Abstract
Background: Pancreatic fibrosis is a characteristic feature of chronic pancreatic injury and is thought to result from a change in the balance between synthesis and degradation of extracellular matrix (ECM) proteins. Recent studies suggest that activated pancreatic stellate cells (PSCs) play a central role in pancreatic fibrogenesis via increased synthesis of ECM proteins. However, the role of these cells in ECM protein degradation has not been fully elucidated.
Aims: To determine: (i) whether PSCs secrete matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) and, if so (ii) whether MMP and TIMP secretion by PSCs is altered in response to known PSC activating factors such as tumour necrosis factor α (TNF-α), transforming growth factor β1 (TGF-β1), interleukin 6 (IL-6), ethanol, and acetaldehyde.
Methods: Cultured rat PSCs (n=3–5 separate cell preparations) were incubated at 37°C for 24 hours with serum free culture medium containing TNF-α (5–25 U/ml), TGF-β1 (0.5–1 ng/ml), IL-6 (0.001–10 ng/ml), ethanol (10–50 mM), or acetaldehyde (150–200 μM), or no additions (controls). Medium from control cells was examined for the presence of MMPs by zymography using a 10% polyacrylamide-0.1% gelatin gel. Reverse transcriptase-polymerase chain reaction (RT-PCR) was used to examine gene expression of MMP9 and the tissue inhibitors of metalloproteinases TIMP1 and TIMP2. Western blotting was used to identify a specific MMP, MMP2 (a gelatinase that digests basement membrane collagen and the dominant MMP observed on zymography) and a specific TIMP, TIMP2. Reverse zymography was used to examine functional TIMPs in PSC secretions. The effect of TNF-α, TGF-β1, and IL-6 on MMP2 secretion was assessed by densitometry of western blots. The effect of ethanol and acetaldehyde on MMP2 and TIMP2 secretion was also assessed by this method.
Results: Zymography revealed that PSCs secrete a number of MMPs including proteinases with molecular weights consistent with MMP2, MMP9, and MMP13. RT-PCR demonstrated the presence of mRNA for metalloproteinase inhibitors TIMP1 and TIMP2 in PSCs while reverse zymography revealed the presence of functional TIMP2 in PSC secretions. MMP2 secretion by PSCs was significantly increased by TGF-β1 and IL-6, but was not affected by TNF-α. Ethanol and acetaldehyde induced secretion of both MMP2 and TIMP2 by PSCs.
Conclusions: Pancreatic stellate cells have the capacity to synthesise a number of matrix metalloproteinases, including MMP2, MMP9, and MMP13 and their inhibitors TIMP1 and TIMP2. MMP2 secretion by PSCs is significantly increased on exposure to the proinflammatory cytokines TGF-β1 and IL-6. Both ethanol and its metabolite acetaldehyde increase MMP2 as well as TIMP2 secretion by PSCs.
Implication: The role of pancreatic stellate cells in extracellular matrix formation and fibrogenesis may be related to their capacity to regulate the degradation as well as the synthesis of extracellular matrix proteins.
Pancreatic fibrosis is a key pathological feature of alcohol induced chronic pancreatitis. There is increasing evidence from recent studies indicating that pancreatic stellate cells (PSCs) play a major role in pancreatic fibrogenesis.1–4 Studies of pancreatic sections from patients with chronic pancreatitis and of animal models of experimental pancreatic fibrosis have suggested that activated PSCs are the primary source of collagen in the fibrotic pancreas.5–9 PSCs, in their quiescent state, can be identified by the presence of vitamin A containing lipid droplets in their cytoplasm and by positive staining for stellate cell selective markers such as desmin and glial fibrillary acidic protein.2 When activated by factors such as cytokines, growth factors, oxidant stress, alcohol (ethanol), or its metabolite acetaldehyde, they transform into myofibroblast-like cells and synthesise increased amounts of the extracellular matrix (ECM) proteins that comprise fibrous tissue, particularly fibrillar collagens and fibronectin.1–4
The ECM plays a central role in the maintenance of normal tissue architecture. It is now evident that ECM turnover (synthesis, secretion, and degradation) is a critical feature of the tissue remodelling that accompanies physiological as well as pathological processes.10 Alteration in the balance between ECM protein synthesis and degradation can result in pathological increases in ECM deposition leading to the development of fibrosis.
While evidence is accumulating regarding the capacity of PSCs to regulate ECM protein synthesis, little is known of the ability of these cells to regulate ECM degradation. The key enzymes involved in ECM protein degradation are matrix metalloproteinases (MMPs). These are a family of at least 25 zinc dependent enzymes that are secreted as inactive (latent) zymogens.11,12 MMPs are classified according to their substrate specificity and structural features into five major groups: gelatinases (MMP2, MMP9), stromelysins (MMP3, MMP10, MMP11), elastases (MMP12, MMP7), collagenases (MMP1, MMP8, MMP13, MMP18), and membrane-type matrix metalloproteinases (MT1-MMP, MT2-MMP, MT3-MMP, MT4-MMP). The propeptide form of MMPs contains a regulatory motif with a free cysteine residue that maintains latency by binding to zinc in the catalytic domain.13 Activation results from dissociation of the regulatory motif from the catalytic domain. In vivo, the plasminogen-plasmin cascade is the major system effecting activation of prometalloproteinases.14 Additional activating mechanisms include mast cell tryptase, cathepsins, elastase, kallikrein, and reactive oxygen intermediates.15,16 MMP2 is also reported to be activated by membrane-type matrix metalloproteinase 1 (MT1-MMP).17 MMP2 and MMP9 both degrade basement membrane collagen (type IV) and are associated with ECM remodelling in wound healing, development, inflammation, fibrosis, angiogenesis, and tumour invasion.18 Degradation of normal basement membrane collagen (collagen-type IV) is thought to facilitate the deposition of pathological fibril forming collagen.19
Activity of MMPs can be inhibited by tissue inhibitors of metalloproteinases (TIMPs). Four subtypes of TIMPs (TIMP1–TIMP4) have been identified to date.20–23 TIMP1 inhibits the activity of several MMPs (MMP1, 3, 8, 9, 10, 11, 13, and 18) while TIMP2 is particularly important in the inhibition of MMP2.
The aim of this study was to examine whether PSCs are a source of MMPs and TIMPs and, if so, to determine the effect of known PSC activating factors on secretion of MMP2 and TIMP2 by these cells.
METHODS
Isolation of pancreatic stellate cells
Rat pancreatic stellate cells were isolated as detailed previously.2 Briefly, the pancreas was digested with a mixture of collagenase P (0.05%), pronase (0.02%), and DNase (0.1%) in Gey’s balanced salt solution. The resultant suspension of cells was centrifuged in a 13.2% Nycodenz gradient at 1400 g for 20 minutes. Stellate cells separated into a hazy band just above the interface of the Nycodenz solution and the aqueous buffer. This band was harvested and the cells were washed and resuspended in Iscove’s modified Dulbecco’s medium (IMDM) containing 10% fetal bovine serum (FBS), 4 mM glutamine, and antibiotics (penicillin 100 U/ml, streptomycin 100 mg/ml). The above technique yields a preparation of stellate cells devoid of contamination by endothelial cells or macrophages, as evidenced by negative staining for the markers factor VIII and ED1, respectively.2
Culture conditions for pancreatic stellate cells
Culture activated PSCs
For experiments using culture activated PSCs, cells were seeded in uncoated plastic culture plates at a density of 20 000 cells per well. Cells were then incubated at 37°C in a 95% air/5% CO2 atmosphere and experiments performed when cells reached 70% confluence.
Quiescent versus activated PSCs
In some experiments, quiescent and activated PSCs from the same cell preparation were compared with respect to MMP2 secretion. Our previous studies have established that PSCs demonstrate an activated phenotype when cultured on uncoated plastic in medium containing 10% FBS for 48 hours.2 To compare quiescent and activated PSCs, freshly isolated cells were seeded at a density of 50–100×103 cells/well in uncoated plastic wells in IMDM with 10% FBS, and incubated for 24 hours (quiescent cells) or 48 hours (activated cells). The medium was then changed to IMDM with 0.2% FBS and cells incubated for a further 24 hours. Secretions were then collected for MMP2 analysis.
Assessment of MMP secretion by pancreatic stellate cells
Zymography
MMP secretion by PSCs was assessed by zymography, as described by Herron and colleagues.24 This method allows the detection of both latent and active forms of MMPs. Proteins are separated by electrophoresis through a polyacrylamide gel containing a substrate (such as gelatin) that can be readily cleaved by MMPs. The presence of MMPs in the sample can be detected as white bands of lysis against the Coomassie blue stained gel. For this study, zymogram gels were prepared by addition of type I gelatin to the standard Laemmli acrylamide polymerisation mixture at a final concentration of 1 mg/ml (0.1%). PSCs were incubated for 24 hours in serum free medium, which was then collected, centrifuged for 10 minutes at 1500 rpm to remove cells and debris, and mixed with non-reducing sample buffer (0.4 M Tris, pH 6.8, 5% sodium dodecyl sulphate (SDS), 20% glycerol, 0.03% bromophenol blue). Samples were electrophoresed at 60 V through a 4% stacking gel and then at 100 V through a 10% resolving gel. Following electrophoresis, gels were washed in 2.5% Triton X-100 with gentle shaking for 30 minutes and then incubated for 30 minutes at room temperature in developing buffer (40 mM Tris-HCl, 0.2 mM NaCl, 6.67 mM CaCl2, 0.1% Triton X-100, pH 7.8). The developing buffer was then replaced with fresh buffer and the gel was incubated overnight at 37°C. The gels were stained for two hours with freshly prepared 0.5% Coomassie Blue R-250 in 10% acetic acid and 40% methanol and destained using fresh Coomassie destaining solution (45% ethanol, 10% acetic acid). Gels were washed (3×15 minutes) with the destaining solution and placed in storage solution (5% methanol, 0.75% acetic acid). As noted earlier, MMP activity was visualised as bands of lysis, which appear white on a dark background.
Western blotting
MMP2 secreted by quiescent and activated PSCs was identified by western blotting using a purified mouse monoclonal antibody. Cultured PSCs (passages 1–3) were used for all experiments. Quadruplicate wells of cells were exposed to the cytokines tumour necrosis factor α (TNF-α 5, 10, and 25 U/ml), transforming growth factor β1 (TGF-β1 0.5 and 1 ng/ml), and interleukin 6 (IL-6 0.001, 0.1, and 10 ng/ml). Cells were also treated with ethanol (10 and 50 mM) or acetaldehyde (150 and 200 μM) in serum free culture medium for 24 hours at 37°C. Cells incubated with serum free culture medium alone served as controls. Serum free culture medium was used to avoid the confounding effects of serum.
Protein concentrations of stellate cell secretions were measured by the method of Lowry and colleagues25 using bovine serum albumin as the standard. Proteins (100 μg) from each sample were separated by gel electrophoresis using a 10% SDS polyacrylamide gel. Known molecular weight protein standards were run alongside the samples. Separated proteins were transferred onto a nitrocellulose membrane using a commercial semi dry blotting apparatus (Biorad, Richmond, California, USA). The membranes were blocked in 5% skim milk in Tris buffered saline (TBS, pH 7.6) with 0.05% Tween-20 (TTBS) for one hour to prevent non-specific binding of antibody. Membranes were then incubated for one hour at room temperature with monoclonal anti-MMP2 (1 μg/ml) in 5% skim milk in TTBS. After three washes of five minutes each with TTBS, membranes were incubated with the horseradish peroxidase (HRP) conjugated secondary antibody (1:500) for 60 minutes at room temperature. Membranes were then rinsed with TTBS (3×5 minutes) and finally with TBS. MMP2 bands were detected by the enhanced chemiluminescence (ECL) technique using the Amersham ECL kit. MMP2 expression was quantified by densitometry of scanned autoradiographs (Scion Image, Maryland, USA). Densitometer readings are expressed as integrated optical densities (arbitrary densitometer units calculated from the density as well as the size of each band/μg protein loaded onto the gel).
RT-PCR
Expression of mRNA for MMP9 in PSCs was examined using reverse transcriptase-polymerase chain reaction (RT-PCR). Total cellular RNA was extracted from PSCs by a modification of the method described by Chomczynski and Sacchi26 using the Tri-reagent kit, as described previously.3 Extracted RNA was quantified by spectrophotometry. The A260/A280 ratio of extracted RNA was routinely in the range 1.7–1.8. Agarose gel electrophoresis of extracted RNA confirmed the integrity of the RNA samples. Total cellular RNA was reverse transcribed using a first strand cDNA synthesis kit according to the manufacturer’s instructions. The resulting cDNA was amplified using the MasterTaq Kit using previously reported primers for MMP927 and glyceraldehyde phosphate dehydrogenase (GAPDH, internal control). Primer sequences were as follows:
Rat MMP9
Forward primer: 5′ AAG GAT GGT CTA CTG GCA C 3′; reverse primer: 5′ AGA GAT TCT CAC TGG GGC 3′.27
Rat GAPDH
Forward primer: 5′ AAT CCC ATC ACC ATC TTC CA 3′; reverse primer: 5′ GGC AGT GAT GGC ATG GAC TG 3′.
The reaction mix contained 4 μl of cDNA, 50 pmol of each primer (forward and reverse), 2 mM MgCl2, 0.2 mM dNTPs, and 2.5 U Taq DNA polymerase. PCR was performed on a Perkin-Elmer thermal cycler with a two minute predenaturation at 94°C, and 35 cycles of amplification consisting of 94°C for one minute (denaturation), 56°C for one minute (annealing), and 72°C for one minute (extension).
Assessment of TIMP secretion by pancreatic stellate cells
Reverse zymography
This method allows the detection of functional TIMPs in a sample. Proteins are separated by electrophoresis in a gelatin-SDS gel containing added gelatinases. Functional TIMPs appear as a dark band, corresponding to the area where gelatin degradation by the gelatinases in the gel is prevented by the inhibitor. Serum free conditioned medium from PSCs (PSC secretion) was desalted and concentrated using a centrifugal filter device with a molecular weight limit of 5000 Da (Ultrafree -0.5; Millipore). Briefly, the centrifugal filter device was rinsed with MilliQ water by centrifugation at 12 000 g for five minutes at 4°C. PSC secretions (400 μl) were then placed into the filter device and centrifuged for 12 000 g for 15 minutes at 4°C. The sample was rinsed twice with MilliQ water and centrifuged as above. This technique resulted in a 20-fold concentration of the PSC secretions. Concentrated secretions were then mixed with non-reducing sample buffer (0.4 M Tris, pH 6.8, 5% SDS, 20% glycerol, 0.03% bromophenol blue). Samples were applied to a 14% polyacrylamide gel containing 1 mg/ml gelatin and 30% (v/v) 10× concentrated conditioned medium of HT1080 cells (as a source of gelatinases). After electrophoresis, gels were washed twice in 2.5% Triton X-100 with gentle shaking for 30 minutes and then incubated for 30 minutes at room temperature in developing buffer (40 mM Tris-HCl, 0.2 mM NaCl, 6.67 mM CaCl2, 0.1% Triton X-100, pH 7.8). This was followed by an overnight incubation at 37°C. Gels were then stained with 0.5% Coomassie blue in 10% acetic acid and 40% methanol and destained with 45% ethanol and 10% acetic acid. Human recombinant TIMP1 and TIMP2 were used as positive controls.
RT-PCR
Expression of mRNA for TIMP1 and TIMP2 in PSCs was examined using RT-PCR, as outlined above. RNA was isolated from PSCs and reverse transcribed. cDNA was amplified using the MasterTaq Kit using previously reported primers for TIMP1 and TIMP2.28,29 GAPDH was used as a positive control, as described previously. Primer sequences for TIMP1 and TIMP2 were as follows:
Rat TIMP1
Forward primer: 5′ ACA GCT TTC TGC AAC TCG 3′; reverse primer: 5′ CTA TAG GTC TTT ACG AAG GCC 3′.28
Rat TIMP2
Forward primer: 5′ ATT TAT CTA CAC GGC CCC 3′; reverse primer: 5′ CAA GAA CCA TCA CTT CTC TTG 3′.29
PCR was performed on a Perkin-Elmer thermal cycler with a two minute predenaturation at 94°C, and 35 cycles of amplification consisting of 94°C for one minute (denaturation), 55°C for one minute (annealing), and 72°C for one minute (extension).
Western blotting
TIMP2 in PSC secretions was identified by western blotting using a purified mouse monoclonal antibody. Cultured PSCs were treated with ethanol (50 mM) or acetaldehyde (200 μm) in serum free culture medium for 24 hours at 37°C using airtight culture plates. Cells incubated with serum free culture medium alone served as controls. PSC secretions were then desalted and concentrated 20-fold, as described earlier. Protein concentrations of the concentrated samples were measured using the Pierce BCA protein assay kit as per the manufacturer’s instructions. Equal amounts of protein for each sample were separated by gel electrophoresis (10% gel) and transferred to nitrocellulose. Known molecular weight protein standards were run alongside the samples. Western blotting was performed as outlined for MMP2. The primary antibody for TIMP2 was used at a concentration of 50 μg/ml.
Statistical analysis
Results are expressed as means (SEM) per experimental protocol. Data were analysed using analysis of variance (ANOVA).30 Fisher’s protected least significant difference was used for comparison of individual groups provided the F test was significant.30,31 Where indicated, data were analysed by the Student’s paired t test. Analyses were performed using the Statview II statistical software package.
Materials
All general chemicals were of analytical reagent grade and were purchased from the Sigma Chemical Company (St Louis, Missouri, USA) as were all cell culture reagents. Collagenase P was purchased from Boehringer Mannheim (Mannheim, Germany). Protease Type XIV (from Streptomyces griseus) was obtained from the Sigma Chemical Company. DNase was purchased from Pharmacia Biotech (Uppsala, Sweden). Nycodenz was obtained from Nycomed Pharma AS (Oslo, Norway). IMDM was purchased from Invitrogen Pty Ltd (Melbourne, Australia). Antibodies were obtained from the following sources: monoclonal antibodies to MMP2 and TIMP2 (Calbiochem-Novabiochem, Cambridge, Massachusetts, USA); monoclonal antibody to TIMP1 (Chemicon, Victoria, Australia); goat antimouse HRP conjugated antibody (Dako Corporation, Carpintaria, California, USA). Rat recombinant TNF-α, TGF-β1, and IL-6 were purchased from Peprotech (Rocky Hill, New Jersey, USA). Primers for MMP9, TIMP1, and TIMP2 were synthesised by Invitrogen Pty Ltd. Prestained broad range protein standards were purchased from Biorad (Richmond, California, USA). ECL kit was obtained from Amersham Pharmacia Biotech (Sydney, Australia). The MasterTaq kit was obtained from Eppendorf Scientific (Westbury, New York, USA). The first strand cDNA synthesis kit was obtained from MBI Fermentas (Vilnius, Lithuania). Human recombinant MMP2, TIMP1, and TIMP2 were obtained from Calbiochem-Novabiochem (Cambridge, Massachusetts, USA). Ultrafree-0.5 centrifugal filter devices were obtained from Millipore (Sydney, Australia). BCA protein assay kit was obtained from Progen (Melbourne, Australia). The HT-1080 cell line was a generous gift from Associate Professor John Rasko, Centenary Institute of Cancer, Medicine, and Cell Biology, Sydney, Australia.
RESULTS
Gelatinolytic activity in conditioned media from PSCs
Zymography of PSC secretions demonstrated the presence of numerous MMPs (fig 1A ▶). As seen on the overloaded zymogram in fig 1A ▶, most gelatinolytic activity appeared to be concentrated in the area corresponding to the molecular weight range 60–80 kDa. The most prominent MMP had a molecular weight of 72 kDa, which was suggestive of MMP2. Additional distinct bands were also observed corresponding to molecular weights of 92 kDa and 57 kDa, which was suggestive of MMP9 and MMP13, respectively.
Figure 1.
Matrix metalloproteinase (MMP) secretion by pancreatic stellate cells (PSCs). (A) Gelatinase activity in conditioned media obtained from PSCs. The overloaded zymogram shows MMP activity in cultured PSC secretions. Conditioned media were obtained from passaged PSC cultures, which were incubated for 24 hours in serum free culture medium. Lanes 1–3: 150 μg of protein from three separate cell preparations. Lanes 4–6: 75 μg of protein from three separate cell preparations. MW, molecular weight. (B) Western blot analysis for MMP2 in PSC secretions. Lane 1: Recombinant MMP2 (positive control). Lane 2: PSC secretions obtained after 24 hours of culture with serum free culture medium.
Western blotting for MMP2
The presence of MMP2 in PSC secretions was confirmed by western blotting using a monoclonal antibody directed against both active and latent forms of MMP2 (fig 1B ▶). A single band was detected which corresponded to the molecular weight of latent MMP2 (72 kDa). MMP2 secretion was demonstrable with both quiescent and activated PSCs (fig 2A,2B ▶). However, MMP2 secretion by cells with the activated phenotype was significantly higher than that by quiescent cells. Densitometer units were 62.4 (3.7) and 83.1 (9.7) for quiescent and activated cells, respectively (p<0.04; n=4 separate cell preparations) (fig 2B ▶).
Figure 2.
Matrix metalloproteinase 2 (MMP2) secretion by quiescent and activated pancreatic stellate cells (PSCs). (A) Representative western blot for MMP2 expression in quiescent and activated PSC secretions from the same cell preparation. MW, molecular weight. (B) Densitometry of all western blots (n=4 separate cell preparations) showed a significant increase in MMP2 levels in culture activated PSCs compared with quiescent cell secretions (*p<0.04, Student’s paired t test).
Expression of MMP9
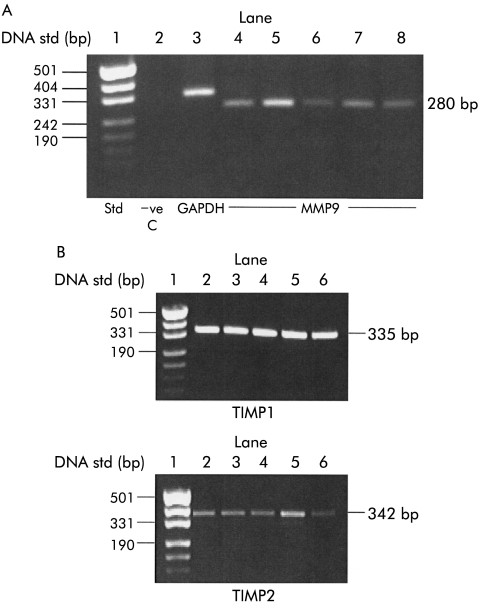
Using RT-PCR, MMP9 expression was observed in five separate PSC preparations (fig 3A ▶). The PCR product was 280 bp, in agreement with the reported literature.27,32
Figure 3.
Expression of matrix metalloproteinase 9 (MMP9) and tissue inhibitors of metalloproteinases (TIMP1/TIMP2) in pancreatic stellate cells (PSCs). (A) Total RNA was extracted from five separate PSC preparations and analysed for MMP9 by reverse transcriptase-polymerase chain reaction (RT-PCR). An RNA ladder was run in lane 1 (std) to determine the size of the PCR products observed. The negative control (−ve C) in lane 2 contained no RNA template in the PCR reaction. Glyceraldehyde phosphate dehydrogenase (GAPDH, lane 3) was used as an internal control. Lanes 4–8 contain PCR products for MMP9 from five separate cell preparations. (B) TIMP1 and TIMP2 expression was analysed in total RNA obtained from five separate PSC preparations by RT-PCR. The top panel shows TIMP1 expression and the bottom panel TIMP2 expression. Both panels contain an RNA ladder in lane 1 to determine the size of the PCR products observed.
Expression of tissue inhibitors of matrix metalloproteinases (TIMP1 and TIMP2)
RT-PCR revealed expression of both TIMP1 and TIMP2 in all PSC preparations tested (n=5 separate cell preparations; fig 3B ▶). PCR products were 335 bp and 342 bp for TIMP1 and TIMP2, respectively, in accordance with the reported literature.32
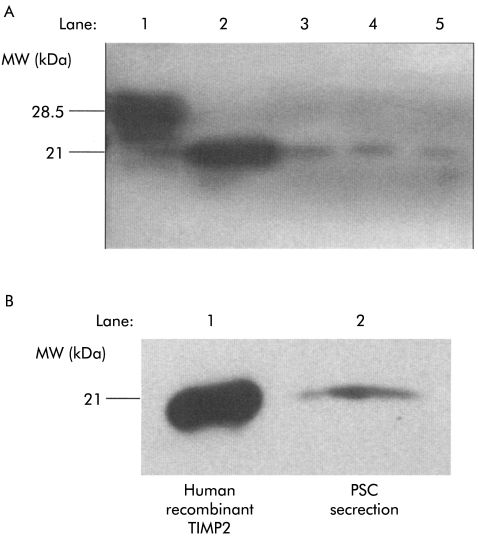
TIMP2 activity in conditioned media from PSCs
Reverse zymography demonstrated the presence of functional TIMP2 in PSC secretions (fig 4A ▶). A single dark band of molecular weight 21 kDa was detected corresponding to the positive control (human recombinant TIMP2). However, despite the use of 20-fold concentrated PSC secretions, this technique failed to demonstrate any band corresponding to the positive control for TIMP1 (fig 4A ▶), suggesting that although PSCs express mRNA for TIMP1, secretion of the corresponding protein is either absent or in amounts too low to be detectable by this method.
Figure 4.
Tissue inhibitor of metalloproteinase (TIMP) secretion by pancreatic stellate cells (PSCs). (A) TIMP1 and TIMP2 activity was analysed using reverse zymography. Lane 1: Human recombinant TIMP1. Lane 2: Human recombinant TIMP2. Lanes 3–5: PSC secretions obtained from three separate cell preparations after 24 hours of culture with serum free culture medium. Bands corresponding to recombinant TIMP2 are visible in PSC secretions. No bands corresponding to recombinant TIMP1 were found in the samples. MW, molecular weight. (B) Representative western blot (n=4 separate cell preparations) for TIMP2 expression in cells after 24 hours of culture with serum free culture medium. Lane 1: Human recombinant TIMP2. Lane 2: PSC secretions.
Western blotting for TIMPs
The presence of TIMP2 in PSC secretions was also confirmed by western blotting using a monoclonal antibody directed against TIMP2 (fig 4B ▶). A single band of molecular weight 21 kDa was detected corresponding to human recombinant TIMP2 (positive control). However, western blotting failed to demonstrate any band corresponding to the positive control for TIMP1 (data not shown).
Effect of TGF-β1, IL-6, and TNF-α on MMP2 secretion
Having determined that PSCs can secrete MMPs and that the predominant MMP is MMP2, the next issue addressed by this study was the effect of known PSC activating factors on MMP2 secretion by these cells. The effect of TGF-β1, IL-6, and TNF-α (at concentrations previously observed to activate PSCs1,33) on MMP2 secretion was analysed by densitometry of western blots. MMP2 secretion by PSCs was significantly increased by TGF-β1 (fig 5A, 5B ▶; n=5 separate cell preparations) at concentrations of 0.5 ng/ml (145.8 (47.8)% of control; p<0.05) and 1 ng/ml (166.6 (40.1)% of control; p<0.05). Similarly, incubation with IL-6 significantly increased MMP2 secretion (fig 6A, 6B ▶; n=5 separate cell preparations) at 0.1 ng/ml (168.2 (41.5)% of control; p<0.05) and 10 ng/ml (176.2 (49.6)% of control; p<0.05). In contrast, TNF-α at concentrations of 5 U/ml (122.2 (19.9)% of control), 10 U/ml (76.9 (13.3)% of control), and 25 U/ml (95.4 (12.2)% of control; n=5 separate cell preparations) had no significant effect on MMP2 secretion by PSCs.
Figure 5.
Effect of transforming growth factor β1 (TGF-β1) on matrix metalloproteinase 2 (MMP2) secretion by pancreatic stellate cells (PSCs). (A) Representative western blot for MMP2 expression in cells incubated for 24 hours with either culture medium alone (Control) or TGF-β (0.5 or 1 ng/ml). (B) Densitometry of all western blots (n=5 separate cell preparations) showed a significant increase in MMP2 levels in PSCs incubated with 0.5 ng/ml and 1 ng/ml TGF-β1 compared with controls (*p<0.05).
Figure 6.
Effect of interleukin 6 (IL-6) on matrix metalloproteinase 2 (MMP2) secretion by pancreatic stellate cells (PSCs). (A) Representative western blot for MMP2 expression in cells incubated for 24 hours with either culture medium alone (Control) or IL-6 (0.01, 0.1, or 10 ng/ml). (B) Densitometry of all western blots (n=5 separate cell preparations) demonstrated a significant increase in MMP2 levels in PSCs incubated with 0.1 ng/ml and 10 ng/ml IL-6 compared with controls (*p<0.05).
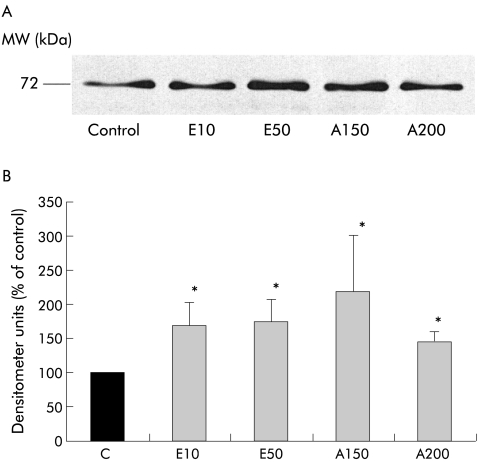
Effect of ethanol and its metabolite acetaldehyde on MMP2 secretion
Ethanol at concentrations of 10 and 50 mmol/l significantly increased secretion of MMP2 by PSCs compared with control cells incubated with serum free culture medium alone (fig 7A, 7B ▶; n=5 separate cell preparations). Densitometer units were 169.2 (33.8)% of control (p<0.05) and 174.3 (31.7)% of control (p<0.05) for 10 mmol/l and 50 mmol/l of ethanol, respectively. Similarly, acetaldehyde (150 and 200 μmol/l) significantly increased MMP2 secretion to 218.46 (83.8)% of control (p<0.05) and 145.5 (13.7)% of control (p<0.05), respectively (fig 7B ▶).
Figure 7.
Effect of ethanol and its metabolite acetaldehyde on matrix metalloproteinase 2 (MMP2) secretion by pancreatic stellate cells (PSCs). (A) Representative western blot for MMP2 expression in cells incubated for 24 hours with either culture medium alone (Control), ethanol (10 and 50 mmol/l; E10 and E50, respectively) or acetaldehyde (150 and 200 μmol/l; A150 and A200, respectively). (B) Densitometry of all western blots (n=5 separate cell preparations) showed that both ethanol (E10 and E50) and acetaldehyde (A150 and A200) significantly increased MMP2 secretion by PSCs compared with controls (*p<0.05).
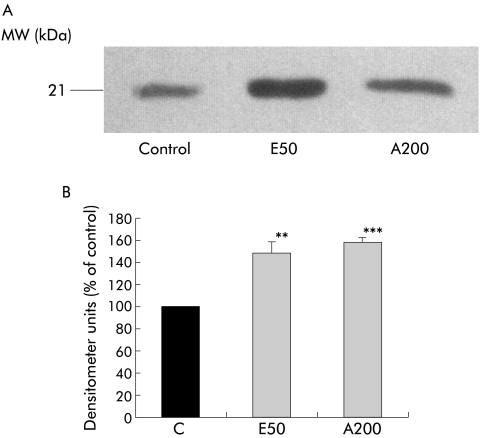
Effect of ethanol and its metabolite acetaldehyde on TIMP2 secretion
Both ethanol (50 mM) and acetaldehyde (200 μM) significantly increased TIMP2 secretion by PSCs compared with control cells incubated with serum free culture medium alone (fig 8A, 8B ▶; n=3 separate cell preparations). Densitometer units were 148.6 (9.5)% of control (p<0.01) and 157.9 (3.7)% of control (p<0.006) for ethanol and acetaldehyde, respectively (fig 8B ▶).
Figure 8.
Effect of ethanol and its metabolite acetaldehyde on tissue inhibitor of metalloproteinase 2 (TIMP2) secretion by pancreatic stellate cells (PSCs). (A) Representative western blot for TIMP2 expression in cells incubated for 24 hours with either culture medium alone (Control), ethanol (50 mmol/l; E50), or acetaldehyde (200 μmol/l; A200). (B) Densitometry of all western blots (n=3 separate cell preparations) showed that both ethanol (E50) and acetaldehyde (A200) significantly increased TIMP2 secretion by PSCs compared with controls (E50, **p<0.01; A200 ***p<0.006).
DISCUSSION
This study has shown that PSCs have the capacity to synthesise and secrete a number of matrix degrading enzymes, including MMP2 (the predominant MMP in PSC secretions), MMP9, and MMP13. MMP2 secretion was demonstrable with both quiescent and activated PSCs but the amount of MMP2 produced by quiescent PSCs was significantly lower than that produced by the activated phenotype. PSC activating factors such as ethanol and acetaldehyde and the cytokines TGF-β1 and IL-6 increased MMP2 secretion by cells. This study has also demonstrated the presence of messenger RNA for the tissue inhibitors of metalloproteinases TIMP1 and TIMP2 in PSCs. However, at the protein level, only TIMP2 was detectable in PSC secretions. Production of this inhibitor by PSCs was significantly increased by both ethanol and acetaldehyde.
The finding that PSCs can synthesise and secrete MMPs is an important one because it suggests that in addition to their well documented role in ECM synthesis, PSCs may also play a role in ECM degradation. The predominant MMP secreted by PSCs was latent MMP2. This is in accordance with studies by Knittel and colleagues32 demonstrating that hepatic stellate cells (HSCs) are a major source of MMP2 in the liver and that the MMP2 secreted is largely in the latent form. In the present study, zymography results suggested that PSCs also secrete MMP9. The capacity of PSCs to synthesise MMP9 was supported by RT-PCR studies. These findings are in agreement with previous reports that HSCs can synthesise and secrete MMP9.32 Zymography results presented in this study also suggested the presence of MMP13 in PSC secretions. This finding concurs with a recent study demonstrating that HSCs express messenger RNA for MMP13.34 Both MMP2 and MMP9 are known to degrade basement membrane collagen while MMP13 is known to degrade fibrillar collagen.
It may appear paradoxical that cells that promote fibrogenesis by deposition of excess fibrillar collagen also secrete proteases that degrade fibrillar collagen (MMP13). However, as has been documented by studies in the liver, fibrogenesis is a complex process resulting from an overall imbalance between ECM synthesis and ECM degradation.35 Studies with HSCs have established that when activated, HSCs synthesise increased ECM proteins, particularly fibrillar collagen, but shut down expression of proteases such as MMP13 (which degrades fibrillar collagen).15,36–38 Together, these changes may facilitate the profibrogenic action of activated stellate cells.
Increased MMP2 secretion by PSCs may be profibrogenic in two ways. Firstly, increased degradation of normal basement membrane collagen (type IV) by MMP2 may facilitate the deposition of pathological fibrillar collagen in the gland. Secondly, MMP2 may exert a proliferative effect on PSCs (in a manner similar to that reported for HSCs39), leading to an increase in the number of activated PSCs at sites of injury and consequent increase in collagen synthesis.
Regulation of ECM synthesis and degradation by MMPs and their inhibitors (TIMPs) is a complex process. In general, TIMPs inhibit MMP activity by binding to the active site of MMPs with a 1:1 stoichiometry, in a reversible and non-covalent manner.35 However, TIMPs have also been shown to play a role in regulating the activation of MMPs from their latent forms. For example, TIMP2, at low concentrations, is known to facilitate activation of MMP2 by acting as a bridging molecule linking the C terminal of proMMP2 to the N terminal of MT1-MMP (the MMP that activates proMMP2).35 On the other hand, at high concentrations, TIMP2 binds to proMMP2 and inhibits its activation by MT1-MMP.35
The present study has shown that culture activated PSCs express mRNA for both TIMP1 and TIMP2. In order to assess secretion of the corresponding proteins by cells, reverse zymography was performed. This technique demonstrated the presence of TIMP2 in PSC secretions which concurs with findings in HSCs.38,40 However, despite the use of 20-fold concentrated PSC secretions, TIMP1 could not be detected in our samples, suggesting that TIMP1 mRNA may not be translated in PSCs or, if translated, may be secreted in amounts too low to be detectable by standard techniques. The lack of demonstrable TIMP1 in PSC secretions contrasts with reports of the presence of TIMP1 in HSC secretions by reverse zymography.40
TIMP2 secretion was found to be induced by the PSC activating factors ethanol and acetaldehyde. This finding is similar to previously reported results with HSCs demonstrating a significant increase in TIMP expression during cell activation.38,40 Our study revealed that the amount of MMP2 secreted by PSCs was of an order of magnitude higher than the amount of TIMP2 secretion, as MMP2 was easily detected by zymography of unconcentrated PSC secretions but TIMP2 could only be detected by reverse zymography of 20-fold concentrated PSC secretions. Thus even though the ethanol and acetaldehyde induced increase in MMP2 was accompanied by an increase in TIMP2, the increase in the latter may not be sufficient to inhibit net MMP2 activity, particularly given that TIMP2 binds to MMP2 in a 1:1 stoichiometry. Furthermore, in view of the fact that low concentrations of TIMP2 can activate MMP2, the observed increase in TIMP2 may potentiate the matrix degradative effect of MMP2.
The proinflammatory cytokines examined in this study (TNF-α and IL-6) are known to be elevated in both pancreatic tissue and serum of patients with acute pancreatitis.41 Serum levels of these cytokines correlate directly with the severity of disease.42–45 Overexpression of TGF-β has been described in human chronic pancreatitis.46,47 In vitro studies have demonstrated that PSCs are activated (as indicated by increased cell proliferation, α-smooth muscle actin, and/or collagen synthesis1,33) by these cytokines. It was therefore of interest to examine the effect of these cytokines on MMP2 secretion by PSCs. Our study revealed that TGF-β1 and IL-6 both significantly increased MMP2 secretion while TNF-α had no effect. Our findings with TGF-β1 are similar to those described in a recent study of rat PSCs by Shek and colleagues.48 With respect to HSCs, the effect of TGF-β1 appears to be controversial with both upregulation49 and no change in MMP2 expression reported.32 To the best of our knowledge, there have been no published studies to date regarding the effects of TNF-α and IL-6 on MMP secretion by PSCs. However, our results with TNF-α concur with reports in HSCs32 showing that TNF-α had no significant effect on MMP2 expression or secretion by these cells.
We have previously demonstrated that PSCs are activated by clinically relevant concentrations of ethanol (a major association of chronic pancreatitis) and its metabolite acetaldehyde.3 In the current study, we found that these compounds induced MMP2 and TIMP2 secretion by PSCs. To date, there are no reports of the effect of ethanol on MMP and TIMP secretion by either PSCs or HSCs. However, acetaldehyde has been shown to upregulate MMP2 gene expression in HSCs cultured from normal human livers.50
In summary, this study has demonstrated that PSCs have the capacity to synthesise and secrete matrix degrading enzymes and their inhibitors, lending support to the concept that these cells may play a role in the regulation of ECM degradation. The fact that PSCs secrete MMP2 even in their quiescent phase suggests that in the normal pancreas, PSCs play a role in maintenance of the normal ECM by regulating both ECM synthesis and degradation. In the diseased pancreas, increased MMP2 secretion by PSCs in response to factors such as TGF-β1, IL-6, ethanol, and acetaldehyde could result in increased basement membrane degradation, which in turn could facilitate the deposition of fibrillar (pathological) collagen leading to the development of fibrosis in the gland. Further studies in this area may help identify novel molecular targets that could be modulated to alter the function of PSCs with respect to ECM degradation thereby preventing/ameliorating the development of fibrosis in chronic pancreatitis.
Acknowledgments
We thank Associate Professor John Rasko (Centenary Institute of Cancer, Medicine, and Cell Biology, Sydney, Australia) for supplying the HT-1080 cell line. This project was supported by grants from the National Health and Medical Research Council of Australia, the Department of Veterans’ Affairs, Australia, and the Australian Brewers’ Foundation.
Abbreviations
ANOVA, analysis of variance
ECL, enhanced chemiluminescence
ECM, extracellular matrix
FBS, fetal bovine serum
GAPDH, glyceraldehyde phosphate dehydrogenase
HRP, horseradish peroxidase
HSCs, hepatic stellate cells
IL, interleukin
IMDM, Iscove’s modified Dulbecco’s medium
PSC, pancreatic stellate cell
MMP, matrix metalloproteinase
MT-MMP, membrane-type matrix metalloproteinase
RT-PCR, reverse transcriptase-polymerase chain reaction
SDS, sodium dodecyl sulphate
TBS, Tris buffered saline
TGF-β, transforming growth factor β
TIMP, tissue inhibitor of metalloproteinase
TNF-α, tumour necrosis factor α
TTBS, Tris buffered saline and Tween 20
REFERENCES
- 1.Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 1999;44:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas—identification, isolation, and culture. Gut 1998;43:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apte MV, Phillips PA, Fahmy RG, et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology 2000;118:780–94. [DOI] [PubMed] [Google Scholar]
- 4.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421–32. [DOI] [PubMed] [Google Scholar]
- 5.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol 1999;155:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casini A, Galli A, Pignalosa P, et al. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol 2000;192:81–9. [DOI] [PubMed] [Google Scholar]
- 7.Neuschwander-Tetri BA, Burton FR, Presti ME, et al. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci 2000;45:665–74. [DOI] [PubMed] [Google Scholar]
- 8.Vogelmann R, Ruf D, Wagner M, et al. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-β1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol 2001;280:G164–72. [DOI] [PubMed] [Google Scholar]
- 9.Emmrich J, Weber I, Nausch M, et al. Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion 1998;59:192–8. [DOI] [PubMed] [Google Scholar]
- 10.Iredale JP. Matrix turnover in fibrogenesis. Hepatogastroenterology 1996;43:56–71. [PubMed] [Google Scholar]
- 11.Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991;5:2145–54. [PubMed] [Google Scholar]
- 12.Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993;4:197–250. [DOI] [PubMed] [Google Scholar]
- 13.Springman EB, Angleton EL, Birkedal-Hansen H, et al. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci U S A 1990;87:364–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy G, Atkinson S, Ward R, et al. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann NY Acad Sci 1992;667:1–12. [DOI] [PubMed] [Google Scholar]
- 15.Iredale JP, Benyon RC, Arthur MJP, et al. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology 1996;24:176–84. [DOI] [PubMed] [Google Scholar]
- 16.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A 1990;87:5578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994;370:61–5. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran M, Hewitt R, Kleiner DJ, et al. MMP-2: expression, activation and inhibition. Enzyme Protein 1996;49:7–19. [DOI] [PubMed] [Google Scholar]
- 19.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000;275:2247–50. [DOI] [PubMed] [Google Scholar]
- 20.Murphy G, Cawston TE, Reynolds JJ. An inhibitor of collagenase from human amniotic fluid. Purification, characterization and action on metalloproteinases. Biochem J 1981;195:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem 1989;264:17374–8. [PubMed] [Google Scholar]
- 22.Pavloff N, Staskus PW, Kishnani NS, et al. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem 1992;267:17321–6. [PubMed] [Google Scholar]
- 23.Leco KJ, Apte SS, Taniguchi GT, et al. Murine tissue inhibitor of metalloproteinases-4 (TIMP-4): cDNA isolation and expression in adult mouse tissues. FEBS Letters 1997;401:213–7. [DOI] [PubMed] [Google Scholar]
- 24.Herron G, Werb Z, Dwyer K, et al. Secretion of metalloproteinases by stimulated capillary endothelial cells. J Biol Chem 1986;261:2810–13. [PubMed] [Google Scholar]
- 25.Lowry O, Rosebrough N, Farr A, et al. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 27.Okada A, Santavicca M, Basset P. The cDNA cloning and expression of the gene encoding rat gelatinase B. Gene 1995;164:317–21. [DOI] [PubMed] [Google Scholar]
- 28.Okada A, Garnier JM, Vicaire S, et al. Cloning of the cDNA encoding rat tissue inhibitor of metalloproteinase 1 (TIMP-1), amino acid comparison with other TIMPs, and gene expression in rat tissues. Gene 1994;147:301–2. [DOI] [PubMed] [Google Scholar]
- 29.Cook TF, Burke JS, Bergman K, et al. Cloning and regulation of rat tissue inhibitor of metalloproteinase-2 in osteoblastic cells. Arch Biochem Biophys 1994;311:313–20. [DOI] [PubMed] [Google Scholar]
- 30.Snedecor G, Cochran W. Statistical methods, 8th edn. Ames, IO: Iowa State University Press, 1989.
- 31.Feldman DJ, Hofmann R, Gagnon J, et al. Statview II. Berkeley, CA: Abacus Concepts Inc, 1987.
- 32.Knittel T, Mehde M, Kobold D, et al. Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non-parenchymal cells of rat liver: regulation by TNF-α and TGF-β1. J Hepatol 1999;30:48–60. [DOI] [PubMed] [Google Scholar]
- 33.Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 2002;50:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe T, Niioka M, Hozawa S, et al. Gene expression of interstitial collagenase in both progressive and recovery phase of rat liver fibrosis induced by carbon tetrachloride. J Hepatol 2000;33:224–35. [DOI] [PubMed] [Google Scholar]
- 35.Benyon RC, Arthur MJP. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis 2001;21:373–84. [DOI] [PubMed] [Google Scholar]
- 36.Iredale JP, Goddard S, Murphy G, et al. Tissue inhibitor of metalloproteinase-1 and interstitial collagenase expression in autoimmune chronic active hepatitis and activated human hepatic lipocytes. Clin Sci 1995;89:75–81. [DOI] [PubMed] [Google Scholar]
- 37.Leyland H, Gentry J, Arthur MJP, et al. The plasminogen-activating system in hepatic stellate cells. Hepatology 1996;24:1172–8. [DOI] [PubMed] [Google Scholar]
- 38.Benyon RC, Iredale JP, Goddard S, et al. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 1996;110:821–31. [DOI] [PubMed] [Google Scholar]
- 39.Benyon RC, Hovell CJ, Da Gaca M, et al. Progelatinase A is produced and activated by rat hepatic stellate cells and promotes their proliferation. Hepatology 1999;30:977–86. [DOI] [PubMed] [Google Scholar]
- 40.Iredale JP, Murphy G, Hembry RM, et al. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J Clin Invest 1992;90:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norman J, Franz M, Riker A. Rapid elevation of pro-inflammatory cytokines during acute pancreatitis and their origination within the pancreas. Surg Forum 1994;45:148–60. [Google Scholar]
- 42.Paajanen H, Laato M, Jaakkola M, et al. Serum tumour necrosis factor compared with C-reactive protein in the early assessment of severity of acute pancreatitis. Br J Surg 1995;82:271–3. [DOI] [PubMed] [Google Scholar]
- 43.Heath DI, Cruickshank A, Gudgeon M, et al. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut 1993;34:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Exley AR, Leese T, Holliday MP, et al. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut 1992;33:1126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leser HG, Gross V, Scheibenbogen C, et al. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology 1991;101:782–5. [DOI] [PubMed] [Google Scholar]
- 46.Van Laethem JL, Deviere J, Resibois A, et al. Localization of transforming growth factor β1 and its latent binding protein in human chronic pancreatitis. Gastroenterology 1995;108:1873–81. [DOI] [PubMed] [Google Scholar]
- 47.Slater SD, Williamson RC, Foster CS. Expression of transforming growth factor-β1 in chronic pancreatitis. Digestion 1995;56:237–41. [DOI] [PubMed] [Google Scholar]
- 48.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-β1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 2002;160:1787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milani S, Herbst H, Schuppan D, et al. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol 1994;144:528–37. [PMC free article] [PubMed] [Google Scholar]
- 50.Casini A, Ceni E, Salzano R, et al. Acetaldehyde regulates the gene expression of matrix-metalloproteinase-1 and -2 in human fat-storing cells. Life Sci 1994;55:1311–16. [DOI] [PubMed] [Google Scholar]