Abstract
Background: Superoxide (O2−) generation through the activity of reduced nicotinamide dinucleotide (NADH) or reduced nicotinamide dinucleotide phosphate (NADPH) oxidases has been demonstrated in a variety of cell types, but not in human colonic epithelial cells.
Aims: To measure O2− production and effects of modulators of NAD(P)H oxidase activity and inhibitors of potential O2− generating enzymes in cultures of human colonic epithelial cells. Expression of the catalytic subunits of NAD(P)H oxidase, Nox1 and gp91phox (phox, phagocytic oxidase), and the membrane bound subunit p22phox was assessed.
Methods: The transformed colonic epithelial cell lines (DLD-1, HT-29, and Caco-2) were studied at subconfluence, confluence, and after differentiation. Primary colonic epithelial cells were isolated from mucosal biopsies from the normal human colon. Extracellular O2− production was measured by the cytochrome c reduction assay or luminol enhanced luminescence. Nox1, gp91phox, and p22phox mRNA expression was assessed in colonic epithelial cells and blood neutrophils by reverse transcriptase-polymerase chain reaction.
Results: Production rates of O2− were higher in subconfluent transformed cells (mean (SEM) 35.8 (4.2) nmol/mg of protein/h) and primary cells (40.4 (5.9)) than in confluent transformed cells (6.0 (0.9); p<0.01). The oxidoreductase inhibitor diphenylene iodonium significantly inhibited O2− production whereas NADPH and NADH increased production rates. In contrast, O2− was unaffected by phorbol myristate ester, NG-nitro-l-arginine methyl ester, indomethacin, or allopurinol. Nox1 mRNA was expressed in all colonic epithelial cells whereas gp91phox was detected only in HT-29 cells and neutrophils. p22phox was expressed in all cell types.
Conclusions: Cultures of transformed and primary epithelial cells from human colon may produce extracellular O2− through an NAD(P)H oxidase expressing Nox1 and p22phox.
Keywords: epithelial cell, colon, large intestine, oxidoreductases, superoxides, NAD(P)H
Synthesis of the superoxide anion (O2−), a key antimicrobial process in phagocytes, has not been demonstrated in human colonic epithelial cells. As nitrotyrosine, the reaction product of O2− with nitric oxide (NO), is abundant in the inflamed epithelium from patients with ulcerative colitis, the colonic epithelial cells themselves may have the ability to produce O2−.1,2
In phagocytes, O2− is generated by a membrane bound enzyme complex, reduced nicotinamide dinucleotide phosphate (NADPH) oxidase, which consists of a transmembrane electron transporting component, the cytochrome b558 heterodimer, comprising a catalytic subunit, gp91phox (phox—phagocytic oxidase), and p22phox.3 On stimulation, a combination of cytosolic subunits translocates to the membrane and subsequently the oxidase complex reduces extracellular oxygen to O2− by use of an electron from intracellular NADPH. Stimulators of phagocyte NADPH oxidase include phorbol myristate ester (PMA) which acts promptly via activation of protein kinase C.4
O2− is also produced by non-phagocytic cells, such as endothelial cells,5 vascular smooth muscle cells,5 and fibroblasts,6 and has been suggested to act as a regulator of genes involved in proliferation, apoptosis, and inflammation.7 In non-phagocytic cells, NADPH as well as reduced nicotinamide dinucleotide (NADH) may serve as a substrate for the oxidase, which is subsequently termed NAD(P)H oxidase, where appropriate. Several alternative catalytic subunits of NAD(P)H oxidase, Nox1–5, have been described,8 some of which appear to be tissue specific. Thus, Nox1 was abundantly expressed in the colon but less in vascular smooth muscle cells.9,10 Nox1 mRNA was detected in colonic mucosal biopsies and in the colonic epithelial cell line Caco-2 but was absent in leucocytes.9
Other sources of O2− exist, such as NO synthases (NOS), xanthine oxidase, cyclooxygenase (COX), and mitochondrial electron transport chain enzymes, which play a quantitatively minor role in the production of O2−.11
O2− production has not been observed in the human colon but cultures of crypt epithelium from rat colon produce O2− under basal conditions.12 Also, cultured gastric pit cells from guinea pigs generate O2− which was unaffected by PMA, suggesting another source of O2− than gp91phox.11
In the present study, we have measured O2− production and expression of the NAD(P)H oxidase subunits Nox1, gp91phox, and p22phox in cultures of transformed colonic epithelial cell lines and primary epithelial cells from the normal human colon.
MATERIAL AND METHODS
Reagents, media, and PCR primers
Reagents were purchased from Sigma Chemical Company (St Louis, Missouri, USA) unless otherwise stated and all culture media were from Gibco (Gaithersburg, Maryland, USA). O2− measurements were performed in Krebs-Ringer buffer (mM) (NaCl 130, KCl 5, CaCl2 0.9, MgCl2 1.2, sodium dihydrogenphosphate-dihydrate 15, and glucose 5), pH 7.4, in which all reagents were dissolved, except for PMA, which was dissolved in dimethyl sulphoxide. For the polymerase chain reaction (PCR), the following primer pairs (Amersham Bioscience, Little Chalfont, UK) were used: Nox1, upstream: 5′-CCAGGATTGAAGTGGATGGT-3′; downstream: 5′-CGGTGAGGAAGAGACGGTAG-3′ (PCR product of 297 bp); gp91phox, upstream: 5′-GAATGGGGAAAAATAAAGGAATG-3′; downstream: 5′-ACCCCTTCTTCTTCATCTGTAGC-3′ (product of 202 bp); and for p22phox, upstream: 5′-CGCTTCACCCAGTGGTACTT-3′; downstream: 5′-GAGAGCAGGAGATGCAGGAC-3′ (product of 200 bp).
Concentrations of lipopolysaccharide of all buffers and culture media were found to be below 5 pg/ml by the limulus amoebocyte lysate assay combined with rocket immunoelectrophoresis.13
DLD-1, HT-29, and Caco-2 colonic epithelial cell lines
The undifferentiated human colonic carcinoma cell lines DLD-1 and HT-29 (American Type Culture Collection (ATCC), Manassas, Virginia, USA) were cultured in McCoy’s 5a medium with 10% (v/v) fetal calf serum in 5% CO2 at 37°C. Caco-2 cells (ATTC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with phenol red, glucose 5.5 mM, and Glutamax, supplemented with 10% (v/v) fetal calf serum. Prior to the study, 105 or 106 cells were seeded in 24 well plates (TTP, Trasadinger, Switzerland) and cultured for 24 hours to obtain subconfluent (approximately 15% confluence for all cell lines) and confluent cells (>95%), respectively. However, O2− was undetectable in preliminary experiments with cells cultured in 2.5 ml of DMEM in the presence of the above additives containing either 5.5 or 25 mM glucose for 24 hours (no change of media).14 Therefore, cell lines were cultured in 2.5 ml of DMEM with the above additives containing glucose 5.5 mM, which was changed every eight hours. To enable culture in higher volumes, cells were seeded in Pico glass vials (Packard, Groningen, Netherlands) and cultured for 24 hours in 8 ml of DMEM with the above additives containing glucose 5.5 mM, which was changed every eight hours. To assess the role of butyrate, cell lines were cultured for 24 hours in 2.5 ml of DMEM with the above additives containing glucose 5.5 mM and butyrate 5 mM with or without change of media.
For differentiated monolayers, culture of Caco-2 cells was continued for a total of 14 days, during which DMEM with the above additives containing glucose 5.5 mM was changed every second day. During the last 24 hours prior to the study, media were changed every eight hours.
Cell metabolism was estimated by measuring glucose, l-lactate, and pH in the media by an autoanalyser (ABL-625, Radiometer, Copenhagen, Denmark), and mitochondrial enzymatic function was assessed by the dimethylthiazol diphenyltetrazolium bromide (MTT) assay.15
Primary colonic epithelial cells from subjects with an uninflamed bowel
Participants
Permission for the study was obtained from the regional ethics committee and all participants gave informed written consent.
Healthy non-smoking subjects, who were free of medications for at least one month, were recruited among hospital staff. Patients referred to endoscopy for symptoms of irritable bowel syndrome or haematochezia to exclude colorectal cancer were included if they had a normal colonoscopy and uninflamed mucosa at histopathological examination.
Endoscopy
In healthy subjects, sigmoidoscopy was performed without prior bowel preparation and eight biopsy specimens were taken from the sigmoid colon and placed in 10 ml of ice cold Krebs-Ringer buffer for immediate isolation of the epithelium. In patients, routine colonoscopy was performed after bowel preparation with an oral polyethylene glycol (The Pharmacy, Bispebjerg Hospital, Denmark) 3000 solution (96 g dissolved in 1.5 litre of tap water) the night before. Eight biopsy specimens were taken from the transverse colon as described above. Additional biopsies were sampled for histopathological evaluation and, if abnormal, the subject was excluded.
Isolation and culture of primary colonic epithelial cells
The isolation procedure has been assessed previously.15 In brief, biopsy specimens were incubated with 1 mM of EDTA/EGTA for 10 minutes followed by mechanical separation, coarse filtering, and washing. Prior to the study, cells were suspended in Krebs-Ringer buffer, pH 7.4, transferred to Eppendorf tubes, and incubated for one hour at 37°C.
Isolation of neutrophils from peripheral blood
Neutrophils were isolated from peripheral blood of healthy subjects for mRNA extraction.16 In brief, blood was sampled in EDTA containing vials and subjected to sequential Dextran sedimentation (Lymphoprep; Nycomed, Oslo, Norway) and gradient centrifugation. Contaminating erythrocytes were lysed using hypotonic saline, and normal osmolality was restored by the addition of hypertonic saline followed by centrifugation at 2000 g for 10 minutes at 4°C.
Superoxide detection
Superoxide dismutase inhibitable cytochrome c reduction assay
Extracellular O2− production was measured by incubation of cells in duplicate with 1 mg/ml cytochrome c from horse heart in Krebs-Ringer buffer, pH 7.4, with or without superoxide dismutase (SOD) 200 U/ml from bovine erythrocytes, and incubated for one hour at 37°C.17 The reaction was stopped by removal of the media from the wells (transformed cells) or by centrifugation at 5000 g at 4°C for five minutes (primary cells), and absorbance of the supernatants was read at 550 nm against distilled water on a spectrophotometer (Shimadzu UV A160, Kyoto, Japan). The difference in absorbance between supernatants of comparable cells incubated with or without SOD was converted to equivalent O2− production using the molar extinction coefficient for cytochrome c (21×103/mol/cm). The detection limit, expressed as mean production of O2− from heat inactivated HT-29 cells+2 SD (n=10), was 0.25 nmol/mg of protein/h and day to day interassay variation, expressed as the coefficient of variation of basal values from 105 HT-29 cells (n=10), was 15%. For pharmacological studies, transformed or primary colonic epithelial cells were incubated with cytochrome c as described above, with or without potential modulators of NAD(P)H oxidase activity: diphenylene iodonium (DPI) 10 μM, NADPH or NADH 10 μM, 100 μM or 1 mM, or PMA 0.05, 0.5, or 5 μg/ml. In additional experiments, transformed cells were incubated with or without potential inhibitors of other O2− generating enzymes: NG-nitro-l-arginine methyl ester (l-NAME), indomethacin, or allopurinol 10 μM, 100 μM, or 1 mM. HT-29 cells, which were heat inactivated or cultured for 24 hours without change of media or supplementation of butyrate, served as negative controls.
Luminol enhanced luminescence assay
To study the kinetics of extracellular O2− production, suspended epithelial cells were incubated in triplicate with luminol 500 μM in Krebs-Ringer buffer, pH 7.4, at 37°C, and luminescence was measured at fixed time points using a luminometer (FB12; Berthold Detection Systems, Pforzheim, Germany). At 60 minutes, NADPH 1 mM was added and luminescence was measured for another hour. In each experiment, values were subtracted from those obtained in experiments with comparable cells treated with SOD 200 U/ml to obtain the luminescence caused by extracellular O2−. Values were corrected for background (Krebs-Ringer buffer with luminol) and given as relative light units per mg of protein per 10 seconds. Day to day interassay variation, expressed as the coefficient of variation of basal values from 105 HT-29 cells (n=10), was 21%.
Expression analysis
RT-PCR analysis
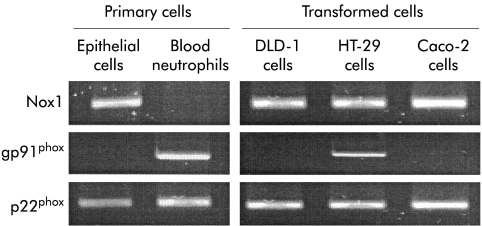
Total RNA was extracted from freshly isolated epithelial cells or blood neutrophils by use of TRIzol (Gibco) and cDNA was generated using the First-Strand cDNA Synthesis Kit (Amersham Bioscience). PCR was performed using AmpliTaq Gold (Applied Biosystems, Foster City, California, USA) and cycles of 35 seconds of denaturation at 94°C, 35 seconds of annealing at 58°C, and 30 seconds of extension at 72°C, all performed in a Mastercycler Gradient (Eppendorf, Hamburg, Germany). The number of cycles for each product is given in the legend to fig 4 ▶. After amplification, PCR products were separated on 2% agarose gels containing 1 μg/μl ethidium bromide and visualised by UV light (Image Master, Amersham Bioscience).
Figure 4.
Expression of Nox1, gp91phox, and p22phox mRNA in transformed colonic epithelial cell lines, primary colonic epithelial cells, and peripheral neutrophils from healthy subjects. Extracted RNA was subjected to reverse transcriptase-polymerase chain reaction using 30 (Nox1 and p22phox) or 37 (gp91phox) cycles of amplification (see methods). The products were separated on 2% agarose gels and visualised by ethidium bromide.
For control of primer specificity, restriction enzyme analysis was performed using enzymes identified by use of the pDraw32 software (shareware, http://home.get2net.dk/acaclone). The PCR products for Nox1, gp91phox, and p22phox were incubated with the relevant enzymes (Apo-1, Sau-96-1, and Mnl-1, respectively; New England Biolabs, Beverly, Massachusetts, USA) for one hour, as instructed by the manufacturer, loaded onto 3% agarose gels, and analysed as above.
Statistical analysis
Results are expressed as means (SEM). The normality assumption for this model was tested with Komolgorov-Smirnov statistics and Levine statistics were used to test for equal variance. Data were analysed by t test for paired or unpaired variables, where appropriate.18 Values of p<0.05 (two tailed) were considered significant.
RESULTS
Metabolism of colonic epithelial cell lines
Concentrations of glucose and lactate, and the pH of the media varied depending on the concentration of cells, the initial concentration of glucose in the media, addition of butyrate, and the frequency of changing the media during culture (table 1 ▶). In media of 105 cells, no changes in pH or lactate values were observed after 24 hours and the concentration of glucose remained high if the initial concentration of glucose was raised to 25 mM (see table 1 ▶). The values of glucose, lactate, and pH in media with 5.5 mM glucose were unaffected by supplementation with butyrate. In unchanged media of 106 cells, the concentration of glucose decreased while lactic acidosis occurred. In contrast, values for glucose, lactate, and pH remained essentially unaffected if the media were changed every eight hours. Addition of butyrate to cultures with 106 cells and unchanged media reduced the decrease in glucose and lactic acidosis. The combination of changing media and supplementing butyrate caused an increase in glucose and aggravation of lactic acidosis (see table 1 ▶).
Table 1.
Concentrations (mean (SEM)) of glucose and lactate and pH in media of 105 or 106 transformed colonic epithelial cells after 24 hours of culture
| 105 cells | 106 cells | |||||
| Glucose (mM) | Lactate (mM) | pH | Glucose (mM) | Lactate (mM) | pH | |
| Initial concentration of glucose | ||||||
| Media unchanged | ||||||
| 5.5 mM | 5.0 (0.1) | 2.1 (0.2) | 7.46 (0.02) | 1.8 (0.1)* | 8.0 (0.1)* | 7.33 (0.01)* |
| 5.5 mM+butyrate | 5.1 (0.1) | 1.7 (0.1) | 7.48 (0.02) | 2.8 (0.3) † | 6.2 (0.4) † | 7.31 (0.02) |
| 25 mM | 21 (0.4) | 2.3 (0.2) | 7.44 (0.02) | 17 (0.4)* | 7.4 (0.1)* | 7.23 (0.01)* |
| Media changed every 8 hours | ||||||
| 5.5 mM | 5.3 (0.1) | 1.9 (0.2) | 7.45 (0.02) | 4.3 (0.1) | 2.5 (0.2) | 7.41 (0.02) |
| 5.5 mM+butyrate | 5.2 (0.1) | 1.4 (0.1) | 7.45 (0.03) | 3.6 (0.2) † | 4.2 (0.2) † | 7.32 (0.01) † |
HT-29 cells were cultured for 24 hours in 2.5 ml of Dulbecco’s modified Eagle’s medium with 10% fetal calf serum containing glucose 5.5 mM (with or without butyrate 5 mM) or 25 mM. During culture, the media were left unchanged or changed every eight hours, as indicated.
n=5 for all experiments.
*p<0.05 compared with cells cultured in media containing glucose 5.5 mM without butyrate and changed every eight hours (paired t test).
†p<0.05 compared with cells cultured at identical conditions without butyrate (paired t test).
MTT values were unaffected by incubation with cytochrome c 1 mg/ml for one hour (per cent of basal values, mean (SEM), n=3 for all experiments: 103% (4)), DPI 10 μM (96% (3)), NADPH 10 μM, 100 μM, and 1 mM (97% (4), 107% (3), and 98% (6), respectively) or NADH (96% (2), 95% (2), and 103% (7)), PMA 0.05 μg/ml, 0.5 μg/ml, and 5 μg/ml (112% (5), 102% (4), and 107% (4)) and l-NAME 10 μM, 100 μM, and 1 mM (105% (3), 103% (1), and 100% (3)), indomethacin (112% (5), 102% (2), and 106% (5)), or allopurinol (104% (5), 103% (4), and 115% (5)).
Superoxide production in transformed and primary colonic epithelial cells
Transformed colonic epithelial cells
In heat inactivated cells and in cells cultured for 24 hours without change of media or supplementation of butyrate, O2− was undetectable regardless of the method of detection or stimulation (NADPH, NADH, or PMA).
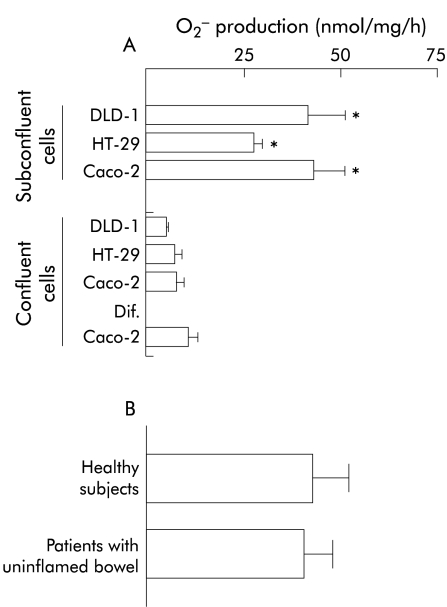
If the media were changed every eight hours or supplemented with butyrate, the transformed cell lines generated extracellular O2− under basal conditions. The rate of O2− production was sixfold higher in subconfluent cells than in undifferentiated or differentiated confluent cells (p<0.001; fig 1A ▶) but unaffected by differentiation of confluent Caco-2 cells (see fig 1A ▶). Rates of production were also unaffected by grade of subconfluence (5%, 15%, and 50% confluence, data not shown) or by culture in a high volume (8 ml v 2.5 ml) of medium (data not shown).
Figure 1.
Superoxide (O2−) production in cultures of transformed colonic epithelial cells and primary colonic epithelial cells from normal human colon. Extracellular O2− production was measured by superoxide dismutase inhibitable cytochrome c reduction and expressed as nmol per mg of protein per hour (see methods). (A) O2− production form DLD-1, HT-29, and Caco-2 colonic epithelial cells. Undifferentiated cells were studied at subconfluence or confluence. For differentiation, Caco-2 cells were cultured for 14 days. Mean (SEM) values of five experiments are given: *p<0.05 compared with confluent cells (paired t test). (B) O2− production in primary colonic epithelial cells from subjects with an uninflamed bowel. Mean values (SEM) of five healthy subjects and 16 patients with an uninflamed bowel are given.
Primary colonic epithelial cells
Cultures of primary colonic epithelial cells from five healthy subjects and 16 patients with an uninflamed colonic mucosa were studied by the cytochrome c reduction assay. In addition, cells from three patients with an uninflamed bowel and three healthy subjects were analysed by luminescence.
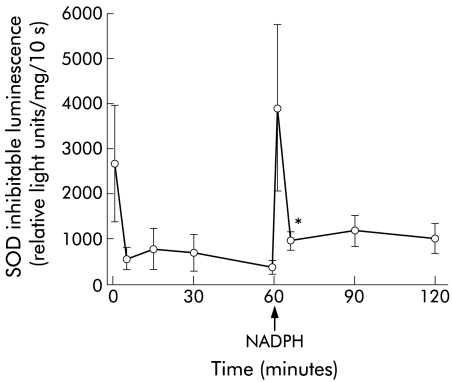
Extracellular O2− was generated under basal conditions, as detected by the cytochrome c reduction assay or luminescence (figs 1B, 2 ▶ ▶). There were no statistically significant differences in production rates between cells from healthy subjects and patients with uninflamed bowel (fig 1B ▶). Using a luminescence technique, constant basal production of O2− was observed (fig 2 ▶).
Figure 2.
Kinetics of superoxide production in cultures of primary colonic epithelial cells from normal human colon and effect of extracellular reduced nicotinamide dinucleotide phosphate (NADPH). O2− production was measured at fixed time points by superoxide dismutase (SOD) inhibitable luminol enhanced luminescence and expressed as relative light units per mg of protein per 10 seconds (see methods). After 60 minutes, NADPH 1 mM was added to the culture media. Mean (SEM) values for cells from six subjects, three patients with an uninflamed bowel and three healthy controls, are given. *p=0.02 compared with 60 minute values (paired t test).
Pharmacological intervention
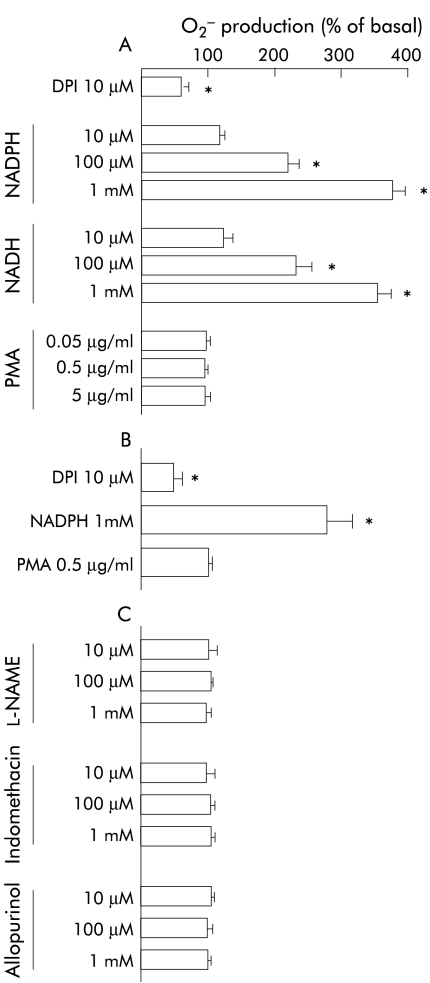
In transformed cell lines, similar responses to intervention were observed and there were only minor differences between responses observed in subconfluent and confluent cells. In subconfluent cells, the oxidoreductase inhibitor DPI reduced O2− generation by 50% (p=0.02) whereas NADPH and NADH caused a dose dependent increase in production rates (see fig 3A ▶). In contrast, O2− generation was unaffected by activation of protein kinase C with PMA and inhibition of NOS with l-NAME, COX, with indomethacin or xanthine oxidase with allopurinol (fig 3A, C ▶). In undifferentiated and differentiated confluent cells, DPI reduced O2− generation by 25% (p=0.05) while NADPH and NADH dose dependently enhanced production rates, which were increased fivefold (p<0.01) at a concentration of 1 mM. Also, in these cells, O2− generation was unaffected by PMA, l-NAME, indomethacin, or allopurinol (data not shown).
Figure 3.
Effects of pharmacological intervention on production of O2− in cultures of transformed or primary colonic epithelial cells expressed as per cent of basal production rates (see fig 1 ▶). Extracellular O2− production was measured by superoxide dismutase inhibitable cytochrome c reduction and expressed as nmol per mg of protein per hour (see methods). In transformed cell lines, similar responses to intervention were observed but only values for experiments with subconfluent Caco-2 cells are given in (A) and (C). (A) Effects of potential modulators of the NAD(P)H oxidase in Caco-2 cells. (B) Effects of potential modulators of the NAD(P)H oxidase in primary colonic epithelial cells. (C) Effects of inhibitors of other potential O2− generating enzymes in Caco-2 cells. Mean (SEM) values of five experiments are given. *p<0.05 compared with basal values (paired t test). DPI, diphenylene iodonium; l-NAME, NG-nitro-l-arginine methyl ester; NADH, reduced nicotinamide dinucleotide; NADPH, reduced nicotinamide dinucleotide phosphate; PMA, phorbol myristate ester.
In primary colonic epithelial cells, DPI reduced basal generation of O2− by 50% (p=0.02; fig 3B ▶). Addition of NADPH caused a threefold increase in O2− production, as detected by the cytochrome c reduction assay (p=0.02; fig 3B ▶) or luminescence (p=0.01; see 60 v 65 minutes, fig 2 ▶) which was observed within seconds (fig 2 ▶). In contrast, the formation of O2− was unaffected by PMA (fig 3B ▶).
Expression analysis
Nox1 and p22phox mRNA was detected in all cultures of transformed cell lines and primary colonic epithelial cells whereas gp91phox mRNA was detected only in HT-29 cells (fig 4 ▶). In contrast, Nox1 mRNA was undetectable in isolated peripheral neutrophils which expressed mRNA for gp91phox and p22phox (fig 4 ▶). After incubation of the PCR products with appropriate restriction enzymes, two smaller fragments of predicted sizes confirmed primer specificity (data not shown).
DISCUSSION
The results of the present study demonstrate that cultures of transformed colonic epithelial cells and primary colonic epithelial cells from the normal human colon produce extracellular O2−, which is attenuated by an oxidoreductase inhibitor. Expression of Nox1 and p22phox suggests that these subunits form part of the oxidase that utilises NADPH/NADH for generation of O2−. The dinucleotides cannot permeate cell membranes freely so the prompt increase in O2− production suggests that the oxidase is localised to the outer cell membrane. On the other hand, NOS, COX, or xanthine oxidase are unlikely to contribute to production of O2− from colonic epithelial cells as the rate of synthesis was unaffected by inhibitors of these enzymes.
Non-epithelial cells are unlikely to contribute significantly to the release of O2− observed in cultures of primary epithelial cells as the purity of cultures used is reported to be >95%.15 Moreover, similar rates of O2− formation and expression of NAD(P)H oxidase subunits were observed in cultures of primary and transformed epithelial cells. Finally, if phagocytes were present, PMA would have been expected to stimulate O2− production.
Our results suggest that Nox1 is the catalytic subunit of NAD(P)H oxidase in colonic epithelial cells as opposed to phagocytes, which catalyse O2− by gp91phox. We detected gp91phox mRNA in HT-29 cells but generation of O2− was unaffected by PMA, suggesting absence of functional gp91phox.
Presently, the regulatory pathways of Nox1 activity are unknown but our results indicate a basal rate of O2− production which is independent of protein kinase C activation by PMA. Similar observations were made in studies on vascular smooth muscle cells which showed Nox1 expression and lack of effect on O2− production by PMA.5
Metabolic dysfunction may impair O2− generation in colonic epithelial cells, as suggested by the observations that transformed cells release O2− only if media containing physiological concentrations of glucose are changed every eight hours or supplemented with butyrate. In cultures with 106 cells, abnormal concentrations of glucose and lactic acidosis were observed if the media were changed once daily. These alterations were partly reversed by butyrate which also restored the production of O2−. In cultures with 105 cells, normal concentrations of glucose and lactate were preserved when cells were cultured in media containing physiological glucose concentrations but production of O2− was undetectable until restored by butyrate. Together, these observations suggest that the consequences of metabolic dysfunction, other than butyrate starvation and abnormal concentrations of glucose and lactate, may contribute to attenuation of O2− formation.
The involvement of O2− in proliferation of colonic epithelial cells is suggested by the observation of high O2− production in subconfluent cells which are characterised by high mitogenic rates. This notion is supported by observations in colonic crypt epithelium of rats in which O2− production contributed to bile acid induced cell proliferation.12 Moreover, fibroblasts transfected with Nox1 showed increased growth rates but reduced growth if transfected with Nox1 antisense mRNA.9 Clearly, further studies are needed to define the role of O2− and Nox1 in colonic epithelial cell proliferation.
In the gut, extracellular production of O2− by epithelial cells may contribute to barrier function. This notion is strengthened by the observation that colitis/enteritis occurs frequently in patients with chronic granulomatous disease who are genetically defective in one of the NAD(P)H oxidase subunits.19 Furthermore, antibacterial activity of Caco-2 cells was reversed by SOD but not by catalase or l-NAME,20 suggesting that O2− from colonic epithelial cells may participate in host defence.
O2− production may also contribute to oxidative stress as may be the case in ulcerative colitis.21 In contrast, antioxidant systems, such as SOD, catalase, and glutathione, which are expressed in colonic epithelial cells, may be depleted during severe inflammation.22 If epithelial concentrations of O2− are increased, damage to cell membranes and organelles may occur directly or through hydrogen peroxide or the hydroxyl radical. Furthermore, the reaction of O2− with NO may result in the formation of reactive nitrogen species, such as peroxynitrite, as suggested by the presence of nitrotyrosine in the inflamed epithelium from patients with ulcerative colitis.1,2
In conclusion, cultures of transformed colonic epithelial cell lines and primary epithelial cells from the normal human colon release extracellular O2− through an NAD(P)H oxidase and express the NAD(P)H oxidase subunits Nox1 and p22phox. Therefore, O2− production may contribute to maintenance of normal colonic barrier.
Acknowledgments
We thank Dr Leif Baek at the Department of Clinical Microbiology, Herlev Hospital, Copenhagen, Denmark, for analysis of LPS. The study was also supported by grants from the Novo Nordisk Foundation. Part of this study was presented at the Digestive Disease Week 2000, San Diego, CA, USA.
Abbreviations
COX, cyclooxygenase
DMEM, Dulbecco’s modified Eagle’s medium
DPI, diphenylene iodonium
l-NAME, NG-nitro-l-arginine methyl ester
MTT, dimethylthiazol diphenyltetrazolium bromide
NADH, reduced nicotinamide dinucleotide
NADPH, reduced nicotinamide dinucleotide phosphate
NO, nitric oxide
NOS, nitric oxide synthase
Nox, NAD(P)H oxidase
phox, phagocytic oxidase
PMA, phorbol myristate ester
RT-PCR, reverse transcriptase-polymerase chain reaction
SOD, superoxide dismutase
REFERENCES
- 1.Singer II, Kawka DW, Scott S, et al. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996;111:871–85. [DOI] [PubMed] [Google Scholar]
- 2.Perner A, Andresen L, Normark M, et al. Expression of nitric oxide synthases and effects of L-arginine and L-NMMA on nitric oxide production and fluid transport in collagenous colitis. Gut 2001;49:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauseef WM. The NADPH-dependent oxidase of phagocytes. Proc Assoc Am Physicians 1999;111:373–82. [DOI] [PubMed] [Google Scholar]
- 4.Mayer AM, Brenic S, Glaser KB. Pharmacological targeting of signaling pathways in protein kinase C-stimulated superoxide generation in neutrophil-like HL-60 cells: effect of phorbol ester, arachidonic acid and inhibitors of kinase(s), phosphatase(s) and phospholipase A2. J Pharmacol Exp Ther 1996;279:633–44. [PubMed] [Google Scholar]
- 5.Görlach A, Brandes RP, Nguyen K, et al. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 2000;87:26–32. [DOI] [PubMed] [Google Scholar]
- 6.Meier B, Cross AR, Hancock JT, et al. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem J 1991;275( Pt 1):241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000;86:494–501. [DOI] [PubMed] [Google Scholar]
- 8.Lambeth JD, Cheng G, Arnold RS, et al. Novel homologs of gp91phox. TIBS 2000;25:459–61. [DOI] [PubMed] [Google Scholar]
- 9.Suh YA, Arnold RS, Lassegue B, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999;401:79–82. [DOI] [PubMed] [Google Scholar]
- 10.Banfi B, Maturana A, Jaconi S, et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 2000;287:138–42. [DOI] [PubMed] [Google Scholar]
- 11.Teshima S, Rokutan K, Nikawa T, et al. Guinea pig gastric mucosal cells produce abundant superoxide anion through an NADPH oxidase-like system. Gastroenterology 1998;115:1186–96. [DOI] [PubMed] [Google Scholar]
- 12.Craven PA, Pfanstiel J, DeRubertis FR. Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation. J Clin Invest 1986;77:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek L. New, sensitive rocket immunoelectrophoretic assay for measurement of the reaction between endotoxin and Limulus amoebocyte lysate. J Clin Microbiol 1983;17:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perner A, Andresen L, Pedersen G, et al. Superoxide production in DLD-1 and HT-29 epithelial cell lines and in primary epithelial cells from normal human colon. Gastroenterology 2000;118:A98. [Google Scholar]
- 15.Pedersen G, Saermark T, Giese B, et al. A simple method to establish short-term cultures of normal human colonic epithelial cells from endoscopical biopsies. Comparison of isolation methods, assessment of viability and metabolic activity. Scand J Gastroenterol 2000;35:772–80. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen OH, Bouchelouche PN, Berild D, et al. Effect of 5-aminosalicylic acid analogous substances on superoxide generation and intracellular free calcium in human neutrophilic granulocytes. Scand J Gastroenterol 1993;28:527–32. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien PJ. Superoxide production. Methods Enzymol 1984;105:370–8. [DOI] [PubMed] [Google Scholar]
- 18.Kuo J, Fox E. Sigma Stat manual. Microcomputer tools for scientist. Revision SSD-1.0. Augsburg: Jandel Scientific, 1992.
- 19.Winkelstein JA, Marino MC, Johnston RBJ, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–69. [DOI] [PubMed] [Google Scholar]
- 20.Deitch EA, Haskel Y, Cruz N, et al. Caco-2 and IEC-18 intestinal epithelial cells exert bactericidal activity through an oxidant-dependent pathway. Shock 1995;4:345–50. [DOI] [PubMed] [Google Scholar]
- 21.Simmonds NJ, Allen RE, Stevens TR, et al. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 1992;103:186–96. [DOI] [PubMed] [Google Scholar]
- 22.Sido B, Hack V, Hochlehnert A, et al. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut 1998;42:485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]