Abstract
Hepatic dendritic cells (DC) unquestionably play important roles in the induction and regulation of immune responses. Due to their paucity, functional characterisation of these important antigen presenting cells has been slow but use of DC growth factors (in particular GM-CSF and Flt3L) that markedly enhance their numbers has proved helpful in furnishing adequate study material. While there is growing evidence that DC function is affected in the pathogenesis of liver disease, most work to date has been performed on non-hepatic DC. Increasing knowledge of hepatic DC biology is likely to improve our understanding of disease pathogenesis and resistance to and therapy of liver disease.
Keywords: dendritic cells, immune regulation, liver disease, transplantation
INTRODUCTION
The liver is an important site of infectious, parasitic, autoimmune, and malignant diseases. Immune responses and their modulation within the liver are critical to the outcome of these conditions and also in liver transplantation. The inherent tolerogenicity of the liver, including its possible role in oral tolerance, poses important questions about how immune reactivity in the liver is regulated. Increasing attention has focused on antigen presenting cells (APC) and the critical roles that they play in both innate and adaptive immunity. APC exist in several forms within the liver and exhibit a spectrum of abilities to capture, process, and present antigen (Ag) to immune effector cells. Although rare, dendritic cells (DC) are the most highly specialised APC, with ability both to instigate and regulate immune reactivity. In addition, DC are well equipped to migrate from peripheral tissue sites such as the liver to regional lymphoid organs, where they present Ag to T cells. In the normal steady state, these events may be important in the maintenance of self tolerance. It is now recognised that the microenvironment in which APC develop or are activated influences their function and their effects on T cell populations. Furthermore, different DC subsets have been identified that exhibit distinct functional capabilities. Progress in uncovering the properties of liver DC has been slow but the recent surge of interest in DC biology and technological advances in their isolation and characterisation have brought these cells to centre stage in the quest for a fuller understanding of immune regulation within and beyond the liver.
LIVER APC POPULATIONS COMPARED
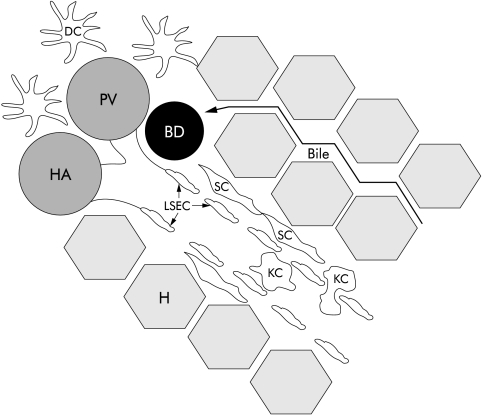
The liver contains several types of APC (fig 1 ▶). Liver sinusoidal endothelial cells (LSEC) line the sinusoids and have a distinct morphology in comparison with vascular endothelial cells that line arterial branches, and central and portal veins.1,2 In contrast with vascular endothelia, LSEC do not express CD31 (PECAM-1, pgIIa endothelial cell adhesion molecule), which is expressed at tight junctions of vascular endothelia, but exhibit higher constitutive levels of CD54 (ICAM-1, intercellular adhesion molecule 1) and CD106 (VCAM-1, vascular cell adhesion molecule).1,2 Also, LSEC have fenestrations up to 100 nm in diameter although passage of particles through these openings is selective. Thus although particles as small as 15 nm fail to enter, lymphocytes can access the space of Disse between the lumen of the sinusoids and hepatocytes. Extracellular matrix and hepatic stellate cells are located in this area. Hepatocytes have been reported to act as APC in certain situations although they are not considered to be primary mediators in immune regulation within the liver.1,3 Kupffer cells (KC), the resident macrophages of the liver, patrol the portal venous system via the sinusoidal lumen and can adhere to LSEC, occasionally causing temporary obstruction of blood flow through the sinusoid (fig 1 ▶).4,5 In normal liver, hepatic DC typically reside only around portal triads6–8 and, like DC in other peripheral sites, are able to efficiently capture, process, and transport Ag to regional lymphoid tissues. All three APC (LSEC, KC, DC) internalise Ag by phagocytosis, receptor mediated endocytosis, or pinocytosis but their phenotypes differ considerably.1,2,9 LSEC and KC express major histocompatibility complex (MHC) Ags, costimulatory and adhesion molecules, and make interleukin (IL)-1 and interferon γ (IFN-γ), suggesting that these cells are at a relatively mature stage.1,2,9,10 Freshly isolated hepatic DC on the other hand are predominantly immature cells, expressing surface MHC but few costimulatory molecules necessary for T cell activation.11–13 Compared with more mature bone marrow (BM) derived or spleen DC, they stimulate naïve allogeneic T cells only poorly.13–15
Figure 1.
Anatomy of sinusoids. The area between the LSEC and hepatocytes, where extracellular matrix and stellate cells reside, is called the space of Disse. Kupffer cells and other immune cells are believed to extravasate through the LSEC fenestrations into the parenchyma. DC normally reside only in the portal areas. BD, bile duct; DC, dendritic cell; H, hepatocyte; HA, hepatic artery; KC, Kupffer cell; LSEC, liver sinusoidal endothelial cell; PV, portal vein; SC, stellate cell.
ROLE OF THE LIVER MICROENVIRONMENT AND HEPATIC DC IN TOLEROGENICITY
The immature phenotype of resident hepatic DC coupled with the inherently unique liver microenvironment potentially makes these APC different from DC in other tissue sites (that is, BM, spleen). Although not considered to be an immune privileged site, such as the anterior chamber of the eye or the testis, there are marked similarities between the cytokine milieu of the liver and these other sites. KC and LSEC constitutively express the anti-inflammatory cytokines IL-10 and transforming growth factor β (TGF-β) that are upregulated on stress, while hepatocytes secrete IL-10 in response to autocrine and paracrine TGF-β.1,2,16,17 Lipocytes, another liver specific cell population that includes Ito and stellate cells, also express increased TGF-β on activation or stress.16 These cytokines not only affect T helper (Th) T cell differentiation directly (skew to Th2) but also can confer tolerogenicity on DC and other APC by inhibiting their maturation and T cell stimulatory function.
“There is now much evidence that DC can be rendered tolerogenic”
Although mature DC, rich in surface MHC and costimulatory molecules, are potent stimulators of immune (T cell) function, there is now much evidence that DC can be rendered tolerogenic. Thus exposure of replicating DC progenitors to IL-10 or TGF-β18 generates DC that are suppressive or tolerogenic. Steinbrink and colleagues19 showed that culture of immature blood derived human DC with IL-10 inhibited their maturation. Similar results have been obtained with DC transduced with either IL-10 or TGF-β.20,21 Lack of adequate costimulatory molecule expression, either due to immaturity or exposure to costimulatory pathway blocking agents, can also result in tolerogenic DC, as shown in both allograft22 and autoimmune disease23 models.
PHENOTYPE OF HEPATIC DC
Many different markers have been used to identify rodent and human DC, including those that are species specific (table 1 ▶). While none are specific to hepatic DC, variations occur in the level of expression of certain markers compared with others. CD11c is a common but not universal marker for DC detection in the murine system. In addition, other markers, such as CD205, have been used by different groups to identify specific murine DC subsets. The two principal subsets identified in mouse liver as well as in lymphoid tissue are the “so-called” myeloid (CD8α-CD11b+) and lymphoid related (CD8α+ CD11b−) subsets of DC. These DC are distinguished by their reciprocal expression of CD8α and CD11b and were thought initially to have distinct lineage and functions.24,25 Recent evidence has shown that these subsets derive from a common precursor and that rigid lineage affiliations between subsets may not exist.26–28 Plasmacytoid DC or type 1 IFN producing cells (a unique cell type of the haematopoietic system) have recently been identified in mouse lymphoid tissues.29–31 These DC are CD11c+CD11b−CD19−B220+ and Gr1+ and may play crucial roles in antiviral immunity. Whether they are present in normal liver has yet to be determined.
Table 1.
Phenotype of liver dendritic cells
| Species | Maturation status | Markers | Comments (ref) |
| Mouse | Immature | CD11c+CD40loCD80loCD86loMHC IIlo | 2 subsets:1,14,41,101 |
| (1) CD8α-CD11b+ | (1) Myeloid related12,102 | ||
| (2) CD8α+CD11b− | (2) Lymphoid related | ||
| B220-CD11c+CD205-F4/80- | Yoneyama65 | ||
| CD205+OX2+ | Gorczynski,37Drakes,43Gorczynski 103 | ||
| CD11b+CD24+CD44+CD45+CD11cloCD16/3 2loCD40lo CD80lo CD86loCD205loF4/80lo | Generated from liver progenitor cells with GM-CSF; called liver derived DC progenitors.32,33 | ||
| Mature | CD11c+MHC IIhiCD86hi | ||
| CD11c+CD54+CD205+MHC II+CD11bmodCD86modCD11a/CD18mod B220-CD3ɛ−Gr1− | Yoneyama65 | ||
| Other | CD205hi B220+CD11c− CD19− | Generated from liver progenitor cells with IL-3 and CD40L. Ig gene rearrangement occurs but no surface expression. Activate then subsequently induce apoptosis of T cells.34 | |
| Rat | Immature | MHC II+ANAE−FcR− | ANAE=α-naphtylacetate esterase=a non-specific esterase; FcR=Fc receptor53 |
| Mature | MHC IhiMHC IIhiCD54+OX62+ | Brenan,36Matsuno,56Saiki92 | |
| (1) ED1+ED2-OX6+, (2) ED1−ED2−OX6+ | 2 subsets of OX62+ cells104 | ||
| MHC II+CD54+OX62+CD90+CTLA-4 Counter-receptor+ | Chen-Woan35 | ||
| CD4+ | Variable expression is common in MHC II+ DC and peripheral tissues of rat51,105 | ||
| Human | Immature | CD11a+ CD45+MHC II+ | Prickett7 |
| CD83loCD86loMHC IIlo | Ninomiya 84 | ||
| Mature | CD200+ | Goddard106 | |
| CD83+CD86+ | Goddard107 |
DC, dendritic cell; GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; MHC, major histocompatibility complex.
DC have been generated in vitro from mouse liver stem/progenitor cells in response to granulocyte macrophage-colony stimulating factor (GM-CSF). These liver derived DC progenitors32,33 are distinct in phenotype from DC freshly isolated from normal liver and are CD11cloCD24+CD44+. Maturation of DC is associated with upregulation of MHC II, CD80, and CD86, with CD205 being an additional marker used by some groups. Lu and colleagues34 have also shown that culture of normal murine hepatic non-parenchymal cells (NPC) with IL-3 and CD40L yields a unique population of DC-like cells that are CD205hiCD11c−B220+CD19−.
Less diversity has been reported to date for DC markers in the rat and human. OX62, an integrin molecule, is commonly used to detect rat DC.35–37 As in mice, maturity is monitored by surface expression of the CD28/CTLA4 ligands CD80 and CD86. Two distinct populations of mature rat hepatic DC have been identified: (1) ED1+ED2−OX6+ and (2) ED1−ED2−OX6+. In humans, DC are commonly MHC II+ and deficient in CD28/CTLA4 ligands while in an immature state. Prickett and colleagues7 found that human liver DC were also CD45+CD11a+CD18+.
Thus it can be seen that there are similarities and disparities among DC populations. Common features to all three species include the lack of or low expression of MHC II and CD28/CTLA4 ligands on immature DC that are increased on maturation. CD11c and OX62 are generally considered the definitive markers for mouse and rat, respectively.
ENUMERATION OF HEPATIC DC
The normal murine liver, one of the larger visceral organs, has a relatively high total interstitial DC content, about 2–5-fold greater than that of other parenchymal organs, such as the kidney or heart.38 However, when the density of MHC II+ DC between these organs is compared, the liver ranks as the lowest.38
Specific DC populations, such as myeloid and lymphoid related subsets, studied in other tissues24,39,40 (table 1 ▶), can be found in normal mouse livers. Previous studies have shown that these subpopulations constitute a low percentage of the total tissue specific DC population. The relative proportions of these two subsets in the liver are similar to those seen in other tissues.12,24,39,40 Each population constitutes 1% of the total normal liver NPC population.12
Liver DC can be isolated from NPC by collagenase digestion followed by metrizamide density centrifugation.12,15,41 Although the total number of DC in the liver is greater than that of other parenchymal organs, there are still few cells to work with in comparison with lymphoid tissue. This paucity of cells is especially evident if a specific DC subset is sought. Administration of recombinant human fms-like tyrosine kinase 3 ligand (Flt3-ligand, Flt3L), an endogenous haematopoietic growth factor, markedly increases the total number of hepatic DC.12 Furthermore, the yield can be further increased by overnight culture of the isolated DC progenitors with GM-CSF. Under such culture conditions, the percentage of both CD8α− and CD8α+ DC can be increased to 10–15% of the total NPC population.12
The phenotype of the DC obtained from Flt3L mobilised mice resembles that of DC isolated from normal liver and in situ.12,15,33,41–43 Drakes and colleagues43 showed that administration of Flt3L did not change the phenotype of freshly isolated hepatic DC, as defined earlier. These Flt3L treated DC, on culture with GM-CSF and IL-4 or exposure to a maturation inducing stimulus, such as extracellular matrix (ECM) protein, increased their surface costimulatory molecule expression and T cell allostimulatory activity.33,43–45
“The leucocyte content of the liver and its DC constituency in particular, appear to play an important role in transplant outcome”
The leucocyte content of the liver and its DC constituency in particular, appear to play an important role in transplant outcome. Thus when donor hepatic leucocytes are either drastically reduced46–48 or greatly augmented,49,50 a switch from tolerance to rejection occurs in murine liver transplantation. In the case of donor leucocyte depletion, transplant tolerance can be restored by replacement of donor leucocytes.47 Thus a balance appears to exist between the number of donor hepatic DC and liver tolerogenicity.
APC FUNCTIONS OF HEPATIC DC
Phagocytosis
Early studies showed that intravenous administration of colloidal carbon8,51,52 or antibody coated human red blood cells53 did not result in phagocytosis by DC. It was speculated that liver DC, unlike KC and LSEC,2,54,55 did not phagocytose these particles in vivo. However, more recently, elegant studies in the rat by Matsuno and colleagues56,57 have shown that carbon laden DC localise in the coeliac nodes within two hours of intravenous administration of carbon particles. Furthermore, it was determined that immature DC were the major population of particle laden cells that entered the hepatic lymph. It was suggested that these phagocytic DC were recruited from the systemic circulation and were not part of the resident DC population. Interestingly, Iyoda and colleagues58 have reported that in mice, only the liver resident CD8α+ DC subset exhibits phagocytic properties in situ.
T cell stimulation
Murine liver DC progenitors cultured overnight with or without GM-CSF stimulate naïve allogeneic T cells.14,15,49 Abe and colleagues13 observed that the allostimulatory activity of immature liver derived DC for memory T cells was not affected by administration of proinflammatory cytokines such as tumour necrosis factor α (TNF-α) or IFN-γ. However, addition of Ag (that is, viral antigen; keyhole limpet haemocyanin) to immature hepatic DC induced upregulation of MHC II, costimulatory molecules, and T cell allostimulatory activity. Khanna and colleagues14 found that although cultured immature mouse liver derived DC were weak stimulators of allogeneic naïve T cells in vitro, their in vivo administration to allogeneic recipients resulted in selectively increased IL-10 production within secondary lymphoid tissue. By contrast, mature BM derived DC elicited increased IFN-γ but not IL-10 production. Immature hepatic DC therefore resemble freshly isolated immature respiratory tract DC that poorly stimulate allogeneic T cells and selectively induce Th2 responses.59 These features of liver derived DC are consistent with hepatic “tolerogenicity” and may play a role in immune response deviation following liver transplantation.
There is as yet little documented information on the T cell stimulatory ability of purified freshly isolated human liver DC. Based on their immature phenotype in situ60 (including lack of CD86) and the known properties of circulating peripheral blood DC with an immature phenotype,61 it is likely however that these cells are weak allostimulators.
DC isolated from lymph
Matsuno and colleagues56 have surveyed and analysed rat hepatic DC after they have exited the liver and entered the lymphatic circulation. By selective lymphadenectomy, it is possible to directly anastomose peripheral lymphatics to the thoracic duct, allowing draining cells to circumvent lymphoid tissues.62 Thus non-lymphoid cells in peripheral lymph can be collected from the thoracic duct. Removal of coeliac nodes allowed enrichment of the lymphatics with hepatic DC, leading Matsuno and colleagues62 to speculate that the liver is perhaps the greatest source of lymph from the gastrointestinal tract. The particle laden DC that entered the lymph were found to be non-phagocytic, even though they appeared immature cytologically. Furthermore, they were found to be strong T cell allostimulators. It has been suggested that these DC are in the early stages of maturation. Little is known of the activation, maturation, and migration of hepatic DC subsequent to Ag uptake.
Portal tract associated lymphoid tissue (PALT)
Portal lymphoid follicles were described in chronic active hepatitis C as early as 1992.63,64 These areas of B and T cell interactions exhibit many histological features classic to lymphoid follicles. More recently, Yoneyama and colleagues65 have identified DC-T cell interactions within these specialised areas of the liver. On infection with Propionibacterium acnes, granulomas form within the liver. DC are mobilised to these sites and can be found to (1) traffic to the hepatic LN; (2) remain in the developing sinusoidal granuloma; or (3) associate with immunoresponsive cells (B and T cells, DC) in a distinct area near the portal triad, termed the PALT by Yoneyama et al.
“Portal inflammation and PALT development have been identified in primary sclerosing cholangitis”
This lymphoid tissue-like area comprises B cell follicles with follicular DC (not BM derived DC but DC specialised for the presentation of Ag captured in immune complexes) interspersed throughout the follicles. CD4+ T cells were found to localise between B cell follicles, but not within these structures, unlike the broad distribution seen within sinusoidal granulomas. Surrounding the B and T cell areas were macrophages. In patients with hepatitis C virus infection, plasma cells and B cells are also found in association with DC within hepatic portal areas, as in lymphoid tissue.66 Similarly, portal inflammation and PALT development have been identified in primary sclerosing cholangitis (PSC).67,68 CCL21 (secondary lymphoid chemokine), a lymph node associated chemokine, is upregulated on CD34+ vascular endothelium of PALT. Expression of CCL21 recruits CCR7+ cells that commonly include DC and naïve T cells.69,70 These findings suggest that there may be important immune cell interactions occurring within PALT, perhaps circumventing the need for DC migration to lymphoid tissue.
Liver derived DC progenitors
In order to generate DC from normal liver, Lu and colleagues33 applied a procedure introduced for the propagation of DC from murine blood or BM. Inaba and colleagues71,72 first showed that culture of normal mouse BM cells with GM-CSF resulted in the propagation of DC. Similarly, culture of liver NPC yielded a population of replicating DC progenitors.33 These immature DC exhibited classic veiled morphology, high surface expression of CD45, CD11b, CD24, and CD44, moderate to low expression of CD11c, CD16/32, CD54, CD205, and F4/80, and low expression of the costimulatory molecules CD40, CD80, and CD86. Furthermore, these cells were resistant to typical DC maturation inducing stimuli, such as the proinflammatory cytokines IFN-γ and TNF-α. Extended culture failed to upregulate MHC II or costimulatory molecules. Instead, these cells matured in response to ECM proteins, such as collagen type 113,33,44,45 (with which DC are associated spatially in normal liver), losing their phagocytic ability and gaining the ability to stimulate naïve allogeneic T cells.
A novel population of mouse liver derived DC-like cells has been propagated in response to IL-3 and CD40L.34 These cells have a phenotype and function distinct from typical immature or mature myeloid or lymphoid related mouse DC. Ig rearrangement occurs within these cells without surface expression of Ig molecules. Furthermore, they have a distinct pattern of surface markers and maintain a DC-like morphology. These CD205brightCD11c−B220+CD19− cells activate T cells and promote their apoptosis. Lu and colleagues34 also showed that a T regulatory type 1 cytokine expression pattern was induced by these DC.
Chemotaxis
Migration of DC to and from peripheral tissue depends on the production of chemokines (CC and CXC) and expression of specific chemokine receptors (CCR and CXCR). Because leucocyte migration is a key event in infection and inflammation, chemokine biology is rapidly becoming an important area of study in relation to elucidation of DC function. Most chemokine receptors are promiscuous and can ligate a variety of different chemokines.73–75
“Because leucocyte migration is a key event in infection and inflammation, chemokine biology is rapidly becoming an important area of study in relation to elucidation of DC function”
In the case of hepatic DC, few studies have been conducted regarding specific chemokine and receptor expression. Drakes and colleagues76 showed that immature and mature liver derived DC exhibited similar chemokines and receptors, although with differing levels of expression. Expression was similar to that detected on BM derived DC. As determined by the RNase protection assay, the chemokine most strongly expressed by both immature and mature liver derived DC was CCL5 (RANTES, regulated upon activation, normal T cell expressed and secreted). However, CCL3 (MIP-1α, macrophage inflammatory protein 1α), CXCL1 (MIP-2), and CCL2 (MCP-1, monocyte chemoattractant protein 1) were also expressed by these liver derived DC. Receptors CCR1 and CCR2 were expressed at comparable levels on these liver DC. CCL5 and CCL3 are among the various chemokines that bind CCR1 while CCL2 binds CCR2. CCL3 expression was greatly enhanced on liver DC maturation and stimulation by bacterial lipopolysaccharide or naïve allogeneic T cells also induced chemotaxis of mature liver derived DC.
Shields et al found that CCR5, for which CCL3 is a ligand, is important in T cell recruitment in both hepatitis C virus (HCV) infected and normal livers.77 Goddard et al similarly observed the importance of CCR5 in T lymphocyte recruitment during the inflammatory response in human liver transplantation.78 The presence of this receptor on T cells coupled with the production of CCL3 by resident liver cells implies the existence of DC-T cell interactions within the liver under normal and inflammatory conditions. Further studies are needed to assess the role of chemokines and their receptors in the regulation of hepatic DC migration and function.
HEPATIC DC AND LIVER DISEASE
Viral hepatitis
Both hepatitis B and C viruses (HBV and HCV, respectively) are major health concerns as these are not only infectious diseases with distinct pathogeneses but are also major prognostic factors for hepatocellular carcinoma (HCC). Although some studies have investigated the role of resident liver DC in defence against these viruses, there is still much to understand.
“A general agreement in the literature is the existence of dysfunctional DC in both HBV and HCV infection”
A general agreement in the literature is the existence of dysfunctional DC in both HBV and HCV infection. HBV transgenic mice that express HBV Ag are used as a model for chronic HBV carriers. These mice show low immune efficiency, as defined by decreased overall specific antibody responses and lowered DC allostimulatory capabilities.79,80 In one study, it was found that defective splenic DC had low costimulatory molecule expression and low IL-12 production.80
Peripheral blood derived DC from chronic HCV patients show impaired maturation.81,82 They fail to respond to TNF-α (that typically induces DC maturation) and are poor T cell allostimulators. These cells also show decreased production of bioactive IL-12 p70.81 Less aggressive HCV infection is associated with inflammation, confined mainly to portal areas where hepatic DC generally reside. In contrast with reports of increased immature blood DC in chronic HCV patients, electron microscopy and surface marker expression have identified the portal infiltrate DC as phenotypically mature.66 These DC are also associated with the formation of new lymphatic capillaries within chronic hepatitis C livers.66 Thus Galle et al speculate a critical role for DC in mediating the HCV disease state based on increased lymphatic drainage and the association of DC with these sites.
One of the chemokines present in portal areas during HCV infection is CCL3.77 This chemokine is produced by T cells, macrophages (KC), and fibroblasts, and attracts DC as well as T cells. Other chemokines present in the portal area at sites of piecemeal necrosis in patients with chronic hepatitis C include CCL5 and DC-CK1, which have been correlated with an active immune response against HCV.83 In fact, DC-CK1 is found in the PALT. It is possible that the production of these cytokines aides DC-T cell interactions.
Hepatocellular carcinoma (HCC)
A prerequisite for effective immune responses against tumours is the need for cells that recognise, process, and present tumour Ag. DC are considered promising biological therapy agents for cancer treatment. In patients with HCC, there is evidence that immature DC with maturation defects are the predominant type of peripheral blood DC.84 Circulating DC show reduced expression of HLA-DR and IL-12 and reduced endocytotic and allostimulatory capacity.84 Additionally, these DC remain immature in the presence of high levels of inflammatory cytokines that normally induce DC maturation.84
“DC are considered promising biological therapy agents for cancer treatment”
By contrast, it has been reported that activated CD83+ DC are increased in the peripheral blood of HCC patients compared with normal patients and patients with liver cirrhosis.85 However, total DC are reduced in the livers of HCC subjects and not localised to cancer nodules.85 Importantly, it had been shown that administration of Flt3L can drastically reduce the number of hepatic metastases in experimental animals.86 Tumour borders exhibited increased infiltration with both DC and T cells as well as increased apoptotic bodies. Thus DC may have an important role in surveillance and clearance of tumour cells in liver cancer.
Granulomatous liver disease
Recent studies have revealed DC recruitment to hepatic sites of experimental granulomas. Yoneyama and colleagues65 observed CD11c+F4/80−B220− DC in P acnes induced granulomas in the perisinusoidal space. These DC later interacted with T cells in the PALT.
Autoimmune diseases
Patients with primary biliary cirrhosis (PBC) have dysfunctional DC with increased production of nitric oxide and lowered allostimulatory capability.61 The number of DC present in portal tracts is greater in PBC patients compared with HCV patients.60 Kaji et al also found that these CD86 positive DC appeared to be more relevant in the earlier stages of PBC as they disappeared from the liver at later stages.
TRANSPLANTATION
The immunobiology of liver transplantation has long been a field of intense study as it may provide valuable insight into the mechanisms underlying transplant tolerance. Liver transplant patients are known to achieve graft acceptance without continued immunosuppressive drug therapy. Moreover, graft failure due to chronic rejection is rare compared with other types of organ transplantation. Furthermore, liver transplantation can protect other organ grafts from the same donor transplanted in conjunction with the liver. Pigs, mice, and some rat strain combinations will accept liver allografts across MHC barriers without immunosuppressive therapy. This acceptance may be lost by removal of donor leucocytes prior to liver transplant46,47 while replacement of donor leucocytes abrogates rejection.47 These findings suggest that donor leucocytes, that include DC, have the capacity to modulate host anti-donor immune reactivity.
“Donor leucocytes, that include DC, have the capacity to modulate host anti-donor immune reactivity”
There are several concepts that attempt to explain the comparative privilege of liver allografts. Starzl et al have proposed the theory of microchimerism (two way silencing of immune reactivity, linked to deletion of alloreactive T cells) to explain promotion of tolerance induction.22,87,88 Microchimerism is the persistence of donor haematopoietic cells within both lymphoid and non-lymphoid tissues of the host. Significantly, donor derived DC can be propagated from the blood or BM of liver transplant recipients.89,90 In mice, this is achieved in liver recipients that accept these grafts without immunosuppression and that develop donor specific tolerance, but not in mice that acutely reject heart grafts from the same donor strain.89
The liver is a haematopoietic organ and thus compared with other transplanted organs may have an advantage in being a continuous source of donor haematopoietic stem/progenitor cells. Many circulating haematopoietic cells also take up residence in the liver. In addition, three hepatic stem cell candidates have been described to date: fetal progenitor bipotential hepatic stem cells, adult hepatocytes, and oval cells—a type of non-parenchymal pluripotent hepatic stem cell.91 The existence of these liver stem/progenitor cells suggests that a hepatic progenitor cell exists for the production of liver specific DC in situ. Importantly, donor interstitial DC appear to self replicate in rat liver graft recipients.92 These donor derived immature DC may promote donor specific tolerance induction.
It has been argued on the other hand that comparatively large numbers of donor leucocytes present in liver allografts cause overstimulation or “abnormal” early activation of recipient T cells that leads to their exhaustive proliferation and deletional tolerance.48
“Conceivably, donor DC may play a role in inducing apoptosis in host T cells via death ligand receptor pathways”
There are many potentially important mechanistic roles for the hepatic DC in determining the outcome of transplantation. Alloreactive host T cell apoptosis in experimental liver transplantation is associated with tolerance whereas less apoptosis is seen with rejection.93–95 Conceivably, donor DC may play a role in inducing apoptosis in host T cells via death ligand receptor pathways.96 It has been shown that there may be such a role for tissue resident migratory DC in immune privileged sites, such as the anterior chamber of the eye97 or testis.98 Neutralisation of IL-12 produced by liver resident DC and other APC in murine livers transplanted from Flt3L treated donors (that are rejected acutely) restores long term allograft survival and enhances alloreactive T cell apoptosis.99 This suggests that suppression/inhibition of donor DC function promotes tolerance induction. The immature state of normal liver derived DC, associated with failure to provide adequate costimulation, may be important in inherent liver tolerogenicity. The immature state/absence of costimulation can also be achieved using immunomodulatory agents, such as IL-1019 or CTLA4-Ig.23 Administration of liver DC progenitors prior to transplantation has been shown to increase allograft survival, although not to induce tolerance.34,100 It remains to be determined whether in a clinically relevant large animal (primate) model, coadministration of immature donor DC with appropriate pharmacological agents or biological immunosuppressants that inhibit their maturation and those of recipient DC would promote the induction of organ transplant tolerance.
Acknowledgments
The authors’ work is supported by National Institutes of Health grants R01 DK49745, R01 AI41011, and U01 AI511698.
Abbreviations
Ag, antigen
APC, antigen presenting cells
BM, bone marrow
CC and CXC, chemokines
CCR and CXCR, chemokine receptors
DC, dendritic cell
ECM, extracellular matrix
Flt3L, fms-like tyrosine kinase 3 ligand
GM-CSF, granulocyte macrophage-colony stimulating factor
HBV, hepatitis B virus
HCC, hepatocellular carcinoma
HCV, hepatitis C virus
KC, Kupffer cell
IL, interleukin
IFN-γ, interferon γ
LSEC, liver sinusoidal endothelial cells
MHC, major histocompatibility complex
NPC, non-parenchymal cells
PALT, portal tract associated lymphoid tissue
PBC, primary biliary cirrhosis
PSC, primary sclerosing cholangitis
TGF-β, transforming growth factor β
TNF-α, tumour necrosis factor α
REFERENCES
- 1.Doherty DG, O’Farrelly C. Dendritic cells: regulators of hepatic immunity or tolerance? J Hepatol 2001;34:156–60. [DOI] [PubMed] [Google Scholar]
- 2.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev 2000;174:21–34. [DOI] [PubMed] [Google Scholar]
- 3.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol 1998;28:221–36. [DOI] [PubMed] [Google Scholar]
- 4.MacPhee P, Schmidt E, Groom A. Evidence for Kupffer cell migration along liver sinusoids, from high-resolution in vivo microscopy. Am J Physiol 1992;263:G17–23. [DOI] [PubMed] [Google Scholar]
- 5.MacPhee P, Schmidt E, Groom A. Intermittence of blood flow in liver sinusoids, studied by high-resolution in vivo microscopy. Am J Physiol 1995;269:G692–8. [DOI] [PubMed] [Google Scholar]
- 6.Daar A, Fuggle S, Hart D, et al. Demonstration and phenotypic characterization of HLA-DR positive interstitial dendritic cells widely distributed in human connective tissues. Transplant Proc 1983;15:311–15. [Google Scholar]
- 7.Prickett TC, McKenzie JL, Hart DN. Characterization of interstitial dendritic cells in human liver. Transplantation 1988;46:754–61. [DOI] [PubMed] [Google Scholar]
- 8.Hart D, Fabre J. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues but not brain. J Exp Med 1981;154:347–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knolle PA, Limmer A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol 2001;22:432–7. [DOI] [PubMed] [Google Scholar]
- 10.Lohse A, Knolle PA, Bilo K, et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 1996;110:1175–81. [DOI] [PubMed] [Google Scholar]
- 11.Morelli AE, O’Connell PJ, Khanna A, et al. Preferential induction of Th1 responses by functionally mature hepatic (CD8a− and CD8a+) dendritic cells: association with conversion from liver transplant tolerance to acute rejection. Transplantation 2000;69:2647–57. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell PJ, Morelli AE, Logar AJ, et al. Phenotypic and functional characterization of mouse hepatic CD8a+ lymphoid-related dendritic cells. J Immunol 2000;165:795–803. [DOI] [PubMed] [Google Scholar]
- 13.Abe M, Akbar SM, Horiike N, et al. Induction of cytokine production and proliferation of memory lymphocytes by murine liver dendritic cell progenitors: role of these progenitors as immunogenic resident antigen-presenting cells in the liver. J Hepatol 2001;34:61–7. [DOI] [PubMed] [Google Scholar]
- 14.Khanna A, Morelli AE, Zhong C, et al. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol 2000;164:1346–54. [DOI] [PubMed] [Google Scholar]
- 15.Woo J, Lu L, Rao AS, et al. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation 1994;58:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bissell D, Wang S-S, Jarnagin W, et al. Cell-specific expression of transforming growth factor-beta in rat liver: evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest 1995;96:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson AW, Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol Today 1999;20:27–32. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi Y, Tsumara H, Miwa M, et al. Contrasting effects of TGF-β1 and TNF-α on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells 1997;15:144–53. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrink K, Wolfl M, Jonuleit H, et al. Induction of tolerance by IL-10-treated dendritic cells. J Immunol 1997;159:4772–80. [PubMed] [Google Scholar]
- 20.Lee W-C, Zhong C, Qian S, et al. Phenotype, function, and in vivo migration and survival of allogeneic dendritic cell progenitors genetically engineered to express TGF-β. Transplantation 1998;66:1810–17. [DOI] [PubMed] [Google Scholar]
- 21.Takayama T, Nishioka Y, Lu L, et al. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation 1998;66:1567–74. [DOI] [PubMed] [Google Scholar]
- 22.Thomson AW, Lu L, Murase N, et al. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells 1995;13:622–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury S, Gallon L, Verburg R, et al. Ex vivo treatment of antigen presenting cells with CTLA4Ig and encephalitogenic peptide prevents experimental autoimmune encephalomyelitis in the Lewis rat. J Immunol 1996;157:3700–5. [PubMed] [Google Scholar]
- 24.Wu L, Li C, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J Exp Med 1996;184:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ardavin C, Wu L, Li C, et al. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature 1993;362:761–3. [DOI] [PubMed] [Google Scholar]
- 26.Martin P, Martinez del Hoyo G, Anjuere F, et al. Concept of lymphoid versus myeloid dendritic cell lineages revisited: both CD8a− and CD8a+ dendritic cells are generated from CD4low lymphoid-committed precursors. Blood 2000;96:2511–19. [PubMed] [Google Scholar]
- 27.Merad M, Fong L, Bogenberger J, et al. Differentiation of myeloid dendritic cells into CD8a-positive dendritic cells in vivo. Blood 2000;96:1865–72. [PubMed] [Google Scholar]
- 28.Traver D, Akashi K, Manz M, et al. Development of CD8a-positive dendritic cells from a common myeloid progenitor. Science 2000;290:2152–4. [DOI] [PubMed] [Google Scholar]
- 29.Bjorck P. Isolation and characterization of murine plasmacytoid dendritic cells. Blood 2001;98:3520–6. [DOI] [PubMed] [Google Scholar]
- 30.Asselin-Paturel C, Boonstra A, Dalod M, et al. Mouse type 1 IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2001;2:1144–50. [DOI] [PubMed] [Google Scholar]
- 31.Nakano H, Yanagita M, Gunn M. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med 2001;194:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drakes ML, Lu L, Subbotin VM, et al. In vivo administration of flt3 ligand markedly stimulates generation of dendritic cell progenitors from mouse liver. J Immunol 1997;159:4268–78. [PubMed] [Google Scholar]
- 33.Lu L, Woo J, Rao AS, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med 1994;179:1823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu L, Bonham CA, Liang X, et al. Liver-derived DEC205+B220+CD19− dendritic cells regulate T cell responses. J Immunol 2001;166:7042–52. [DOI] [PubMed] [Google Scholar]
- 35.Chen-Woan M, Delaney C, Fournier V, et al. In vitro characterization of rat bone-marrow derived dendritic cells and their precursors. J Leukoc Biol 1996;59:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenan M, Puklavec M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J Exp Med 1992;175:1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorczynski L, Chen Z, Hu J, et al. Evidence that an OX-2-positive cell can inhibit the stimulation of type 1 cytokine production by bone marrow-derived B7–1 (and B7–2)-positive dendritic cells. J Immunol 1999;162:774–81. [PubMed] [Google Scholar]
- 38.Steptoe RJ, Patel R, Subbotin V, et al. Comparative analysis of dendritic cell density and total number in commonly transplanted organs: morphometric estimation in normal mice. Transpl Immunol 2000;8:49–56. [DOI] [PubMed] [Google Scholar]
- 39.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell sub-populations identified. J Exp Med 1996;184:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley MT, Inaba K, Witmer-Pack MD, et al. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol 1989;118:108–25. [DOI] [PubMed] [Google Scholar]
- 41.Thomson AW, Lu L, Subbotin V, et al. Propagation of dendritic cell progenitors from mouse liver and their in vivo migration to T-dependent areas of allogeneic lymphoid tissue. Transplant Proc 1994;26:3484–6. [PubMed] [Google Scholar]
- 42.Thomson AW, Lu L, Subbotin V, et al. In vitro propagation and homing of liver-derived dendritic cell progenitors to lymphoid tissues of allogeneic recipients. Transplantation 1995;59:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drakes ML, Lu L, Subbotin V, et al. In vivo administration of flt3 ligand markedly stimulates generation of dendritic cell progenitors from mouse liver. J Immunol 1997;159:4268–78. [PubMed] [Google Scholar]
- 44.Drakes ML, Lu L, McKenna HJ, et al. The influence of collagen, fibronectin, and laminin on the maturation of dendritic cell progenitors propagated from normal or Flt3-ligand-treated mouse liver. Adv Exp Med Biol 1997;417:115–20. [DOI] [PubMed] [Google Scholar]
- 45.Thomson AW, Lu L, Woo J, et al. Exposure to type-I collagen induces maturation of mouse liver dendritic cell progenitors. Adv Exp Med Biol 1995;378:511–18. [DOI] [PubMed] [Google Scholar]
- 46.Sun J, McCaughan GW, Gallagher ND, et al. Deletion of spontaneous rat liver allograft acceptance by donor irradiation. Transplantation 1995;60:233–6. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu Y, Goto S, Lord R, et al. Restoration of tolerance to rat hepatic allografts by spleen-derived passenger leukocytes. Transpl Int 1996;9:593–95. [DOI] [PubMed] [Google Scholar]
- 48.Bishop GA, Sun J, DeCruz D, et al. Tolerance to rat liver allografts. III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol 1996;156:4925–31. [PubMed] [Google Scholar]
- 49.Steptoe RJ, Fu F, Li W, et al. Augmentation of dendritic cells in murine organ donors by Flt3 ligand alters the balance between transplant tolerance and immunity. J Immunol 1997;159:5483–91. [PubMed] [Google Scholar]
- 50.Steptoe RJ, Li W, Fu F, et al. Trafficking of APC from liver allografts of Flt3L-treated donors: augmentation of potent allostimulatory cells in recipient lymphoid tissue is associated with a switch from tolerance to rejection. Transpl Immunol 1999;7:51–7. [DOI] [PubMed] [Google Scholar]
- 51.Steiniger B, Klempnauer J, Wonigeit K. Phenotype and histological distribution of interstitial dendritic cell in the rat pancreas, liver, heart, and kidney. Transplantation 1984;38:169–74. [DOI] [PubMed] [Google Scholar]
- 52.Witmer-Pack MD, Crowley MT, Inaba K, et al. Macrophages, but not dendritic cells, accumulate colloidal carbon following administration in situ. J Cell Sci 1993;105:965–73. [DOI] [PubMed] [Google Scholar]
- 53.Lautenschlager I, Halttunen J, Hayry P. Characteristics of dendritic cells in rat liver. Transplantation 1988;45:936–9. [DOI] [PubMed] [Google Scholar]
- 54.Smedsrod B, Melkko J, Araki N, et al. Advanced glycation end products are eliminated by scavenger-receptor mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J 1997;322:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magnusson S, Berg T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem J 1989;257:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuno K, Ezaki T, Kudo S, et al. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. J Exp Med 1996;183:1865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuno K, Kudo S, Ezaki T. The liver sinusoids as a specialized site for blood-lymph translocation of rat dendritic cells. Adv Exp Med Biol 1997;417:77–81. [DOI] [PubMed] [Google Scholar]
- 58.Iyoda T, Shimoyama S, Liu K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med 2002;195:1289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stumbles P, Thomas J, Pimm C, et al. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med 1998;188:2019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaji K, Tsuneyama K, Nakanuma Y, et al. B7-2 positive cells around interlobular bile ducts in primary biliary cirrhosis and chronic hepatitis C. J Gastroenterol Hepatol 1997;12:507–12. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto K, Akbar SK, Masumoto T, et al. Increased nitric oxide (NO) production by antigen-presenting dendritic cells is responsible for low allogeneic mixed leucocyte reaction (MLR) in primary biliary cirrhosis. Clin Exp Immunol 1998;114:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuno K, Kudo S, Ezaki T, et al. Isolation of dendritic cells in the rat liver lymph. Transplantation 1995;60:765–8. [DOI] [PubMed] [Google Scholar]
- 63.Mosnier JF, Degott C, Marcellin P, et al. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology 1993;17:366–71. [PubMed] [Google Scholar]
- 64.Scheuer P, Ashrafzadeh P, Sherlock S, et al. The pathology of hepatitis C. Hepatology 1992;15:567–71. [DOI] [PubMed] [Google Scholar]
- 65.Yoneyama H, Matsuno K, Zhang Y, et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med 2001;193:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galle MB, DeFranco RM, Kerjaschki D, et al. Ordered array of dendritic cells and CD8+ lymphocytes in portal infiltrates in chronic hepatitis C. Histopathology 2001;39:373–81. [DOI] [PubMed] [Google Scholar]
- 67.Grant A, Goddard S, Ahmed-Choudhury J, et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am J Pathol 2002;160:1445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grant A, Lalor P, Hubscher S, et al. MAdCAM-1 expression is increased in primary sclerosing cholangitis and supports lymphocyte adhesion to hepatic endothelium: a mechanism to explain the recruitment of mucosal lymphocytes to the liver in inflammatory liver disease. Hepatology 2001;33:1065–73. [DOI] [PubMed] [Google Scholar]
- 69.Cyster J. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med 1999;189:447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kellerman S, Hudak S, Oldham E, et al. The CC-chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol 1999;162:3859–64. [PubMed] [Google Scholar]
- 71.Inaba K, Steinman RM, Witmer-Pack MD, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med 1992;175:1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992;176:1693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murphy P, Baggiolini M, Charo I, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharm Rev 2000;52:145–76. [PubMed] [Google Scholar]
- 74.Nelson P, Krensky A. Chemokines, chemokine receptors, and allograft rejection. Immunity 2001;14:377–86. [DOI] [PubMed] [Google Scholar]
- 75.Sallusto F, Mackay C, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 2000;18:593–620. [DOI] [PubMed] [Google Scholar]
- 76.Drakes ML, Zahorchak AF, Takayama T, et al. Chemokine and chemokine receptor expression by liver-derived dendritic cells: MIP-1alpha production is induced by bacterial lipopolysaccharide and interaction with allogeneic T cells. Transpl Immunol 2000;8:17–29. [DOI] [PubMed] [Google Scholar]
- 77.Shields P, Morland C, Salmon M, et al. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific lver compartments within hepatitis C-infected liver. J Immunol 1999;163:6236–43. [PubMed] [Google Scholar]
- 78.Goddard S, Williams A, Morland C, et al. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation 2001;72:1957–67. [DOI] [PubMed] [Google Scholar]
- 79.Akbar S, Onji M, Inaba K, et al. Low responsiveness of hepatitis B virus-transgenic mice in antibody response to T-cell-dependent antigen: defect in antigen-presenting activity of dendritic cells. Immunology 1993;78:468–75. [PMC free article] [PubMed] [Google Scholar]
- 80.Akbar SK, Horiike N, Onji M. Prognostic importance of antigen-presenting dendritic cells during vaccine therapy in a murine hepatitis B virus carrier. Immunology 1999;96:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol 1999;162:5584–91. [PubMed] [Google Scholar]
- 82.Auffermann-Gretzinger S, Keeffe E, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 2001;97:3171–6. [DOI] [PubMed] [Google Scholar]
- 83.Kusano F, Tanaka Y, Marumo F, et al. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest 2000;80:415–22. [DOI] [PubMed] [Google Scholar]
- 84.Ninomiya T, Akbar S, Masumoto T, et al. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol 1999;31:323–31. [DOI] [PubMed] [Google Scholar]
- 85.Chen S, Akbar S, Tanimoto K, et al. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett 2000;148:49–57. [DOI] [PubMed] [Google Scholar]
- 86.Peron J, Esche C, Subbotin V, et al. FLT3-ligand administration inhibits liver metastases: role of NK cells. J Immunol 1998;161:6164–70. [PubMed] [Google Scholar]
- 87.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet 1992;339:1579–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology 1993;17:1127–52. [PMC free article] [PubMed] [Google Scholar]
- 89.Lu L, Rudert WA, Qian S, et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med 1995;182:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rugeles M, Aitouche A, Zeevi A, et al. Evidence for the presence of multilineage chimerism and progenitors of donor dendritic cells in the peripheral blood of bone marrow-augmented organ transplant recipients. Transplantation 1997;64:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feldmann G. Liver transplantation of hepatic stem cells: potential use for treating liver diseases. Cell Biol Toxic 2001;17:77–85. [DOI] [PubMed] [Google Scholar]
- 92.Saiki T, Ezaki T, Ogawa M, et al. Trafficking of host- and donor-derived dendritic cells in rat cardiac transplantation: allosensitization in the spleen and hepatic nodes. Transplantation 2001;71:1806–15. [DOI] [PubMed] [Google Scholar]
- 93.Qian S, Lu L, Fu F, et al. Apoptosis within spontaneously accepted mouse liver allografts. J Immunol 1997;158:4654–61. [PMC free article] [PubMed] [Google Scholar]
- 94.Meyer D, Baumgardt S, Loeffeler S, et al. Apoptosis of T lymphocytes in liver and/or small bowel allografts during tolerance induction. Transplantation 1998;66:1530–6. [DOI] [PubMed] [Google Scholar]
- 95.Sharland A, Shastry S, Wang C, et al. Kinetics of intragraft cytokine expression, cellular infiltration, and cell death in rejection of renal allografts compared with acceptance of liver allografts in a rat model: early activation and apoptosis is associated with liver graft acceptance. Transplantation 1998;65:1370–7. [DOI] [PubMed] [Google Scholar]
- 96.Lu L, Qian S, Hershberger P, et al. Fas Ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol 1997;158:5676–84. [PubMed] [Google Scholar]
- 97.Griffith T, Brunner T, Fletcher S, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995;270:1189–92. [DOI] [PubMed] [Google Scholar]
- 98.Bellgrau D, Gold D, Selawry H, et al. A role for CD95 ligand in preventing graft rejection. Nature 1995;377:630–32. [DOI] [PubMed] [Google Scholar]
- 99.Li W, Lu L, Wang Z, et al. IL-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J Immunol 2001;166:5619–28. [DOI] [PubMed] [Google Scholar]
- 100.Rastellini C, Lu L, Ricordi C, et al. Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation 1995;60:1366–70. [PMC free article] [PubMed] [Google Scholar]
- 101.Thomson AW, Drakes ML, Zahorchak AF, et al. Hepatic dendritic cells: immunobiology and role in liver transplantation. J Leukoc Biol 1999;66:322–30. [DOI] [PubMed] [Google Scholar]
- 102.Thomson AW, O’Connell PJ, Steptoe RJ, et al. Immunobiology of liver dendritic cells. Immunol Cell Biol 2002;80:65–73. [DOI] [PubMed] [Google Scholar]
- 103.Gorczynski RM, Levy G, Chen Z. Hepatic mononuclear cells modulate delivery of immunogenic stimuli by allogeneic dendritic cells. Transplant Proc 1999;31:856–7. [DOI] [PubMed] [Google Scholar]
- 104.Sato T, Yamamoto H, Sasaki C, et al. Maturation of rat dendritic cells during intrahepatic translocation evaluated using monoclonal antibodies and electron microscopy. Cell Tissue Res 1998;294:503–14. [DOI] [PubMed] [Google Scholar]
- 105.Schon-Hegrad M, Oliver J, McMenamin P, et al. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med 1991;173:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goddard S, Barclay N, Adams D. CD200 (OX2) has limited expression in liver, and is expressed by mature dendritic cells from non-lymphoid tissue. Scand J Immunol 2001;54:29. [Google Scholar]
- 107.Goddard S, Hubscher S, Lane P, et al. A comparison of dendritic cells migrated from human liver and skin. Scand J Immunol 2001;54:29. [Google Scholar]