Oesophageal acid exposure (total per cent time pH <4) measured at 5.5 cm above the squamocolumnar junction (conventional reflux) and at 0.5 cm above the squamocolumnar junction (short segment reflux) in individual patients (n = 11) (p<0.001).
Table 1.
Oesophageal acid exposure and DeMeester score measured 55 mm (conventional reflux) and 5 mm (short segment reflux) above the squamocolumnar junction
| % time pH <4 (Total) | % time pH <4 (Upright) | % time pH <4 (Supine) | DeMeester score | |
| Conventional reflux | 1.8% (0.8–4) | 2.3% (0.9–4.3) | 1.3% (0–6.1) | 8 (4–19) |
| Short segment reflux | 11.7% (2.4–36.3) | 12.7% (2.3–32.1) | 10.5% (1.2–42.9) | 45 (12–131) |
| p<0.001 | p<0.001 | p<0.001 | p<0.001 |
Table 2.
Preprandial and postprandial oesophageal acid exposure measured 55 mm (conventional reflux) and 5 mm (short segment reflux) above the squamocolumnar junction
| % time pH <4 (Preprandial) | % time pH <4 (Postprandial) | |
| Conventional reflux | 1.6 (0–6.7) | 2.8 (0–9.3) |
| Short segment reflux | 14.2 (1.5–57.6) | 21.8 (1–55.5) |
| p<0.001 | p<0.001 |
There was no correlation between oesophageal acid exposure close to the squamocolumnar junction and acid exposure measured at the conventional site (r = 0.19).
The number of recorded individual reflux episodes was also higher close to the squamocolumnar junction compared with the conventional position (168 (range 51–350) v 33 (range 14–53); p<0.001). Consequently, the DeMeester score (derived from the number of reflux episodes and per cent time pH <4) calculated from the pH data recorded close to the squamocolumnar junction was significantly greater than at the conventional position (45 (range 12–131) v 8 (range 4–19); p<0.001) (table 1 ▶). The median pH nadir recorded during reflux episodes was lower close to the squamocolumnar junction than at the conventional site (pH 2.0 (range 1.4–2.6) v 2.9 (range 1.9–4.0); p<0.01) (table 3 ▶).
Table 3.
Number, pH nadir, and mean length of reflux episodes, recorded 55 mm (conventional reflux) and 5 mm (short segment reflux) above the squamocolumnar junction
| No of reflux episodes | Reflux pH nadir | Mean length of reflux episode (s) | |
| Conventional reflux | 33 (14–53) | 2.9 (1.9–4.0) | 50 (19–110) |
| Short segment reflux | 168 (51–350) | 2.0 (1.4–2.6) | 54 (25–91) |
| p<0.001 | p<0.01 |
The degree of acid exposure just proximal to the squamocolumnar junction was greater during the postprandial period versus the preprandial period with per cent time pH <4 being 21.8% (range 1.0–55.3) compared with 14.2% (range 1.5–57.6) (p<0.05) (table 2 ▶). Acid exposure just proximal to the squamocolumnar junction was similar during the upright versus the supine period (per cent time pH <4: 12.7% (range 2.3–32.1) v 10.5% (range 1.2–42.9)).
There was no significant difference between mean length of reflux episode measured just proximal to the squamocolumnar junction (54 seconds (range 25–91)) compared with the conventional position (50 seconds (range 50–110)). Subjectively, the appearance of reflux episodes on the pH tracing was similar at both recording positions (fig 4A, B ▶). In addition, there was no significant intraindividual correlation between the number of reflux episodes at the distal versus the proximal electrode positions (r = 0.16, NS).
Figure 4.
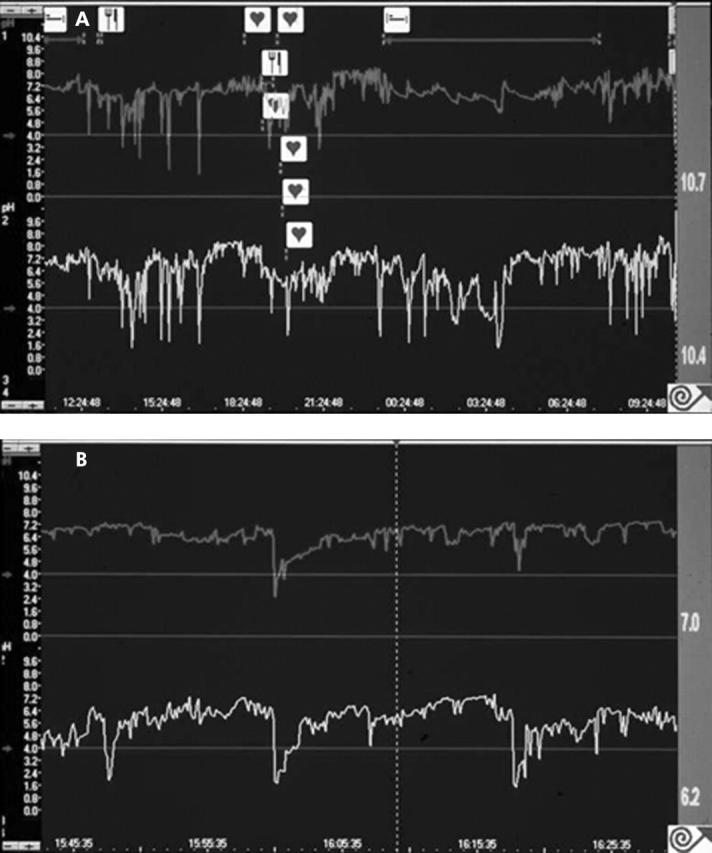
Examples of pH recording. The upper trace in both (A) and (B) displays conventional reflux measured 5.5 cm above the squamocolumnar junction and the lower trace displays short segment reflux measured 0.5 cm above the squamocolumnar junction. Note that tracings in (B) have a more expanded time scale than tracings in (A).
There was no discernable correlation between the character of the patients’ symptoms and the extent of acid exposure of the most distal oesophagus. In particular, per cent time pH <4 just above the squamocolumnar junction in the five patients with predominant retrosternal discomfort (median 6 (range 2.4–14.9)) was similar to that in the six patients with other symptoms (12.5 (3–36.3)). In addition, per cent time pH <4 at the more proximal traditional electrode position in patients with predominant retrosternal discomfort (1.4 (0.8–2.4)) was similar to that in the other patients (2.1 (0.8–4)).
DISCUSSION
The present study indicates that oesophageal acid exposure is much greater just above the squamocolumnar junction than 5 cm proximal to it, where reflux is conventionally measured. All parameters of oesophageal acid exposure were substantially higher at the more distal site, generating a much higher DeMeester score of 45 (range 12–131) compared with 8 (range 4–19). Our findings indicate that acid may reflux onto the most distal squamous oesophageal mucosa without extending more proximally. We would suggest that an appropriate term to describe this phenomenon is “short segment reflux”.
Previous studies comparing acid exposure at different positions in the oesophagus have usually examined acid refluxing above the conventional electrode position 5 cm above the upper border of the LOS. Measuring 10 cm above the LOS will result in a 50% reduction in acid exposure compared with the conventional pH recording position.10,11 There is little information on pH measured closer to the LOS or squamocolumnar junction. Weusten et al, using a multichannel pH probe, reported that 43% of reflux events detected at 3 cm above the LOS failed to reach an electrode at 6 cm above the LOS.12 Loughney et al used dual channel 24 hour pH monitoring to compare the pH at the upper border of the LOS with that at the conventional site 5 cm above the upper border of the LOS.13 They studied patients with long segment Barrett’s, patients with short segment (<3 cm) Barrett’s, and patients with reflux symptoms but normal pH studies. In all three groups they showed higher oesophageal acid exposure at the upper border of the LOS than at the conventional site (per cent time pH <4: 37% v 24%, 13% v 9%, and 4 v 2%, respectively).13 However, this was a relatively modest increase of only 50% compared with the 600% increase in acid exposure seen in our study comparing the conventional site with the most distal oesophagus.
In our study, we positioned the pH electrodes relative to the squamocolumnar junction. Our calculations indicated that the squamocolumnar junction was approximately 11 mm below the upper border of the LOS and thus consistent with that previously reported.14 Our proximal electrode position was similar to the conventional recording position (4.4 cm v 5 cm above the upper border of the LOS). The pH data in this study can therefore be usefully compared with previous studies relying on the manometric LOS distance to position the electrodes.
Our study differs from previous attempts to measure distal oesophageal pH in two ways. Firstly, we anchored the pH electrode to the mucosa with clips to avoid electrode movement during oesophageal shortening. Secondly, in our patients the distal oesophageal electrode was below the upper border of the LOS. Our distal oesophageal electrode was therefore likely to be measuring acid exposure for the first time within, rather than above, the LOS.
As well as comparing total acid exposure we were able to examine the pattern of acid exposure at the two sites. Individual reflux episodes were more frequent and produced a lower minimum pH near the squamocolumnar junction than at the conventional site. The pattern of acid exposure near the squamocolumnar junction showed similarities with conventional acid reflux, being increased during the postprandial compared with the preprandial period. However, we found a difference with respect to posture. Conventional acid reflux is less frequent in the supine versus the upright position whereas our acid exposure measured close to the squamocolumnar junction was similar in both positions.
The mechanism of acid exposure at the two sites needs to be considered. The majority of conventional reflux events are accompanied by TLOSRs, particularly in the presence of a competent LOS. These relaxations tend to occur when upright and after meals and are infrequent when supine overnight.15 Ambulatory studies with 24 hour pH manometry have reported an overall rate of 3 TLOSRs/hour leading to a total of 72 TLOSRs over a 24 hour period.15 Although we did not measure TLOSRs during these pH studies, our average total of 188 short segment reflux events greatly outnumbers this estimate. This suggests that the acid exposure detected close to the squamocolumnar junction (short segment acid reflux) is unlikely to be fully explained by TLOSRs. In addition, acid exposure just above the squamocolumnar junction was not confined to the upright position, as might be expected for a TLOSR related mechanism. Finally, there was no correlation between the quantity of conventional reflux and short segment reflux measured in each patient.
What other mechanisms might contribute to the high level of acid exposure just above the squamocolumnar junction? We have previously shown that buffering of gastric acid by food is markedly reduced in this region, leading to a pocket of highly acidic (fasting pH) gastric juice at the gastro-oesophageal junction.6 Further work on the location of this acid pocket has revealed that after a meal it extends from immediately below the squamocolumnar junction to 1–2 cm up onto the squamous epithelium of the distal oesophagus.6 This postprandial change in pH at and above the squamocolumnar junction may be due to distension of the proximal stomach after a meal. This may cause the LOS to be intermittently prized apart at its distal extent, exposing the oesophageal squamous epithelium to acid. This mechanism was originally proposed by Oberg and colleagues.16 This partial opening of the LOS would allow acid to reach an intrasphincteric electrode close to the squamocolumnar junction but would not be detected by a conventional pH electrode placed above the upper limit of the LOS. Short segment acid reflux may reflect this process and would be consistent with oesophageal acid exposure just above the squamocolumnar junction being greater during the postprandial versus the preprandial period.
Although short segment reflux was greater in the postprandial period (pH <4: 21.8%), it was also observed under fasting conditions (pH <4: 14.2%). Mechanisms in addition to meal distension therefore need to be considered. The high level of acid exposure just proximal to the squamocolumnar junction might be partly the result of intermittent oesophageal shortening.17,18 The latter mainly takes place in the deep muscle layer which is separated from the mucosa by the elastic submucosal layer. If the mucosa does not shorten to the same extent as the deep muscle layer, then the squamocolumnar junction could become located distal to the lower oesophageal sphincter and thus exposed to gastric acid.
The different acid exposure at the two sites might also be due to different neutralising capacities. It is possible that there is more residual saliva present in the lumen above than within the high pressure zone and thus more neutralising capacity.
It is also important to consider the possibility that the increased acid exposure just above the squamocolumnar junction is artefactual and due to the methodology employed. Inadvertent displacement of the clipped pH electrode just above the squamocolumnar junction resulting in the pH catheter separating from the mucosa and moving into the stomach would produce higher levels of detected acid at this electrode. We believe this is unlikely to be the case as we designed the loop and clip to allow only 1 or 2 mm of “play” in the pH catheter. In addition, we repeated endoscopy at the end of the 24 hour study period and were able to confirm that the clips were intact and that the electrode had not moved from its position proximal to the squamocolumnar junction. The methodology also raises concerns about what effect a pH catheter straddling the gastro-oesophageal junction may have on LOS function and oesophageal acid exposure. However, previous studies suggest that oesophageal acid exposure is not influenced by the presence of a pH catheter.19 In addition, other studies by our own group have indicated that the gastro-oesophageal pH step up is not altered by the presence of an endoscopic clip.6
Our observation that the most distal oesophagus has the highest exposure to gastric refluxate is consistent with the anatomical distribution of epithelial damage attributed to reflux.20 Intestinal metaplasia is also more prevalent closer to the gastro-oesophageal junction. Studies of unselected patients undergoing endoscopy found 9–36% with specialised intestinal metaplasia involving less than 3 cm of the distal oesophagus.21–24 The most distal oesophagus is also the site with the highest incidence of adenocarcinoma25–30 and the frequent occurrence of short segment reflux could be an aetiological factor.
Another recent observation has been the high prevalence of intestinal metaplasia of the most distal oesophagus in subjects without any symptoms of reflux disease. Gerson et al reported intestinal metaplasia at the gastro-oesophageal junction or extending less than 3 cm proximal to it in 33% of subjects with no history of reflux symptoms.31 This intestinal metaplasia is likely to be the precursor of adenocarcinoma of the cardia which also shows little association with reflux symptoms.25 The majority of subjects in our study had no reflux symptoms. It is therefore possible that this short segment reflux could account for the high prevalence of intestinal metaplasia and cancer at the cardia which is occurring in subjects without symptoms or evidence of conventional reflux disease.
In conclusion, the most distal oesophagus is exposed to significant amounts of gastric acid, even in subjects without reflux symptoms. This short segment reflux may explain the high incidence of metaplasia and neoplasia of the most distal oesophagus which occurs in the general population.
Abbreviations
LOS, lower oesophageal sphincter
TLOSR, transient lower oesophageal sphincter relaxation
PIP, pressure inversion point
REFERENCES
- 1.Nebel O, Fornes M, Castell DO. Symptomatic gastroesophageal reflux: Incidence and precipitating factors. Dig Dis Sci 1976;21:953–6. [DOI] [PubMed] [Google Scholar]
- 2.Dodds WJ, Dent J, Hogan WJ, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 1982;307:1547–52. [DOI] [PubMed] [Google Scholar]
- 3.Mittal RK, Holloway RH, Penagini R, et al. Transient lower esophageal sphincter relaxation. Gastroenterology 1995;109:601–10. [DOI] [PubMed] [Google Scholar]
- 4.Herwaarden MAV, Samson M, Smout AJPM. Excess gastroesophageal reflux in patients with hiatus hernia is caused by mechanisms other than transient LES relaxations. Gastroenterology 2000;119:1439–46. [DOI] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Shi G, Manka M, et al. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Gastroenterology 2000;118:688–95. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher J, Wirz A, Young J, et al. Unbuffered highly acidic gastric juice exists at the gastroesophageal junction after a meal. Gastroenterology 2001;121:775–83. [DOI] [PubMed] [Google Scholar]
- 7.Kahrilas PJ, Lin SWS, Pouderoux P. Attenuation of esophageal shortening during peristalsis with hiatus hernia. Gastroenterology 1995;109:1818–25. [DOI] [PubMed] [Google Scholar]
- 8.Hirota WK, Loughney TM, Lazas DJ, et al. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: Prevalence and clinical data. Gastroenterology 1999;116:277–85. [DOI] [PubMed] [Google Scholar]
- 9.Johnson LF, DeMeester TR. Development of 24-hour intra-oesophageal pH monitoring composite scoring system. J Clin Gastroenterol 1986;8 (suppl 1) :52–8. [DOI] [PubMed] [Google Scholar]
- 10.Anggiansah A, Sumboonnanonda K, Wang J, et al. Significantly reduced acid detection at 10 centimeters compared to 5 centimeters above the lower oesophageal sphincter in patients with acid reflux. Am J Gastroenterology 1993;88:842–6. [PubMed] [Google Scholar]
- 11.Singh P, Taylor RH, Colin-Jones DG. Simultaneous two level oesophageal pH monitoring in healthy controls and patients with oesophagitis: comparison between two positions. Gut 1994;35:304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weusten BLAM, Akkermans LMA, Vanberge-Henegouwen GP, et al. Dynamic characteristics of gastro-oesophageal reflux in ambulatory patients with gastro-oesophageal reflux disease and normal control subjects. Scand J Gastroenterol 1995;30:731–7. [DOI] [PubMed] [Google Scholar]
- 13.Loughney MAJT, Maydonovitch CL, Wong RKH. Esophageal manometry and ambulatory 24-hour pH monitoring in patients with short and long segment Barrett’s esophagus. Am J Gastroenterol 1998;93:916–19. [DOI] [PubMed] [Google Scholar]
- 14.Csendes A, Maluenda F, Braghetto I, et al. Location of the lower oesophageal sphincter and the squamous columnar mucosal junction in 109 healthy controls and 778 patients with different degrees of endoscopic oesophagitis. Gut 1993;34:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoeman MN, Tippett MD, Akkermans LMA, et al. Mechanisms of gastroesophageal reflux in ambulant health human subjects. Gastroenterology 1995;108:83–91. [DOI] [PubMed] [Google Scholar]
- 16.Oberg S, Peters JH, DeMeester TR, et al. Inflammation and specialised intestinal metaplasia of cardiac mucosa is a manifestation of gastroesophageal reflux disease. Ann Surg 1997;226:522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson WG. Role of mast cell-derived mediators in acid-induced shortening of the esophagus. Gastrointest Liver Physiol 1998;37:G385–8. [DOI] [PubMed] [Google Scholar]
- 18.Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during peristalsis. Gastroenterology 1997;112:1147–54. [DOI] [PubMed] [Google Scholar]
- 19.Decktor DL, Krawet SH, Rodriguez SL, et al. Dual site ambulatory pH monitoring: A probe across the lower esophageal sphincter does not induce gastroesophageal reflux. Am J Gastroenterol 1996;91:1162–6. [PubMed] [Google Scholar]
- 20.Cameron AJ, Arora AS. Barrett’s esophagus and reflux esophagitis: Is there a missing link? Am J Gastroenterol 2002;97:273–8. [DOI] [PubMed] [Google Scholar]
- 21.Johnston MH, Hammond AS, Laskin W, et al. The prevalence and clinical characteristics of short segments of specialized intestinal metaplasia in the distal esophagus on routine endoscopy. Am J Gastroenterol 1996;91:1507–11. [PubMed] [Google Scholar]
- 22.Spechler SJ, Zeroogian JM, Antonioli DA, et al. Prevalence of metaplasia at the gastro-oesphageal junction. Lancet 1994;344:1533–6. [DOI] [PubMed] [Google Scholar]
- 23.Trudgill NJ, Suvarna SK, Kapur KC, et al. Intestinal metaplasia at the squamocolumnar juntion in patients attending for diagnostic gastroscopy. Gut 1997;41:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandurkar S, Talley NJ, Martin CJ, et al. Short segment Barrett’s oesophagus: prevalence, diagnosis and associations. Gut 1997;40:710–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825. [DOI] [PubMed] [Google Scholar]
- 26.Numans ME, van der Graaf Y, de Wit NJ, et al. How useful is selection based on alarm symptoms in requesting gastroscopy? Scand J Gastroenterol 2001;36:437–43. [DOI] [PubMed] [Google Scholar]
- 27.Chow W-H, Finkle WD, McLaughlin JK, et al. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. JAMA 1995;274:474. [PubMed] [Google Scholar]
- 28.Ye W, Chow W-H, Lagergren J, et al. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux disease and after antireflux surgery. Gastroenterology 2001;121:1286–93. [DOI] [PubMed] [Google Scholar]
- 29.Corley DA, Levin TR, Habel LA, et al. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology 2002;122:633–40. [DOI] [PubMed] [Google Scholar]
- 30.Bytzer P, Christensen PB, Damkier P, et al. Adenocarcinoma of the esophagus and Barrett’s esophagus: a populatin-based study. Am J Gastroenterol 1999;94:86. [DOI] [PubMed] [Google Scholar]
- 31.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology 2002;123:461–7. [DOI] [PubMed] [Google Scholar]