Abstract
Background and objective: A proportion of liver transplanted patients with recurrent chronic hepatitis have a sustained virological response to combination therapy with interferon plus ribavirin. However, the long term benefit of antiviral therapy with regard to hepatitis C virus (HCV) RNA clearance remains unknown in patients with HCV recurrence. This study examined the long term biochemical, virological, and histological outcome in transplanted patients with recurrent chronic hepatitis who had a sustained virological response to antiviral therapy.
Patients and methods: Fifty four patients with recurrent hepatitis C were treated with antiviral therapy involving induction by combination therapy (interferon (IFN) plus ribavirin) for six months and maintenance ribavirin therapy for 12 months. Fourteen patients who had recurrent chronic hepatitis and sustained virological response to antiviral therapy were followed for three years after the end of antiviral therapy. Serum alanine aminotransferases were assessed every three months during the observation period. Serum hepatitis C RNA detected by polymerase chain reaction was evaluated every six months during follow up, and protocol biopsy procedures were performed routinely every year. Semiquantitative histopathological assessment of allograft hepatitis was performed using the Knodell score and HCV was also detected by polymerase chain reaction on frozen graft tissue samples.
Results: At the end of antiviral therapy, the sustained response rate was 26%. A complete response (normal serum alanine aminotransferase level and undetectable serum HCV RNA) was achieved in 13/14 (93%) patients three years after the end of treatment. A comparison of liver histology findings before and after a mean of three years after antiviral therapy showed a clear improvement in 12/14 (86%) patients. In 5/14 (36%) patients, the last biopsy showed normal or near normal histological findings. After three years of follow up, the total Knodell score was 3.2 (range 1–8) versus 8.3 (range 5–12) before treatment (p=0.001). Graft HCV RNA was detectable before treatment in all 14 patients and was undetectable at the end of follow up in 13/14 (93%) patients tested.
Conclusion: In patients with biochemical and virological responses induced by ribavirin and interferon, a complete response was sustained in 93% for at least three years after cessation of therapy. This long term response was associated with absence of detectable intrahepatic hepatitis C RNA and marked histological improvement.
Cirrhosis due to hepatitis C virus (HCV) infection is now the most frequent indication for orthotopic liver transplantation (OLT) in Western Europe and the USA,1–3 and disease of the graft is recognised as a severe problem.4 In the absence of effective prophylaxis, HCV is detectable in serum after OLT in approximately 95% of patients.5–7 Because the virus remains present, the new graft is at risk of recurrent disease. Spontaneous remission of the disease after transplantation seems to be rare and clinical recurrence of hepatitis C has been reported in up to 62% of patients after OLT and can lead to progressive liver failure, cirrhosis,8,9 and death.10 At present, combination treatment is the only therapy available which has shown efficacy in eradicating the virus, normalising biochemical parameters, and improving histological liver damage.11,12 However, optimal treatment to induce long term biochemical and histological remission in transplanted patients with HCV recurrence remains a challenge. Indeed, little information is available on histological outcome, and the question of the long term benefit of combination therapy with regard to HCV clearance and histological improvement remains to be answered.
To address this question, we assessed the long term biochemical, virological, and histological outcome of 14 patients with HCV chronic hepatitis on the graft, who had a sustained virological response after combination therapy with interferon (IFN) and ribavirin.
PATIENTS AND METHODS
Study population
Since 1993, patients undergoing OLT for hepatitis C cirrhosis in our centre have been treated with early antiviral therapy after transplantation if they fulfilled the following criteria: (1) survival longer than six months after OLT; (2) presence of chronic hepatitis on the graft biopsy; (3) serum HCV-RNA positive; (4) elevation of alanine aminotransferase (ALT) greater than 1.5 times normal. Immunosuppression was not adjusted in liver recipients with histologically documented recurrent hepatitis C after transplantation.
Among 54 patients with recurrent post-transplant hepatitis C who started combination therapy in our centre between 1992 and 1995, 14 patients (26%) who had a sustained response after therapy were included in the present study. The mean period between liver transplantation and the beginning of treatment was 13 months (range 8–24). Sustained response was defined as: (1) strictly normal serum ALT each month for the first six months after the end of therapy; and (2) negative results on testing for serum HCV RNA six months after the end of combination therapy. All 14 patients were followed up for three years after the end of treatment.
All patients received the same treatment involving two phases: induction for six months with IFN-α2b (Intron A; Schering Inc, Kenilworth, New Jersey, USA) given subcutaneously at a dose of 3 MU three times weekly and oral ribavirin (Rebetol; Schering Inc) 1000 mg in two divided daily doses and maintenance on ribavirin monotherapy for 12 months. In the maintenance phase of treatment, the dose of ribavirin was 800 mg daily for eight patients and 600 mg daily for six patients. All patients had chronic hepatitis on the graft, shown on biopsy within three months before treatment; the mean total Knodell score was 8.3 (range 5–12). All patients had serum ALT levels greater than 1.5 times the upper limit of normal for at least three months, and all patients were negative for hepatitis B surface antigen.
The immunosuppressive regimen used was conventional double therapy with prednisolone 5 mg/day and cyclosporin (Novartis Pharma; Rueil-Malmaison, France) in 10 patients. Only four patients received tacrolimus (Fusisawa, Nanterre, France) and prednisolone at the same dose. No further changes in the immunosuppressive regimen were made during this study other than adjustment of cyclosporin and tacrolimus doses to maintain drug levels within the therapeutic range (cyclosporin whole blood level 150–200 μg/l; tacrolimus whole blood level 5–15 ng/ml). Immunosuppression was not adjusted in liver recipients with histologically documented recurrent hepatitis C after transplantation.
Follow up
Follow up included clinical assessment every three months, measurement of serum ALT levels at least every three months, and detection of serum HCV RNA by polymerase chain reaction (PCR) every six months.
Serum HCV RNA
Qualitative HCV RNA was determined by PCR using primers from the 5′ non-coding region, as described previously.13 The lower limit of sensitivity of this assay is 103 copies/ml. We tested for the presence of HCV RNA in all 14 patients on serum specimens that were kept frozen and collected six months after withdrawal of antiviral therapy and then every six months during the entire follow up period. All serum samples were tested retrospectively for HCV RNA using Amplicor HCV version 2.0 (lower detection limit 100 copies/ml).
HCV viral quantitation
During follow up, serum HCV RNA quantitation was performed using the quantitative branched DNA (bDNA) amplification assay (Quantiplex HCV 2;0; Chiron Diagnostics, Emeryville, California, USA) in patients with persistent detectable HCV RNA levels. The lower limit of sensitivity of this assay is 0.2×106 viral equivalents/ml.
HCV RNA was not quantitated in graft specimens prior to treatment or during follow up.
Genotype and serotype of HCV
HCV genotyping was performed in 13 patients for whom pretreatment serum samples were available. Genotyping was done using the reverse hybridisation assay (LiPA; InGeN, Rungis, France) after amplification with the PCR assay. The classification system of Simmonds was used.14 The following genotypes were identified: 1b (n=12), 1a (n=1), and 3a (n=1).
Liver histological studies
In all 14 patients, the last post-treatment biopsy was done at least three months after the end of antiviral therapy and repeated every year. Additional liver biopsies were performed as clinically indicated for abnormal liver function tests. Biopsy specimens were stained with haematoxylin and eosin. Two other stainings (eosin Sirius red, Masson’s trichrome) were used for fibrosis staging. Biopsy specimens obtained at the end of treatment and during follow up were assessed for fibrosis (score 0–4) and activity (score 0–18) according to the scoring system of Knodell and colleagues.15 Liver biopsy specimens from each patient were evaluated by an experienced liver transplantation pathologist blinded to HCV RNA status. The pathologist was not blinded as to whether the biopsy was pre or post-treatment. After the end of therapy, 59 graft biopsies were performed during follow up and a mean of 4.2 biopsy specimens (range 3–6) were available per patient. Duration of histological follow up was three years after the end of therapy. A diagnosis of recurrent hepatitis was based on the presence of portal, periportal, and lobular inflammation, with lobular acidophilic bodies and/or lobular hepatocytolysis, all in the absence of endothelitis. Knodell’s score includes four items (periportal necrosis with or without bridging necrosis, intralobular degeneration and focal necrosis, portal inflammation, and fibrosis).15 Recurrent chronic hepatitis C was defined as mild if the total histology score was <6, moderate if the score was 6–9, and severe if the score was >9. Histological improvement was assessed by comparing the pretreatment biopsy specimen with the last biopsy obtained after treatment. Histological improvement was defined as improved if the histology total score was at least 2 points lower in the post-treatment specimen or was the same in the two specimens, and as deteriorated if the score was higher in the post-treatment specimen.
Graft HCV RNA
A graft tissue specimen was collected at the end of treatment in 14 patients and during follow up at each biopsy. Only one patient could not be tested at the end of follow up. All tissues samples were immediately frozen in liquid nitrogen and kept at −80°C. Qualitative HCV RNA was determined by PCR using primers from the 5′ non-coding region.13 The lower limit of sensitivity of this assay is 500 copies/ml.
Statistical analysis
Results are expressed as mean (range) (minimum and maximum). Pretreatment and post-treatment values were compared using the Wilcoxon signed rank test. p values less than 0.05 were considered statistically significant.
RESULTS
The characteristics of the 14 patients with a sustained response are shown in table 1 ▶. There were 10 males and four females with a mean age of 50.9 years at entry into the study. All patients were followed for three years after the end of antiviral therapy. Among the 54 patients treated, the overall rate of side effects of antiviral therapy was found to be 36%. The most frequently reported adverse events included asthenia and anaemia, representing 82% of side effects. Six patients (11%) stopped ribavirin therapy because of anaemia. One patient developed chronic rejection 12 months after withdrawal of IFN. Thrombocytopenia and leucopenia were also detected and represented 3% and 4% of side effects, respectively.
Table 1.
Demographic, biochemical, virological, and histological characteristics before treatement in 14 patients who had a complete and sustained response to antiviral therapy
| Characteristic | Value |
| Age* (y) | 50.9 (32–66) |
| Sex (M/F) | 10/4 |
| Source of infection (%) | |
| Blood transfusion | 72 |
| Intravenous drug use | 24 |
| Unknown | 4 |
| ALT (IU/l)* | 129 (89–234) |
| Serum HCV RNA level (Meq/ml)* | 2.9 (0.9–2.7) |
| Genotype | |
| 1b | 12/14 |
| Mean total histology score* | 8.3 (5–12) |
*Values are mean (range).
HCV, hepatitis C virus; ALT, alanine aminotransferase.
Clinical outcome
At the end of follow up, all 14 patients included were alive. Seven of 14 (50%) patients who had fatigue before treatment said that fatigue disappeared after treatment. Three of 14 patients developed severe side effects due to immunosuppressive therapy: one developed cytomegalovirus disease without hepatitis during the second year of follow up. Of three patients with severe chronic hepatitis before treatment, none developed cirrhosis. The only patient with cirrhosis on the graft before treatment remained stable without decompensation. No hepatocellular carcinoma, as assessed by ultrasonography every six months, occurred in this patient during follow up. During follow up, no acute or chronic rejection was observed or detected on the graft biopsy.
Serum HCV RNA
By definition, no patient had detectable serum HCV RNA six months after the end of antiviral therapy. During the long term follow up period, serum HCV RNA was persistently undetectable in 13 of 14 patients tested (93%). In one patient, a late relapse with reappearance of serum HCV RNA at seven months post-treatment and a slight increase in serum ALT level (1.5 times the upper limit of normal) 22 months after the end of antiviral therapy was observed. This relapser was the only patient with positivity for HCV RNA on the graft at entry into the study.
Liver HCV RNA
Before treatment, HCV RNA was detectable in graft specimens of all 14 patients tested. After follow up for three years, 13/14 (93%) patients were negative for HCV RNA on the graft. One serum HCV RNA negative patient was HCV RNA positive on the graft at inclusion. This patient became serum HCV RNA positive seven months after the end of treatment and developed a biochemical and histological relapse 22 months after withdrawal of ribavirin monotherapy. At this time, serum HCV RNA level measured by bDNA was 22 Meq/ml.
Liver histological findings
Before treatment, graft biopsies showed that chronic hepatitis was mild in four patients (29%), moderate in seven (50%), and severe in three patients (21%).
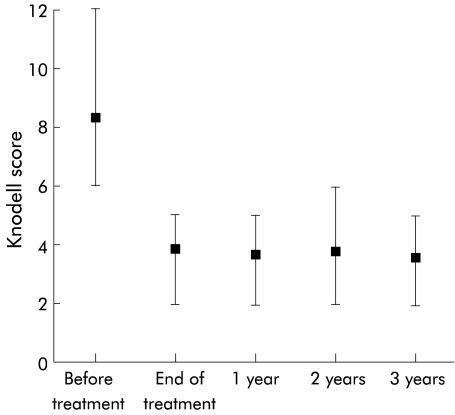
Pretreatment and post-treatment graft biopsy specimens were compared for 14 patients who had their last post-treatment liver biopsy done three years after the end of treatment. Analysis of biopsy specimens showed that all of the improvement was obtained at the end of treatment, and the Knodell score remained relatively stable over the three year post-treatment period (fig 1 ▶). No significant change was observed in fibrosis score (fig 2 ▶). Before therapy, the total Knodell score of these 14 patients was 8.3 (range 5–12). The last allograft biopsy, done three years after treatment, showed a mean total Knodell score of 3.2 (range 1– 8) (p=0.001). Five of 14 patients (36%) had normal or near normal histological findings (score >2), four (29%) had mild chronic hepatitis, four (29%) had moderate chronic hepatitis C, and one (6%) had severe chronic hepatitis. Comparison of histology scores for the pretreatment biopsy and the last biopsy specimen showed improvement (decrease >2) in 11/14 (79%) patients, no change in only one patient (7%), and deterioration in two patients (14%).
Figure 1.
Total Knodell score before, at the end of treatment, and 1–3 years after antiviral therapy. Graft biopsies were done every year during follow up after the end of therapy.
Figure 2.
Knodell score for fibrosis before, at the end of treatment, and 1–3 years after antiviral therapy. Graft biopsies were done every year during follow up after the end of therapy.
DISCUSSION
The major objective of treatment of recurrent chronic hepatitis C after OLT is to prevent progression to cirrhosis and thereby prevent loss of the graft. The combination of IFN-α and ribavirin is effective and achieves a sustained virological response in 25% of patients.12 However, it has become important to assess the long term effects to determine whether the treatment has indeed modified the natural history of HCV infection after OLT.
We have described the virological and histological course of 14 liver transplanted patients who were selected because they were serum HCV RNA negative at the end of antiviral therapy. Ninety three per cent of the included patients had persistently normal ALT levels and negative HCV RNA in the prolonged follow up period after treatment. In these patients, absence of HCV RNA in serum and in the graft, together with normalisation of serum ALT levels and marked histological improvement after treatment, very likely indicated eradication of the chronic HCV infection and subsequent interruption of the disease progression, with a low risk of further relapse or development of cirrhosis on the graft. In non-immunocompromised patients with chronic hepatitis, previous studies16,17 have shown similar results but until now no similar data were reported in the liver transplanted population. To confirm these results, further studies recruiting more patients are needed.
This study used both ALT normalisation and negative HCV RNA as end points. Indeed, the natural course of patients with sustained ALT normalisation and positive HCV RNA is not clear; sometimes late relapse may occur. Furthermore, chronic HCV viraemia with persistently normal ALT activity may show chronic hepatitis in graft biopsy specimens. Using these criteria, we did not observe biochemical or virological relapse in 93% of patients three years after cessation of the combination therapy (3/13 patients with a sustained complete response have been followed up for more than four years as of this writing). Although the sustained disappearance of detectable serum HCV RNA may not be sufficient to rule out a persistent low level viral infection, normal serum ALT levels in 13/14 patients who were negative for HCV RNA and were followed for three years suggests sustained inhibition of viral replication. Thus normal serum ALT levels in the absence of detectable serum HCV RNA during the six month post-treatment follow up period seem to be reliable indicators of sustained response.
Although four patients had severe chronic hepatitis C without cirrhosis before antiviral therapy, no new cases of cirrhosis appeared during follow up; this suggests that combination therapy following ribavirin monotherapy for one year has long term benefit in transplanted patients with HCV chronic hepatitis. In our study, one patient with graft cirrhosis had a sustained virological response, confirming that the long term response is possible even in patients with cirrhosis. Furthermore, no decompensation and no hepatocellular carcinoma occurred in this patient. However, in our study, the number of patients with cirrhosis was too small to draw any conclusions about the impact of antiviral therapy on the risk of graft failure and hepatocellular carcinoma.
After three years of follow up after withdrawal of antiviral therapy, 5/14 patients (36%) had normal or near normal histological findings. Histological improvement was present at the end of treatment and the HAI score remained stable thereafter (fig 1 ▶). This improvement was due to lowered inflammatory scores as fibrosis scores were essentially unchanged, as shown on fig 2 ▶. However, severe liver inflammation without signs of chronic rejection persisted for up to three years in one patient, despite normal serum ALT values and negative results on testing for serum and liver HCV RNA. Two explanations for this result can be postulated. Firstly, the constant presence of inflammatory infiltrates may be due to persistence of some degree of graft cellular immune response to HCV which probably takes many years to disappear. Secondly, the technique may not be sufficiently sensitive to detect low level viral infection and the explanation that a continuing low level HCV RNA replication persists in the graft cannot be entirely excluded. Another hypothesis for this case with inflammation on biopsy, several years after being negative for HCV-RNA, is that it is unrelated to HCV and may be due to other mechanisms such as a drug induced problem, de novo autoimmune hepatitis, or less likely an atypical rejection.
The absence of detectable graft HCV-RNA in 13/14 patients is consistent with the view that HCV infection may be cleared with antiviral therapy even in immunocompromised patients. These results suggest that persistence of graft HCV RNA at the end of antiviral therapy was the most important factor for relapse. This is similar to results reported in non-transplanted patients.18,19 The presence of a low level of HCV RNA in the liver, not detected on PCR, cannot be ruled out because the technique used in this study was not sufficiently sensitive. In our study however, no relapse occurred among the 13 patients without graft HCV RNA at the end of treatment and during follow up.
In the only one case of late relapse observed in this study, serum HCV RNA was negative at the end of treatment. This was not a false positive result of PCR because serum was also tested using PCR (Amplicor), with the same result. This case may suggest that the treatment does not completely suppress viral replication and very low replication rates remain in the compartment of replication competent cells (mainly hepatocytes). Another interesting finding in this case was detection of serum and graft HCV RNA despite the absence of hepatitis over 15 months. This observation of late relapse raises the question of the cytopathogenicity of HCV for hepatocytes and suggests that mechanisms other than direct cytotoxicity may be implicated in HCV induced graft damage.
In conclusion, this is the first study in the transplantation setting to show that persistent suppression of HCV replication can be obtained after early treatment and patients may be cured of the disease. Absence of detectable graft HCV RNA at the end of treatment in patients with recurrent chronic hepatitis C seems to be a reliable indicator of long term biochemical and virological response. Furthermore, disappearance of detectable viraemia leads to significant histological improvement.
Abbreviations
HCV, hepatitis C virus
OLT, orthotopic liver transplantation
ALT, alanine aminotransferase
PCR, polymerase chain reaction
b-DNA, branched DNA
IFN, interferon
REFERENCES
- 1.Ascher NL, Lake JR, Emond J, et al. Liver transplantation for hepatitis C virus related cirrhosis. Hepatology 1994;20:24–7S. [DOI] [PubMed] [Google Scholar]
- 2.Ferrel LD, Wright TL, Roberts J, et al. Hepatitis C viral infection in liver transplant. Hepatology 1992;16:875–6. [DOI] [PubMed] [Google Scholar]
- 3.Shah G, Demetris AJ, Gaveler JS, et al. Incidence, prevalence and clinical course of hepatitis following liver transplantation. Gastroenterology 1992;103:323–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizollon T, Mutimer D, Ducerf C, et al. Hepatitis C virus recurrence after liver transplantation. Gut 1999;44:575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feray C, Samuel D, Thiers V, et al. Reinfection of liver graft by hepatitis C virus after liver transplantation. J Clin Invest 1992;89:1361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S, Terrault NA, Ferrell L, et al. Severity of liver disease in liver transplantation recipients with hepatitis C virus infection: relationship to genotype end level of viremia. Hepatology 1996;24:1041–6. [DOI] [PubMed] [Google Scholar]
- 7.Wright T L, Donegan E, Hsu H H, et al. Recurrent and acquired hepatitic viral infection in liver transplant recipients. Gastroenterology 1992;103:317–22. [DOI] [PubMed] [Google Scholar]
- 8.Gane E J, Portmann B C, Naoumov N V, et al. Long term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996;334:815–20. [DOI] [PubMed] [Google Scholar]
- 9.Feray C, Caccamo L, Alexander GJ, et al. European collaborative study on factors influencing outcome after liver transplantation for hepatitis C (European concerted action on viral hepatitis. Eurohep group). Gastroenterology 1999;117:619–35. [DOI] [PubMed] [Google Scholar]
- 10.Schluger LK, Sheiner PA, Thung SN, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 1996;23:971–6. [DOI] [PubMed] [Google Scholar]
- 11.Bizollon T, Palazzo U, Ducerf C, et al. Pilot study of the combination of alpha interferon and ribavirin as therapy of recurrent hepatitis C after liver transplantation. Hepatology 1997;26:500–4. [DOI] [PubMed] [Google Scholar]
- 12.Samuel D, Bizollon T, Feray C, et al. Combination of interferon alpha 2bplus ribavirin for recurrent HCV infection after liver transplantation: a randomized controlled study. Hepatology 2000;32:259A. [Google Scholar]
- 13.Parvaz P, Guichard S, Chevallier P, et al. Hepatitis C: description of a highly sensitive method for clinical detection of viral RNA. J Virol Meth 1994;47:83–94. [DOI] [PubMed] [Google Scholar]
- 14.Simmonds P. Variability of hepatitis C virus. Hepatology 1995;21:570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic hepatitis. Hepatology 1981;1:431–5. [DOI] [PubMed] [Google Scholar]
- 16.Marcellin P, Boyer N, Gervais A, et al. Long-term histologic improvement and loss of detectable intra-hepatic HCV RNA in patients with chronic hepatitis c and sustained response to interferon-alpha therapy. Ann Int Med 1997;127:875–81. [DOI] [PubMed] [Google Scholar]
- 17.Morisco F, Tucillo C, Lasevoli P, et al. Chronic hepatitis C long-term responders to human leukocyte interferon-alpha therapy: persistence of a sustained biochemical and virological response during 5 years of surveillance. Euro J Gastroenterol Hepatol 1998;10:399–403. [DOI] [PubMed] [Google Scholar]
- 18.Kondo M, Tanaka K, Ikeda M, et al. Hepatic HCV-RNA as a predictor of outcome after interferon therapy in patients with chronic hepatitis C. J Gastroenterol Hepatol 1996;11:236–40. [DOI] [PubMed] [Google Scholar]
- 19.Shindo M, Arai K, Sokawa Y, et al. Hepatitis C RNA as a predictor of a response to interferon-alpha therapy. Ann Intern Med 1995;122:586–91. [DOI] [PubMed] [Google Scholar]