Abstract
Background: Mechanisms underlying abnormalities of circulation and renal function in cirrhosis are not completely understood. Our previous study revealed that primary afferent denervation by neonatal capsaicin treatment prevented the development of hyperdynamic circulation in portal hypertensive and cirrhotic rats.
Aims: The present study aimed to clarify the role of capsaicin sensitive nerves in the development of renal dysfunction and ascites formation in cirrhosis.
Methods: Rat pups were injected with capsaicin (50 mg/kg) or vehicle and allowed to grow. When they reached adulthood, cirrhosis was induced by bile duct ligation while controls received sham operation. Cardiac output and regional blood flows were measured by radioactive microspheres, glomerular filtration rate by 3H inulin clearance, and urine volume, sodium excretion, and ascites formation were determined. Immunohistochemical staining for Fos in the brain stem cardiovascular regulatory nuclei, the nucleus of the solitary tract, and ventrolateral medulla was measured as an index of central neuronal activation.
Results: Increased cardiac output and renal blood flow, and decreased systemic vascular resistance, arterial pressure, renal vascular resistance, and glomerular filtration rate, as well as ascites, were found in vehicle treated cirrhotic rats. Neonatal capsaicin treatment completely blocked the development of hyperdynamic circulation and ascites, and improved renal function in cirrhotic rats. This was associated with complete abrogation of brain stem neuronal activation in capsaicin treated cirrhotic rats.
Conclusions: These results indicate that intact primary afferent innervation is necessary for the development of not only the hyperdynamic circulation but also the renal dysfunction and ascites formation characteristic of cirrhosis.
Keywords: bile duct ligation, kidney function, ascites, sodium and water retention, primary afferent nerves, Fos, c-fos
Cirrhosis is associated with hyperdynamic circulation, consisting of peripheral vasodilatation and increased cardiac output. Regional blood flow to many vascular beds is also increased. Renal sodium and water retention eventually leads to ascites formation. Mechanisms underlying the hyperdynamic circulation and renal dysfunction remain incompletely understood. However, a theory proposed in 1988, the “peripheral vasodilation” hypothesis,1 attempts to link these phenomena together. This theory suggests that peripheral arteriolar vasodilatation is the primary initiating factor that leads to effective arterial underfilling of the circulation, sensed by the kidney as an inadequate volume and thus inducing salt and water retention.
Factors responsible for arterial vasodilatation in cirrhosis remain unclear but excessive activity or levels of vasodilators such as glucagons,2–4 nitric oxide,5–7 prostaglandins,8–10 and calcitonin gene related peptide (CGRP)11,12 have been suggested. Our previous study found that the hyperdynamic circulation in portal hypertensive and cirrhotic rats is blocked by neonatal capsaicin treatment.11 Capsaicin is the active ingredient of the pungent capsicum peppers, and in acute doses activates the primary afferent nerves whereas in higher doses given to neonatal animals it permanently ablates these nerves. It has proven remarkably useful over the past three decades as a pharmacological tool to explore the physiology of primary afferent nerves.
The aim of the present study was to investigate further the effects of capsaicin sensitive nerves on the development of hyperdynamic circulation and renal dysfunction, including ascites formation, in rats with cirrhosis induced by bile duct ligation (BDL).
MATERIALS AND METHODS
The protocol was approved by the University of Calgary Faculty of Medicine Animal Care Committee and the experimental procedures were carried out in accordance with guidelines established by the Canadian Council on Animal Care.
Capsaicin treatment
Newborn Sprague-Dawley rat pups (Charles River Canada, St Laurent, Canada) on the second day of life, under light halothane anaesthesia, were subcutaneously injected with 50 mg/kg capsaicin (Sigma Chemicals, St Louis, Missouri, USA) dissolved in a vehicle of 80% physiological saline, 10% Tween 80, and 10% ethanol (2% in oxygen), according to methods described previously.13,14 A control group received an equivalent volume of only the vehicle. Rats were then returned to their dams and reared until a body weight of 150–200 g, under a constant photoperiod (12 hour light:dark cycles) at 20°C with free access to food and water.
Cirrhosis induction
Cirrhosis was induced by the method of BDL, as previously described.15 Briefly, under halothane anaesthesia, through a midline laparotomy, the common bile duct was doubly ligated with 4-0 silk thread and sectioned between the ligatures. Incisions were closed with silk, and the animals were given an intramuscular injection of benzathine penicillin G (30 000 IU) immediately after operation to prevent sepsis. Control rats (sham) underwent exactly the same surgical procedures except for ligation of the bile duct. Animals were then kept for 4–5 weeks when a body weight of 300–400 g had been attained, by which time an obvious cirrhosis had developed. Chronic cholestasis was also evidenced by marked elevations in aspartate aminotransferase, alanine aminotransferase, and bilirubin in BDL rats, as we have previously reported.16 Capsaicin treatment did not change any of these parameters (data not shown). Thus there were four groups of rats: sham vehicle treated (n=7); sham capsaicin treated (n=7); BDL vehicle treated (n=11); and BDL capsaicin treated (n=14).
Experimental procedures
Rats were housed individually in metabolic cages and food but not water was removed from the cages for 24 hours before the study. During this time urine volume of each rat was measured gravimetrically. Thereafter rats were prepared for the study as previously described.17 Briefly, under halothane anaesthesia, an intravenous cannula was inserted into the left femoral vein using PE-50 tubing. The left femoral artery was cannulated with a PE-50 catheter to allow collection of blood samples and measurement of mean arterial pressure and heart rate using a pressure transducer (Gould P23XL, Oxnard, California, USA) connected to a recorder (Gould Instruments, Cleveland, Ohio, USA). The left ventricle was cannulated with PE-50 tubing via the right carotid artery and the correct position was confirmed by pressure tracings. Catheters were subcutaneously tunnelled and exteriorised at the dorsal surface of the neck.
Animals were then subjected to a 3 cm midline incision. The presence or absence of ascites was determined by gently swabbing the peritoneal cavity with preweighed cotton gauze pads, which were weighed afterwards to determine ascites volume. Pilot studies in unoperated and sham operated rats of similar body weight showed that all had <2 ml of fluid in the peritoneal cavity, so ascites was therefore defined as >2 ml of peritoneal fluid.
A PE-10 catheter was inserted into the left ureter and exteriorised through the abdominal wall. The distal end of the catheter was threaded subcutaneously and exteriorised along the leg. All incisions were closed with silk ligatures following local application of 5% lidocaine gel to minimise postoperative pain. Animals were then placed in individual metabolic cages for a recovery period of two hours. After blood pressure and heart rate had been stable for at least one hour, the studies were performed.
Glomerular filtration rate was assessed by 3H inulin (Amersham Life Science, Arlington Heights, Illinois, USA) clearance.18 A solution of 2 μCi 3H inulin/ml was prepared with physiological saline and infused at 33 μl/min through the left femoral vein for one hour. Two successive 30 minute periods were carried out for the clearance study. Urine was collected continuously in preweighed liquid scintillation tubes while blood samples were obtained at the midpoint of each period at a rate of 0.8 ml/min for one minute. Volume losses from blood sampling were replaced by physiological saline. The radioactivity of the blood sample was determined by a liquid scintillation beta counter (Wallack RackBeta, Turku, Finland). Urine volume was determined gravimetrically assuming a density of 1.0 g/ml. Urinary sodium concentration was determined by an ion selective electrode (Hitachi Instruments).
Cardiac output and regional blood flows were measured by radioactive microspheres and the reference sample method.19 A precounted sonicated aliquot of ∼60 000–80 000 microspheres of 15±3 μm diameter labelled with 113Sn (New England Nuclear, Boston, Massachusetts, USA) was injected over 15 seconds into the left ventricle. The spheres were flushed with 0.7 ml of physiological saline. Beginning 5 seconds before microsphere injection, the reference sample was withdrawn from the femoral artery at 0.8 ml/min for one minute into a syringe connected to a motor driven withdrawal pump. Radioactivity of the sample was counted by a gamma counter (Wallack 1480 Wizard 3, Turku, Finland).
Calculations
Cardiac index (ml/min/100 g body weight) was calculated as (total counts per minute (cpm) injected × 0.8 ml/min/reference sample cpm)/100 g body weight. Blood flow (ml/min/100 g body weight) of each organ was calculated as (organ cpm × 0.8 ml/min/reference sample cpm)/100 g body weight. Portal tributary blood flow was calculated as the sum of the flows in the stomach, spleen, small intestine, colon, and mesentery with pancreas. Hepatic arterial flow was calculated as liver cpm as described above. Systemic vascular resistance (mm Hg /ml/min/100 g body weight) was calculated as mean arterial pressure/cardiac index. Renal vascular resistance (mm Hg/ml/min/100 g body weight) was calculated as mean arterial pressure/renal blood flow.
Inulin clearance data from the two successive 30 minute periods in each study were averaged. Glomerular filtration rate (ml/min/100 body weight) was equated to clearance of 3H inulin /100 g body weight.
Hepatic collagen determination
Liver tissue was immediately fixed with 10% formalin in phosphate buffered saline (PBS). Samples were later embedded in paraffin and sectioned (3 μm). After being mounted on glass slides and deparaffinised, sections were immersed for 10 seconds in saturated aqueous picric acid containing 0.1% Fast Green FCF (Sigma) and rinsed with distilled water before staining for 15 minutes with 0.1% Sirius Red F3BA (Polysciences Inc., Warrington, Pennsylvania, USA). Sirius Red and Fast Green selectively bind to collagenous and non-collagenous proteins, respectively. Hepatic fibrosis was estimated as previously described.20 Coded slides were independently assessed under light microscopy by two blinded observers. The extent of hepatic fibrosis (scored from 1 to 4) was estimated according to the following criteria: (1) collagen restricted to portal areas; (2) mild expansion of loose periportal collagen into surrounding parenchyma associated with bile ductular proliferation; (3) moderate expansion of periportal collagen into surrounding parenchyma with significant organisation of fibrous tissue and mild portoportal bridging; and (4) extensive portoportal and portocentral fibrous bridging. There was excellent agreement between the two observers; scores never differed by more than 1 point.
Brain stem medullary immunohistochemical staining for Fos
For the Fos studies, separate groups of animals were prepared as described previously.21,22 In brief, rats were anaesthetised with 50 mg/kg intraperitoneal sodium pentobarbitol (MTC Pharmaceuticals, Mississauga, Ontario, Canada). To avoid Fos expression due to stress, rats that struggled or squealed during injection were removed from further study. The right femoral artery and vein were cannulated with PE 50 tubing (Becton-Dickenson, Parsippany, New Jersey, USA). A few minutes before any incision, topical lidocaine 2% ointment (Astra Pharma, Mississauga, Ontario, Canada) was applied locally to avoid Fos expression due to pain. Haemorrhage was induced by manually withdrawing 12 ml blood/kg body weight (estimated 20% of blood volume) at a rate of 2 ml/min. The right femoral arteries and veins were also cannulated in unchallenged controls, but no blood withdrawn.
After 90 minutes, rats were intravenously infused with sodium pentobarbital (50 mg/kg), perfused with 1 l/kg body weight of cold PBS at pH 7.4, followed by 1 l/kg of ice cold 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. Brains were carefully removed from the rats and post fixed overnight in 4% paraformaldehyde at 4°C and then cryoprotected in 30% sucrose in 0.1 M phosphate buffer for 1–3 days at 4°C. Serial 40 μm sections of brains were cut using a cryostat, and sections were analysed for Fos immunoreactivity. Adjacent sections were stained with cresyl violet to reveal the nuclear boundaries.
Fos was detected using rabbit anti-Fos polyclonal antisera (Oncogene Science, Manhasset, New York, USA). In brief, sections were incubated in a blocking serum consisting of 1.5% normal goat serum (Vector Labs, Burlingame, California, USA) diluted in PBS containing 0.4% Triton X-100 for one hour at room temperature. The blocking serum was removed and sections were incubated with the primary antibody, rabbit anti-Fos polyclonal antisera diluted 1:20 000 with blocking serum, for 48–72 hours at 4°C. Sections were incubated with secondary antibody, biotinylated goat antirabbit IgG (1:200, Vector), washed, incubated in Vecastain Elite ABC Reagent (Vector) and diaminobenzidine-nickel peroxidase substrate (Vector) for colour development, and mounted on chrome-alum coated slides.
FOS quantification
The diaminobenzidine-nickel stained Fos cells were identified by their size, shape, and colour. The relevant brain nuclei were identified using the cresyl violet stained sections. The main parts of the nucleus tractus solitarius (NTS) and ventrolateral medulla (VLM) were chosen from three representative sections of each area. Counting was done visually at 200× magnification. The final value used was the mean of the three sections. All slides were analysed together in order to reduce variability in counting.
Statistical analysis
Results are expressed as mean (SEM). The ascites data were analysed by Fisher’s exact test and other data by one way analysis of variance with a Newman-Keuls post hoc test for multiple comparisons. The significance level was set at p<0.05.
RESULTS
Systemic and splanchnic haemodynamics
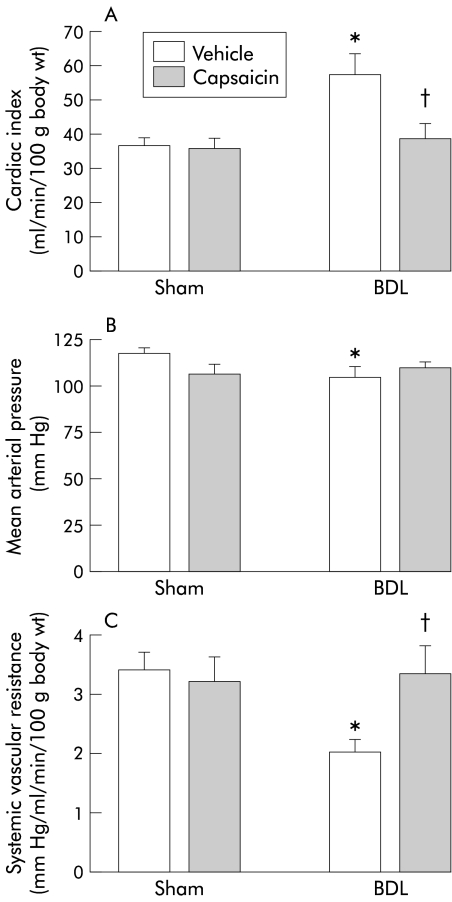
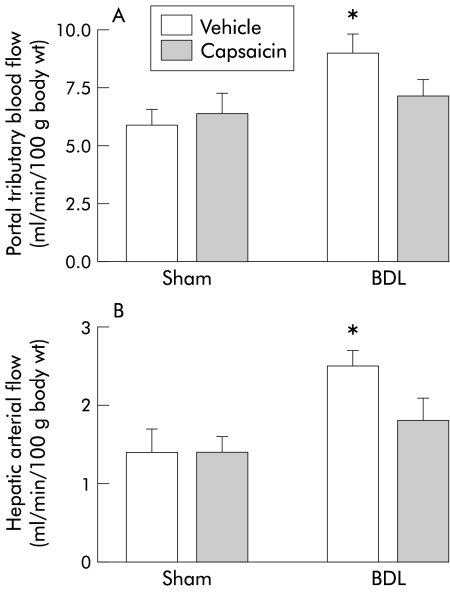
Vehicle treated BDL rats exhibited the expected hyperdynamic circulation, with increased cardiac index, hepatic arterial flow, and portal tributary blood flow, and decreased mean arterial pressure and systemic vascular resistance, compared with sham operated vehicle rats (figs 1, 2 ▶ ▶). Capsaicin treated BDL rats displayed similar systemic haemodynamics as sham operated controls, confirming our previous observations that hyperkinetic circulation is absent in this group (figs 1, 2 ▶ ▶).
Figure 1.
Systemic haemodynamics in the four groups of rats. Cardiac index (A), mean arterial pressure (B), and systemic vascular resistance (C) in bile duct ligated (BDL) or sham operated rats treated with capsaicin or vehicle. Data are mean (SEM). Significant difference: *compared with the sham vehicle group; †compared with BDL vehicle rats.
Figure 2.
Splanchnic haemodynamics in the four groups of rats. Portal tributary blood flow (A) and hepatic arterial flow (B) in bile duct ligated (BDL) or sham operated rats treated with capsaicin or vehicle. Data are mean (SEM). *Significant difference compared with the sham vehicle group.
Renal function and haemodynamics
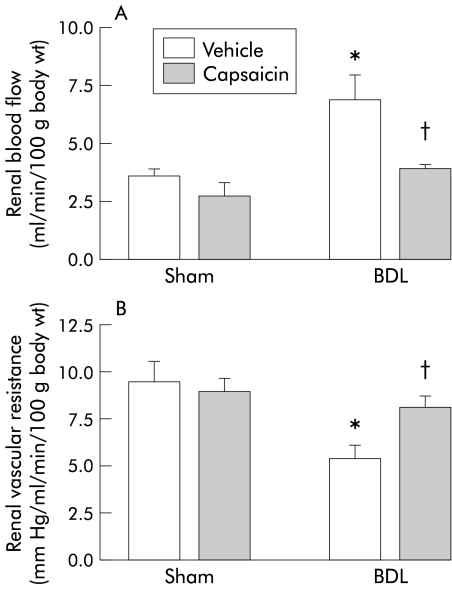
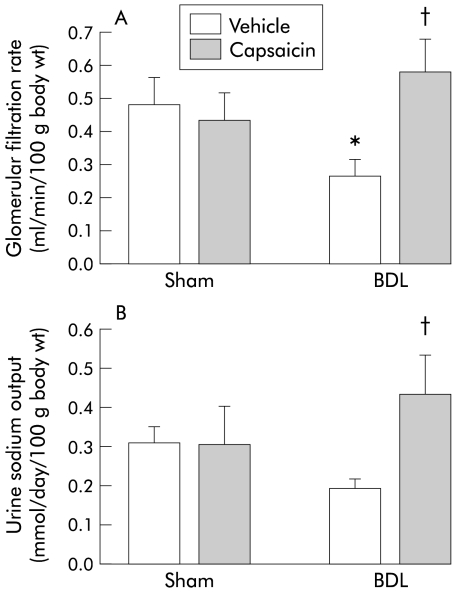
In vehicle treated cirrhotic rats compared with the corresponding vehicle treated sham controls, renal vascular resistance was decreased and renal blood flow increased (fig 3 ▶). In contrast, glomerular filtration rate was significantly decreased in BDL vehicle treated rats compared with sham vehicle treated rats (fig 4 ▶). Capsaicin treatment completely eliminated the abnormalities of renal function observed in the BDL vehicle group. These parameters were similar between BDL capsaicin treated and sham vehicle treated groups. Renal function in sham capsaicin treated rats remained unchanged compared with sham vehicle treated rats (figs 3, 4 ▶ ▶).
Figure 3.
Renal haemodynamics in the four groups of rats. Renal blood flow (A) and renal vascular resistance (B) in bile duct ligated (BDL) or sham operated rats treated with capsaicin or vehicle. Data are mean (SEM). Significant difference: *compared with sham vehicle group; †compared with BDL vehicle rats.
Figure 4.
Renal function in the four groups of rats. Glomerular filtration rate (A) and urine sodium concentration (B) in bile duct ligated (BDL) or sham operated rats treated with capsaicin or vehicle. Data are mean (SEM). Significant difference: *compared with sham vehicle group; †compared with BDL vehicle rats.
Urine sodium output was lower in vehicle treated BDL rats compared with sham vehicle treated animals but this was not significant (fig 4 ▶). However, capsaicin treatment significantly increased urine sodium excretion in BDL rats compared with the vehicle BDL group (fig 4 ▶). Urine volumes showed marked individual variability but the means did not differ significantly between the four groups (in ml/day): sham 50 (9); sham capsaicin 19 (4); BDL 57 (8); BDL capsaicin 48 (7).
Seven of 11 BDL vehicle treated rats showed ascites of 3–19 ml whereas none of 14 BDL capsaicin treated animals had ascites (p<0.05; Fisher’s exact test). No ascites was detected in the two sham operated groups (table 1 ▶).
Table 1.
Effect of capsaicin treatment on ascites formation
| Sham vehicle | BDL vehicle | Sham capsaicin | BDL capsaicin | |
| Ascites present | 0 | 7* | 0 | 0† |
| Ascites absent | 7 | 4 | 7 | 14 |
| Total (n) | 7 | 11 | 7 | 14 |
BDL, bile duct ligated.
Ascites was defined as <2 ml of peritoneal fluid.
Significantly different (p<0.05) from: *sham vehicle group; †BDL vehicle group (Fisher’s exact test).
Hepatic fibrosis
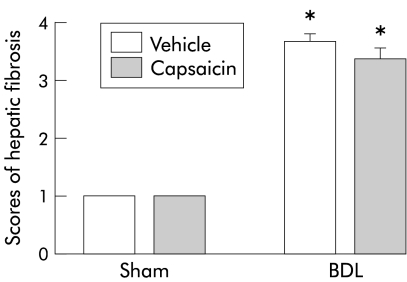
In both vehicle and capsaicin treated sham rats, normal amounts of hepatic collagen were seen in the portal areas (fig 5 ▶). In contrast, both the BDL vehicle and BDL capsaicin rat livers showed fibrous expansion and parenchymal loss. Bridging (portocentral) fibrosis was frequently observed. There were no significant differences in fibrosis scores between BDL vehicle and BDL capsaicin groups (fig 5 ▶).
Figure 5.
Hepatic collagen deposition in the four groups of rats. Hepatic fibrosis scores in bile duct ligated (BDL) or sham operated rats treated with capsaicin or vehicle. Data are mean (SEM). *Significant difference compared with the corresponding sham group.
Medullary neuronal Fos immunoreactivity
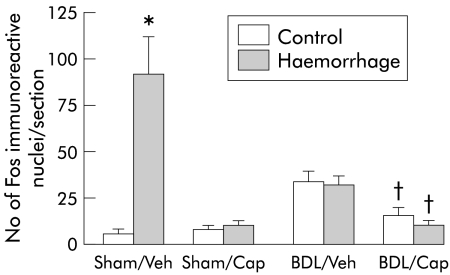
Compared with sham vehicle treated rats, BDL vehicle rats showed increased Fos expression in the NTS (fig 6 ▶), similar to previous studies.21,22 In contrast, capsaicin treated BDL rats had very low levels of Fos expression, comparable with both vehicle and capsaicin treated sham operated rats. The expected reactivity of the NTS to haemorrhage was also completely eliminated in the BDL and sham operated capsaicin treated rats, as well as the BDL vehicle group (fig 6 ▶). Fos immunoreactivity in the VLM showed a similar pattern in all groups (data not shown).
Figure 6.
Number of Fos immunoreactive neurones in the nucleus of the solitary tract. Bile duct ligated (BDL) or sham operated rats treated with capsaicin (Cap) or vehicle (Veh) with (haemorrhage) or without haemorrhage challenge (control). Data are mean (SEM) of eight animals in each group. Significant difference: *compared with sham vehicle control group; †compared with BDL vehicle rats.
DISCUSSION
The results of the present study indicated that capsaicin treated cirrhotic rats failed to demonstrate the expected derangement of the circulation and renal function. Even formation of ascites was blocked in these animals. This suggests that primary afferent nerves can modulate not only haemodynamics but also renal function in cirrhosis.
In the first part of the study, we decided to reconfirm that in our laboratory neonatal capsaicin treatment successfully abrogates the hyperdynamic circulation of cirrhosis. This was because following our initial observation of this capsaicin effect in both BDL cirrhotic and portal hypertensive rats,11 Fernandez et al failed to demonstrate this finding in portal hypertensive rats.23 Thus the current results again showing absence of hyperdynamic circulation in capsaicin treated BDL rats were reassuring. We believe that significant differences in our experimental protocols and those of Fernandez et al explain the discrepancy, in particular our conscious animal protocols versus their use of anaesthetic. Moreover, Fernandez et al did not study the BDL rat model. Finally, several other studies indirectly support our position that primary afferent nervous tone is altered in this BDL model. Specifically, Ferraz et al found that gastric microcirculatory responses to acute capsaicin application were depressed24 in the same BDL model, and Casini et al reported that neonatal capsaicin treatment significantly reduced hepatic fibrosis in the eight week BDL rat.25 A preliminary study has reported significant effects of an ablative dose of capsaicin on hepatic blood flow in cirrhotic rats.26
We chose to study the chronic BDL rat model because in most centres, and certainly in our hands, the majority of these animals develop easily detectable ascites. The haemodynamics and renal functional changes in the BDL rat have been extensively characterised previously. In those respects, our results in the vehicle treated BDL rats generally agree with the literature.27–29 However, because the four week BDL rat has dramatically increased renal blood and plasma flows, unlike the cirrhotic patient, direct extrapolation of the present results to the human condition would be speculative.
There are four possible explanations for normalisation of renal function in our capsaicin treated BDL rats. Firstly, could the results be due to a direct effect of capsaicin itself, independent of its effect on the primary afferent nerves? The available evidence concerning the renal effects of neonatal capsaicin treatment suggests in fact that the opposite result might occur. Previous studies indicated that neonatal capsaicin administration to normal rat pups results in an antinatriuretic and antidiuretic effect in adult life.30–32 The mechanism underlying this possible renal inhibitory effect of capsaicin remains unclear. However, acute capsaicin dosing appears to have no effect on renal function,30–32 so the effect of neonatal treatment is likely mediated through primary afferent denervation. In any case, we did not observe any renal or haemodynamic effect of capsaicin in our sham operated control rats, and the effects on renal function in our BDL rats were opposite to the effects described in normal rats. Accordingly, we believe that capsaicin per se did not cause the changes in renal function in the present study.
Secondly, as Casini et al noted decreased hepatic fibrosis in their eight week BDL rats, the question arises whether the present results are simply due to a decrease in portal pressure. However, based on our data and other results, this possibility seems extremely unlikely. Chronic bile duct ligation induces a progressive cirrhosis/liver failure,33,34 and the eight week BDL rat is probably very different from the four week BDL model used in our study. Moreover, in a previous study, we found no difference in portal pressure between capsaicin and vehicle treated four week BDL rats.11 Finally, the present results showed a similar extent of hepatic fibrosis in the two groups.
Thirdly, could the results be directly due to denervation of the primary afferent innervation? It is clear from previous studies that the neonatal dose of capsaicin used in the present study was sufficient to permanently ablate the primary afferent neurones and induce lifelong denervation.13,14 Previous studies provide circumstantial evidence in favour of the notion that the renal effects noted in the present study were due to afferent denervation. Over the past decade, DiBona et al have elegantly demonstrated that in the BDL rat, renal sympathetic nervous tone is increased, and this leads to salt and water retention.35 Ablation of the renal efferent nerves corrects the renal functional abnormalities, including sodium retention.36 In general, the neural pathway controlling the cardiovascular system is comprised of the primary afferent innervation, the brain stem medullary cardiovascular regulatory nuclei, predominantly the NTS and VLM, and the effector arm composed of sympathetic and parasympathetic efferent nerves.
In the last part of our study, we aimed to test the hypothesis that capsaicin induced interruption of the afferent loop would disrupt the normal functioning of this pathway by examining Fos immunohistochemical staining in the brain stem medullary nuclei. It has been clearly established that neuronal activation is associated with induction of the c-fos gene and its protein product Fos.37–39 c-fos is an immediate early gene that has important roles in cellular signal transduction and transcriptional regulation.40 Immunohistochemical detection of Fos has been used as a metabolic marker to identify neuronal groups and trace neuronal pathways that have been activated by physiological and pathophysiological stimuli.41,42 In a previous study, we demonstrated a baseline increase in Fos staining in the NTS and VLM of BDL cirrhotic rats.22 Moreover, an intense stimulus such as hypotensive haemorrhage did not affect the level of neuronal activation in BDL animals whereas controls showed the expected increase.22
In addition to a role as a marker of neuronal activation, there is increasing evidence to suggest that c-fos mediated signal transduction and transcription can directly activate neurones.43,44 We have recently demonstrated that c-fos mediated signalling is a sine qua non for the development of hyperdynamic circulation in the prehepatic portal hypertensive rat.45 In that study, microinjections of c-fos antisense oligonucleotides into the NTS eliminated the hyperdynamic circulation in portal hypertensive rats.45 All of these findings indicate that the c-fos pathway is a key mediator of the hyperdynamic circulation of portal hypertension and cirrhosis.
In the present study, neonatal capsaicin treatment of BDL rats markedly diminished baseline Fos staining in BDL rats through to the scant levels seen in normal controls. This result was not unexpected, as our previous study with subacute (seven days prior to the study) administration of capsaicin to the adult rat had shown similar blunting of Fos staining in the NTS and VLM.21 The present results indicated that neonatal capsaicin treatment also abolished the central neural response to cardiovascular challenge in BDL rats. However, because the hyperdynamic circulation was also eliminated in these capsaicin treated animals, it is impossible to completely rule out a major or permissive effect of such elimination as a cause of the observed changes in renal function.
Thus the fourth possibility is that elimination of the hyperdynamic circulation induced the beneficial effects on renal function. This is consistent with the “peripheral vasodilatation” hypothesis.1 According to this hypothesis, peripheral vasodilatation leads to decreased effective circulating volume, thus inducing the cirrhotic kidney to retain salt and water, by the pressure diuresis mechanism.46,47
Exactly how neonatal capsaicin denervation of primary afferent nerves blocks the development of systemic vasodilatation in cirrhotic rats remains unclear. As central neural activation is necessary for the hyperdynamic circulation to develop, the current finding of abrogated central activation in capsaicin treated BDL rats strongly suggests that the peripheral to central signal that leads to central activation and thus increased efferent cardiovascular nervous activity is carried by primary afferent nerves. The exact nature of the peripheral to central signal remains speculative; we have previously suggested that portal or mesenteric venous hypertension or congestion might be the initial peripheral signal.45 Primary afferent nerves also contain vasodilatory peptides such as substance P and CGRP. These substances might exert a local effect, particularly in the gut circulation.
In the present study, because capsaicin treatment induced the dual and probably intertwined phenomena of afferent denervation and abrogation of hyperdynamic circulation, we cannot definitively ascertain which mechanism (vasodilatation versus denervation) is operative. Indeed, it is possible that both mechanisms may contribute: we speculate that primary afferent denervation interrupts the neural control pathway, leading to decreased activity in the brain stem cardiovascular regulatory centres, thereby eliminating systemic and renal vasodilatation and removing the major stimulus for renal sodium retention.
In conclusion, neonatal capsaicin treatment prevented the development of hyperdynamic circulation and renal dysfunction as well as ascites formation in cirrhosis. The beneficial renal effect might be mediated through denervation of capsaicin sensitive primary afferent nerves or by elimination of systemic peripheral vasodilatation.
Acknowledgments
This study was funded by an operating research grant from the Canadian Institutes of Health Research. Dr Li and Dr Song were supported by fellowship awards from the Canadian Association of Gastroenterology and the Canadian Association for Study of Liver, respectively. Dr Lee was supported by an Alberta Heritage Foundation for Medical Research Senior Scholarship award.
Abbreviations
BDL, bile duct ligated
NTS, nucleus of the solitary tract
VLM, ventrolateral medulla
CGRP, calcitonin gene related peptide
cpm, counts per minute
PBS, phosphate buffered saline
REFERENCES
- 1.Schrier RW, Arroyo V, Bernardi M, et al. Peripheral arterial vasodilation hypothesis: A proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151–7. [DOI] [PubMed] [Google Scholar]
- 2.Benoit JN, Barrowman JA, Harper SL, et al. Role of humoral factors in the intestinal hyperemia associated with chronic portal hypertention. Am J Physiol 1984;247:E486–93. [DOI] [PubMed] [Google Scholar]
- 3.Kravetz D, Bosch J, Arderiu MT, et al. Effects of somatostatin on splanchnic hemodynamics and plasma glucagon in portal hypertensive rats. Am J Physiol 1988;254:G322–8. [DOI] [PubMed] [Google Scholar]
- 4.Benoit JN, Zimmerman B, Premen AJ, et al. Role of glucagon in splanchnic hyperemia of chronic portal hypertension. Am J Physiol 1986;251:G674–8. [DOI] [PubMed] [Google Scholar]
- 5.Pizcueta MP, Pique JM, Fernandez M, et al. Modulation of the hyperdynamic circulation of cirrhotic rats by nitric oxide inhibition. Gastroenterology 1992;103:1909–15. [DOI] [PubMed] [Google Scholar]
- 6.Sieber CC, Lopez-Talavera JC, Groszmann RJ. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology 1993;104:1750–4. [DOI] [PubMed] [Google Scholar]
- 7.Niederberger M, Martin PY, Gines P, et al. Normalization of nitric oxide production corrects arterial vasodilatation and hyperdynamic circulation in cirrhotic rats. Gastroenterology 1995;109:1624–30. [DOI] [PubMed] [Google Scholar]
- 8.Sitzmann JV, Bulkley GB, Mitchell MC, et al. Role of prostaglandin in the splanchnic hyperemia contributing to portal hypertension. Ann Surg 1989;209:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton G, Phing RC, Hutton RA, et al. The relationship between prostacyclin activity and pressure in the portal vein. Hepatology 1982;2:236–42. [DOI] [PubMed] [Google Scholar]
- 10.Guarner C, Soriano G, Such J, et al. Systemic prostacyclin in cirrhotic patients: relationship with portal hypertension and changes after intestinal decontamination. Gastroenterology 1992;102:303–9. [PubMed] [Google Scholar]
- 11.Lee SS, Sharkey KA. Capsaicin treatment blocks development of hyperkinetic circulation in portal hypertensive and cirrhotic rats. Am J Physiol 1993;264:G868–73. [DOI] [PubMed] [Google Scholar]
- 12.Hori N, Okanoue T, Sawa Y, et al. Role of calcitonin gene-related peptide in the vascular system on the development of the hyperdynamic circulation on conscious cirrhotic rats. Hepatology 1997;26:1111–19. [DOI] [PubMed] [Google Scholar]
- 13.Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically-induced selective degeneration of chemosensitive primary sensory neurons. Nature 1977;270:741–3. [DOI] [PubMed] [Google Scholar]
- 14.Sharkey KA, Williams RG, Dockray GJ. Sensory substance P innervation of the stomach and pancreas. Demonstration of capsaicin-sensitive sensory neurons in the rat by combined immunohistochemistry and retrograde tracing. Gastroenterology 1984;87:914–21. [PubMed] [Google Scholar]
- 15.Lee SS, Girod C, Braillon A, et al. Hemodynamic characterization of the chronic bile duct-ligated rat: effect of pentobarbital sodium. Am J Physiol 1986;251:G176–80. [DOI] [PubMed] [Google Scholar]
- 16.Ma Z, Zhang Y, Huet PM, et al. Differential effects of jaundice and cirrhosis on beta-adrenoceptor signaling in three rat models of cirrhotic cardiomyopathy. J Hepatol 1999;30:485–91. [DOI] [PubMed] [Google Scholar]
- 17.Lee SS, Chilton EL, Pak JM. Adenosine receptor blockade reduces splanchnic hyperemia in cirrhotic rats. Hepatology 1992;15:1107–11. [DOI] [PubMed] [Google Scholar]
- 18.Anderson WP, Korrer PI, Selig SE. Mechanisms involved in the renal response to intravenous and renal artery infusions of noradrenaline in conscious dogs. J Physiol 1981;321:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuchiya M, Ferrone RA, Walsh GM, et al. Regional blood flows measured in conscious rats by combined Fick and microsphere methods. Am J Physiol 1978;235:G357–60. [DOI] [PubMed] [Google Scholar]
- 20.Rioux KP, Sharkey KA, Wallace JL, et al. Hepatic mucosal mast cell hyperplasia in rats with secondary biliary cirrhosis. Hepatology 1996;23:888–95. [DOI] [PubMed] [Google Scholar]
- 21.Song DS, Sharkey KA, Breitman DR, et al. Disordered central cardiovascular regulation in portal hypertensive and cirrhotic rats. Am J Physiol 2001;280:G420–30. [DOI] [PubMed] [Google Scholar]
- 22.Breitman DR, Lee SS. Blunted responsiveness of the neuronal activation marker Fos in brainstem cardiovascular nuclei of cirrhotic rats. Hepatology 1997;26:1380–5. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez M, Casadevall M, Schuligoi R, et al. Neonatal capsaicin treatment dose not prevent splanchnic vasodilatation in portal hypertensive rats. Hepatology 1994;20:1609–14. [DOI] [PubMed] [Google Scholar]
- 24.Ferraz JG, McKnight W, Sharkey KA, et al. Impaired vasodilatory responses in the gastric microcirculation of anesthetized rats with secondary biliary cirrhosis. Gastroenterology 1995;108:1183–91. [DOI] [PubMed] [Google Scholar]
- 25.Casini A, Lippe IT, Evangelista S, et al. Effect of sensory denervation with capsaicin on liver fibrosis induced by common bile duct ligation in rat. J Hepatol 1990;11:302–12. [DOI] [PubMed] [Google Scholar]
- 26.Mach T, Zejc M, Sendur R, et al. Sensory neurons and neuropeptides in the regulation of hepatic blood flow and hepatoprotection. J Hepatol 2001;34(suppl 1):S203. [Google Scholar]
- 27.Criado M, Flores O, Ortiz MC, et al. Elevated glomerular and blood mononuclear lymphocyte nitric oxide production in rats with chronic bile duct ligation: role of inducible nitric oxide synthase activation. Hepatology 1997;26:268–77. [DOI] [PubMed] [Google Scholar]
- 28.Jonassen TE, Christensen S, Sorensen AM, et al. Effects of chronic octreotide treatment on renal changes during cirrhosis in rats. Hepatology 1999;29:1387–95. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz MC, Garcia-Sanz A, Bentley MD, et al. Microcomputed tomography of kidneys following chronic bile duct ligation. Kidney Int 2000;58:1632–40. [DOI] [PubMed] [Google Scholar]
- 30.Manzini S, Bacciarelli C, Perfumi M, et al. Impairment of renal urinary excretion in neonatal, but not in adult capsaicin-pretreated rats. Neurosci Lett 1992;135:1–4. [DOI] [PubMed] [Google Scholar]
- 31.Holzer-Petsche U, Lembeck F. Systemic capsaicin treatment impairs the micturition reflex in the rat. Br J Pharmacol 1984;83:935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perfumi M, Massi M. Resiniferatoxin provides further evidence for a role of capsaicin-sensitive sensory neurons in the control of the kidney function. Arch Int Pharmacodyn Ther 1994;327:232–45. [PubMed] [Google Scholar]
- 33.Better OS, Bomzon A. Effects of jaundice on the renal and cardiovascular system. In: Epstein M, ed. The kidney in liver disease, 3rd Edn. Baltimore: William and Wilkins, 1988:508–28.
- 34.Martinez-Prieto C, Ortiz MC, Fortepiani LA, et al. Haemodynamic and renal evolution of the bile duct-ligated rat. Clin Sci 2000;98:611–17. [PubMed] [Google Scholar]
- 35.DiBona GF, Sawin LL, Jones SY. Characteristics of renal sympathetic nerve activity in sodium-retaining disorders. Am J Physiol 1996;271:R295–2. [DOI] [PubMed] [Google Scholar]
- 36.DiBona GF, Sawin LL. Hepatorenal baroreflex in cirrhotic rats. Am J Physiol 1995;269:G29–33. [DOI] [PubMed] [Google Scholar]
- 37.Dun NJ, Dun SL, Chiaia NL. Hemorrhage induces Fos immunoreactivity in rat medullary catecholaminergic neurons. Brain Res 1993;608:223–32. [DOI] [PubMed] [Google Scholar]
- 38.Miura M, Takayama K, Okada J. Neuronal expression of Fos protein in the rat brain after baroreceptor stimulation. J Auton Nerv Syst 1994;50:31–43. [DOI] [PubMed] [Google Scholar]
- 39.Murphy, AZ, Ennis M, Shipley MT, et al. Directionally specific changes in arterial pressure induce differential patterns of fos expression in discrete areas of the rat brainstem: a double-labeling study for Fos and catecholamines. J Comp Neurol 1994;349:36–50. [DOI] [PubMed] [Google Scholar]
- 40.Curran T, Miller AD, Zokas L, et al. Viral and cellular fos proteins: a comparative analysis. Cell 1984;36:259–68. [DOI] [PubMed] [Google Scholar]
- 41.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 1989;29:261–5. [DOI] [PubMed] [Google Scholar]
- 42.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 1988;240:1328–31. [DOI] [PubMed] [Google Scholar]
- 43.Zhu B, Herbert J. Angiotensin II interacts with nitric oxide-cyclic GMP pathway in the central control of drinking behaviour: mapping with c-fos and NADPH-diaphorase. Neuroscience 1997;79:543–53. [DOI] [PubMed] [Google Scholar]
- 44.Chikada N, Imaki T, Seki T, et al. Distribution of c-fos mRNA in the brain following intracerebroventricular injection of nitric oxide (NO)-releasing compounds: possible role of NO in central cardiovascular regulation. J Neuroendocrinol 2000;12:1112–23. [DOI] [PubMed] [Google Scholar]
- 45.Song D, Liu H, Sharkey KA, et al. Hyperdynamic circulation in portal-hypertensive rats is dependent on central c-fos gene expression. Hepatology 2002;35:159–66. [DOI] [PubMed] [Google Scholar]
- 46.Guyton AC, Coleman TG, Cowley AW. Arterial pressure regulation: overriding dominance of the kidney in the long-term regulation and in hypertension. Am J Med 1972;52:584–94. [DOI] [PubMed] [Google Scholar]
- 47.Atucha NM, Cegarra M, Ramirez A, et al. Pressure diuresis and natriuresis in cirrhotic rats. Am J Physiol 1993;265:G1045–9. [DOI] [PubMed] [Google Scholar]