Abstract
Background and aims: Despite the progress made in understanding the immunological aspects of the pathogenesis of coeliac disease (CD), the early steps that allow gliadin to cross the intestinal barrier are still largely unknown. The aim of this study was to establish whether gliadin activates a zonulin dependent enterocyte intracellular signalling pathway(s) leading to increased intestinal permeability.
Methods: The effect of gliadin on the enterocyte actin cytoskeleton was studied on rat intestinal epithelial (IEC-6) cell cultures by fluorescence microscopy and spectrofluorimetry. Zonulin concentration was measured on cell culture supernatants by enzyme linked immunosorbent assay. Transepithelial intestinal resistance (Rt) was measured on ex vivo intestinal tissues mounted in Ussing chambers.
Results: Incubation of cells with gliadin led to a reversible protein kinase C (PKC) mediated actin polymerisation temporarily coincident with zonulin release. A significant reduction in Rt was observed after gliadin addition on rabbit intestinal mucosa mounted in Ussing chambers. Pretreatment with the zonulin inhibitor FZI/0 abolished the gliadin induced actin polymerisation and Rt reduction but not zonulin release.
Conclusions: Gliadin induces zonulin release in intestinal epithelial cells in vitro. Activation of the zonulin pathway by PKC mediated cytoskeleton reorganisation and tight junction opening leads to a rapid increase in intestinal permeability.
Keywords: gliadin, enterocyte cytoskeleton, tight junction, intestinal permeability, coeliac disease
Coeliac disease (CD) is an autoimmune enteropathy triggered by ingestion of gluten containing grains in genetically susceptible individuals. The gliadin fraction of wheat gluten represents the environmental factor responsible for the development of the intestinal damage typical of the disease.1 While in recent years we have witnessed significant progress on the immunological aspects of CD pathogenesis,2 no major achievements have been made in understanding the early steps that allow gliadin to cross the intestinal epithelial barrier to be recognised by the intestinal immune system.3 Gliadin deamidation by tissue transglutaminase has been demonstrated to enhance the recognition of gliadin peptides by HLA DQ2/DQ8 T cells in genetically predisposed subjects and it might initiate the cascade of autoimmune reactions which are finally responsible for mucosal destruction through production of cytokines and matrix metalloproteinases.3,4 These reactions imply that gliadin and/or its breakdown peptides in someway cross the intestinal epithelial barrier and reach the lamina propria of the intestinal mucosa where they are recognised by antigen presenting cells. Under physiological circumstances the intestinal epithelial barrier is described as being almost impermeable to macromolecules.5 However, CD is characterised by enhanced paracellular permeability across intestinal epithelium— that is, “leaky gut”, a condition that would allow passage of macromolecules through the paracellular spaces.6–8 We have recently reported that zonulin, a modulator of tight junction (tj) permeability,9 is upregulated during the acute phase of CD.10 Following binding to its surface receptor, zonulin induces a protein kinase C (PKC) mediated polymerisation of intracellular actin filaments which are directly connected to structural proteins of the tj hence regulating epithelial permeability.9–11 The complex actin cytoskeleton network of the enterocyte is known to be involved in the intracellular trafficking of molecules as well as in the regulation of paracellular permeability by its direct interaction with the tj structural proteins.12–14 This study was aimed at establishing the interplay between gliadin and the enterocyte, with specific emphasis on the effect of gliadin on zonulin release and subsequent activation of intracellular signalling leading to the disassembly of intercellular tj.
METHODS
IEC-6 cell cultures
Rat intestinal epithelial cells (IEC-6 cells) were grown in cell culture flasks (Falcon Labware, Reston, Virginia, USA) at 37°C in an atmosphere of 95% air and 5% CO2. The medium consisted of Dulbecco’s modified Eagle’s medium (Gibco, Rockville, Maryland, USA) containing 4500 mg/l d-glucose, pyridoxine hydrochloride, 5% heat inactivated (56°C, 30 minute) fetal bovine serum, 0.1 U/ml bovine insulin, 4 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin.
Gliadin peptides
Gliadin (Sigma, St Louis, Missouri, USA) was freshly prepared in a 70% ethanol solution (20 mg/ml) and used at serial dilutions in the cell culture medium, ranging from the 1:20 dilution (final concentration: gliadin 1 mg/ml; ethanol 3.5%) to the 1:200 dilution (final concentration: gliadin 0.1 mg/ml; ethanol 0.35%). The pH was adjusted to 7.4 when necessary by 1 M NaOH buffer. Similar ethanol concentrations were added to the final concentration of bovine serum albumin (BSA) and zein from maize (Sigma) used as negative controls. Ethanol concentration was never more than 3.5% in the final solution in order to avoid any direct effect of ethanol on cultured cells. Synthetic peptides 31–55 and 22–39 (Biopolymer Laboratories, University of Maryland, Baltimore, Maryland, USA) were also obtained to be tested for their permeating activity in the Ussing chamber assay.
Fluorescence microscopic analysis of intracellular F-actin
Cells were washed in phosphate buffered saline (PBS) and gently detached with 2–3 minutes of exposure to 0.25% trypsin, 1 mM EDTA solution (Gibco brl). Cells (2×104 cells/ml) were suspended in medium and seeded onto eight chamber slides (Nalge Nunc International) for 24 hours. Gliadin was added at increasing concentrations (0.1–1.0 mg/ml) and exposure times (15, 30, 45, 60 minutes). Cells were then washed twice in PBS, fixed in 3.7% paraformaldeyde in PBS (pH 7.4) for 15 minutes at room temperature, permeabilised with 0.5% TritonX-100 in PBS (Sigma) for 10 minutes at room temperature, and stained by incubation with 0.3 μM fluorescein phalloidin (Sigma) in PBS at 37°C for 30 minutes. After two additional washes, coverslips were mounted with glycerol-PBS (1:1) at pH 8.0. The results were analysed with a fluorescence microscope (Zeiss, Thornwood, New York, USA). In selected experiments, gliadin was substituted with zein at a final concentration of 0.1 mg/ml.
F-actin quantitation by spectrofluorimetry
Intracellular F-actin was fluorometrically measured by spectrofluorimetry. After detaching the cells as described above, IEC-6 cells (0.2×106 cells/ml medium) were seeded onto 24 well plates and cultured for 72 hours (37°C, 5% CO2); incubation with gliadin (0.1 mg/ml) was performed for varying exposure times (15, 30, 45, 60 minutes) at 37°C and 4°C; cells were washed twice with cytoskeleton stabilisation buffer (KCl 75 mM, MgSO4 3 mM, ethylene glycol tetraacetic acid 1 mM, imidazole 10 mM, DTT 0.2 mM, aprotinin 10 μg/ml, PMSF 0.1 mM), fixed with 3.7% formaldehyde/buffer for 15 minutes, permeabilised (0.2% Triton X-100/buffer, five minutes), washed twice, and stained with NBD-phallacidin 0.3 μM for 30 minutes at room temperature; after two washes, extraction of NBD-phallacidin was initiated by addition of ice cold high pressure liquid chromatography grade methanol and continued overnight at −20°C.
Fluorescence measurements were carried out using an SLM Model 8000 spectrofluorometer (Spectronic Instruments, Inc., Rochester, New York, USA). Excitation was at 464 nm and emission was observed at 536 nm. Excitation and emission slits were set at 8 nm.
Zonulin quantitation by sandwich enzyme linked immunosorbent assay (ELISA)
A sandwich ELISA was developed in order to measure zonulin concentration in cell culture supernatants using affinity purified anti-zonula occludens toxin (Zot) antibodies, produced as previously described.9 Five different serial dilutions of a 200 μg/ml Zot solution (0.7, 3.1, 12.5, 50, and 200 ng/ml) were prepared in PBS-T (0.05% Tween-20 in PBS) and used to generate the standard curve. Firstly, a 10 μg/ml anti-Zot IgG solution in PBS was added to each well (100 μl/well) of a 96 well microplate. After incubation for 48 hours at +4°C, the plate was washed three times with PBS-T and blocked overnight with PBS-T (300 μl/well) containing 1% BSA. After draining the blocking solution, five Zot serial standards and the cell culture medium samples were added in duplicate (100 μl/well) and incubated for two hours at room temperature with continuous shaking. Following three washes with PBS-T, 0.5 μg/ml biotinylated anti-Zot antibody solution in PBS-BSA1%-PEG 4% was added to each well (100 μl/well) and incubated for one hour at room temperature while shaking. After washing six times in PBS-T, a 15 minute incubation was performed with extravidin (Sigma) diluted 1:16 000 in 0.1 M Tris-HCl, 1 mM MgCl2, BSA 1% at pH 7.3 at room temperature. The plate was washed again three times with PBS-T and incubated for 30 minutes at 37°C with 0.1 ml of p-nitrophenyl phosphate substrate in glycin buffer (pH 10.7, containing 0.1 M NaCl, 0.1 mM ZnCl2, 1 mM MgCl2). Absorbance at 405 nm was measured with a microplate autoreader (Molecular Devices Thermomax Microplate Reader, USA). To define the intra- and interassay precision of the ELISA sandwich method, the coefficient of variation (CV) was calculated using three replicates from two samples with different concentrations of zonulin on three consecutive days. The interassay test of the ELISA sandwich method produced CV values of 9.8%. CV of the intra-assay test was 4.2% on day 1, 3.3% on day 2, and 2.9% on day 3.
Inhibitors
To explore the gliadin activated pathway(s) leading to cytoskeleton rearrangement, experiments were performed by spectrofluorimetry with specific inhibitors, including cycloheximide (50 μg/ml), an inhibitor of protein synthesis, and CGP41251 (1 μM), a specific inhibitor of PKC.14 Spectrofluorimetry and Ussing chamber experiments were also performed in the presence of the synthetic peptide FZI/0 (1 μg/ml; Biopolymer Laboratories, University of Maryland, Baltimore, Maryland, USA), which specifically competes for binding of zonulin to the receptor.11
Ussing chambers
Experiments were carried out as previously described.14 Briefly, adult male New Zealand white rabbits (2–3 kg) were sacrificed by cervical dislocation. A 20 cm segment of ileum was removed, rinsed free of the intestinal content, opened along the mesenteric border, and stripped of muscular and serosal layers. Eight sheets of mucosa so prepared were then mounted in lucite Ussing chambers, connected to a voltage clamp apparatus (EVC 4000; World Precision Instruments, Saratosa, Florida, USA), and bathed with freshly prepared buffer containing (mM): NaCl 53; KCl 5; Na2SO4 30.5; mannitol 30.5; Na2HPO4 1.69; NaH2PO4 0.3; CaCl2 1.25; MgCl2 1.1; NaHCO3 25. The bathing solution was maintained at 37°C with water jacketed reservoirs connected to a constant temperature circulating pump and gassed with 95% O2 and 5% CO2. Potential difference was measured, and short circuit current and tissue resistance (Rt) were calculated as previously described.14
Tissues were exposed to either gliadin (in the presence or absence of the zonulin inhibitor synthetic peptide FZI/0 added at a final concentration of 1 μg/ml) or two gliadin derived synthetic peptides (the non-toxic peptide AA 22–39 or the toxic peptide 31–55, final concentration 0.1 μg/ml) and the electrical parameters monitored every 10 minutes.
Statistical analysis
Student’s t test was used for both paired and unpaired analysis. Statistical significance was reached when p values were less than 0.05.
RESULTS
Fluorescence microscopic analysis of intracellular F-actin
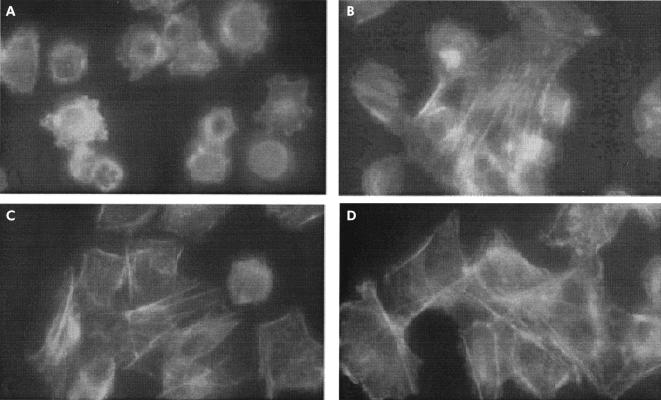
Incubation of IEC-6 cells with gliadin led to reorganisation of intracellular actin filaments which was visible by fluorescent microscopy after only 15 minutes of gliadin incubation and was characterised by a redistribution of F-actin to the cell subcortical compartment (fig 1A ▶). No significant changes were observed when IEC-6 cells were exposed to similar concentrations of either zein protein (fig 1B ▶) from maize (a non-toxic grain for CD patients) or BSA negative control (fig 1C ▶). The gliadin effect on the actin cytoskeleton was reversible as two hours after withdrawal of gliadin the actin cytoskeleton returned to its basal state (fig 1D ▶).
Figure 1.
Effect of gliadin (0.1 mg/ml) on IEC-6 cell cytoskeleton. (A) Fluorescence microscopy of gliadin exposed IEC-6 cells. Incubation for 15 minutes of cultured cells with gliadin caused a reorganisation of actin filaments characterised by redistribution to the cell subcortical compartment and subsequent cell rounding. A normal F-actin fluorescence pattern was observed when cells were exposed to similar concentrations of either zein, a protein from maize (B), or bovine serum albumin negative control (C). The gliadin effect on the actin cytoskeleton was reversible as two hours after withdrawal of gliadin from the culture medium the actin cytoskeleton returned to its basal state (D). Magnification: 40×.
F-actin quantitation by spectrofluorimetry
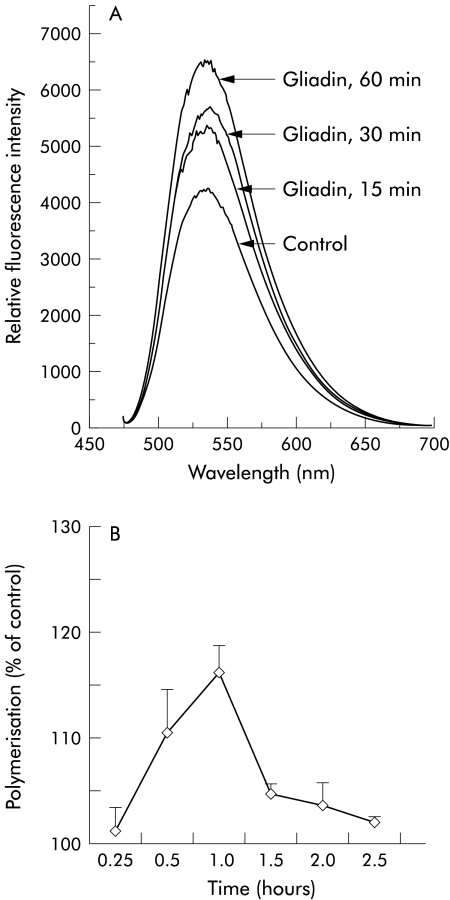
F-actin quantitation by spectrofluorimetry showed that gliadin induced a significant increase in the cellular content of polymerised actin filaments (fig 2A ▶). Figure 2B ▶ shows the time profile of actin polymerisation with a peak at 60 minutes (p<0.0001) and a return to baseline values after 2.5 hours. The temperature of +4°C failed to inhibit the gliadin induced F-actin change (data not shown), ruling out the possibility that actin polymerisation is associated with gliadin endocytosis.15
Figure 2.
F-actin quantitation by spectrofluorimetry in IEC-6 cells. (A) Gliadin (0.1 mg/ml) induced a time dependent increase in the cellular content of actin filaments, beginning as early as 15 minutes after exposure to the protein. Fluorescence was measured as relative fluorescence intensity units. (B) IEC-6 cells were exposed to gliadin 0.1 mg/ml at increasing time intervals, NBD-phallacidin extracted at the indicated time interval, and measured by spectrofluorimetry. The time profile of actin polymerisation showed a peak at 60 minutes. Actin polymerisation was expressed as per cent of control. n=4 for each time point.
Effect of gliadin on actin polymerisation is independent of new protein synthesis
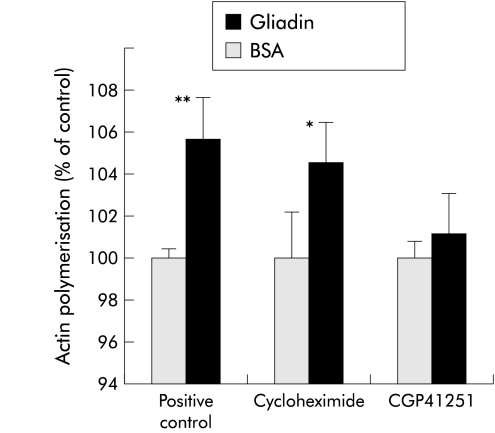
To establish whether the effect of gliadin on actin polymerisation is dependent on new protein synthesis, experiments were conducted in the presence or absence of the protein synthesis inhibitor cycloheximide. Pretreatment of cells with cycloheximide (50 μg/ml for 30 minutes prior to and throughout one hour of gliadin exposure) did not inhibit gliadin induced actin polymerisation (fig 3 ▶).
Figure 3.
Effect of cycloheximide and CGP41251 on gliadin induced cytoskeleton rearrangement. IEC-6 cells exposed to gliadin were pretreated 30 minutes before and throughout gliadin exposure with either the protein synthesis inhibitor cycloheximide or the protein kinase C (PKC) inhibitor CGP41251. Gliadin exposed cells without pretreatment served as a positive control, while bovine serum albumin (BSA) exposed cells served as negative controls. Pretreatment with cycloheximide did not affect gliadin induced actin polymerisation, suggesting that this phenomenon is independent of new protein synthesis. In contrast, pretreatment with CGP41251 completely blocked gliadin induced actin polymerisation, suggesting that the effect of gliadin on the cell cytoskeleton is PKC mediated. The results were expressed as percentage of actin polymerisation obtained in BSA exposed cells. *p<0.05, **p<0.01 compared with negative control.
Pretreatment with PKC inhibitor CGP41251
As the effects of both zonulin and Zot on actin polymerisation are PKC-α dependent9,14 we elected to establish the effect of the PKC-α inhibitor CGP41251 on gliadin induced actin polymerisation. The effect of gliadin on F-actin was almost totally inhibited by the PKC inhibitor CGP 41251 (1 μM) when added 30 minutes prior to and throughout one hour of gliadin exposure (fig 3 ▶).
Role of zonulin in gliadin induced actin polymerisation
As the effect of gliadin on the cell cytoskeleton was similar to that previously seen with both zonulin and its prokaryotic analogue Zot,16,17 we decided to determine whether gliadin induced actin polymerisation was mediated by zonulin.
Gliadin induced zonulin release from enterocytes
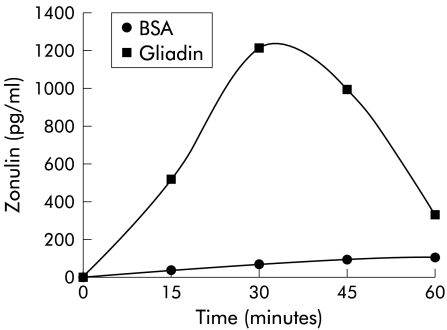
Zonulin concentration was measured by a sandwich ELISA in media of gliadin exposed cells at increasing time intervals. Zonulin was detectable in cell culture supernatants as soon as 15 minutes post-incubation with 0.1 mg/ml gliadin, reached a peak at 30 minutes, and returned to baseline after 60 minutes (fig 4 ▶). The amount of zonulin secreted was dependent on the gliadin concentration as incubation of cells with 1 mg/ml gliadin caused a peak release of zonulin of 2200 pg/ml. No detectable zonulin was found in BSA exposed cells (fig 4 ▶). Zonulin release temporarily preceded and kinetically resembled the time profile of actin polymerisation following gliadin exposure (fig 2B ▶), suggesting that gliadin induced actin polymerisation is zonulin dependent.
Figure 4.
Effect of gliadin on zonulin release from IEC-6 cells. Gliadin 0.1 mg/ml induced release of zonulin in the cell medium that peaked at 30 minutes post-gliadin incubation and returned to baseline when cells were incubated with gliadin for 60 minutes. BSA, bovine serum albumin. Each point represents the average of four determinations.
Effect of the zonulin inhibitor FZI/0 on gliadin dependent actin polymerisation
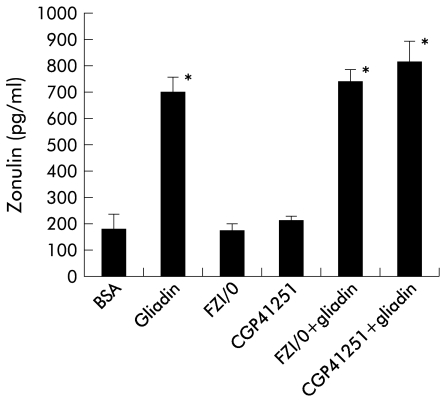
We have recently demonstrated that the effect of zonulin on the cell cytoskeleton and tj permeability can be inhibited by FZI/0, a synthetic peptide that mimics the zonulin binding domain and therefore blocks zonulin activated intracellular signalling.11 The peak of gliadin induced actin polymerisation (106.8±0.5% of baseline) was inhibited by pretreatment with FZI/0 (1 μg/ml) for 30 minutes prior to and throughout the experiment (101.8±0.5; p<0.05). Pretreatment with either the synthetic peptide FZI/0 or the PKC inhibitor CGP41251 did not inhibit zonulin release following gliadin incubation (fig 5 ▶). These results suggest that the inhibitory effect of FZI/0 on gliadin induced actin polymerisation is due to blockage of the zonulin receptor rather than an effect on zonulin release by enterocytes following gliadin exposure.
Figure 5.
Effect of the synthetic peptide FZI/0 or the protein kinase C inhibitor CGP41251 on gliadin induced zonulin release from IEC-6 cells. When added to IEC-6 cells, neither FZI/0 nor CGP41251 induced zonulin secretion. Both molecules did not affect gliadin mediated zonulin secretion, suggesting that their inhibitory effect on gliadin mediated actin polymerisation occurs downstream of secretion of zonulin from IEC-6 cells. The effects of gliadin and bovine serum albumin (BSA) on zonulin release are shown as positive and negative controls, respectively.
Effect of gliadin on intestinal permeability
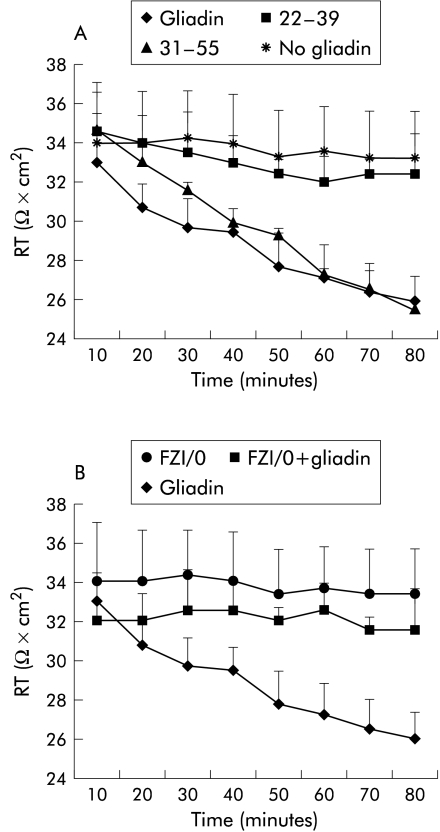
Addition of α-gliadin to rabbit intestinal mucosa mounted in Ussing chambers led to a reduction in Rt, which became significant after 30 minutes of incubation. The same effect was observed with the gliadin toxic peptide 31–55 but not with the gliadin non-toxic peptide 22–39 (fig 6A ▶). The gliadin permeating effect was reversible, with tissue Rt returning to baseline within 30 minutes of removal of the peptide from the mucosal bathing solution (data not shown). The effect of gliadin on Rt was inhibited by pretreatment with the synthetic peptide FZ1/0 (1 μg/ml; fig 6B ▶), suggesting that the effect of gliadin on Rt is zonulin dependent.
Figure 6.
Effect of gliadin on tissue epithelial electrical resistance (Rt) in rabbit intestinal mucosa mounted in Ussing chambers. (A) Addition of the α-gliadin peptide led to a significant reduction in Rt which was detected after a few minutes of incubation. The same effect was observed with the gliadin toxic peptide 31–55 but not with the gliadin non-toxic peptide 22–39. No change in Rt was observed in the absence of gliadin (no gliadin). (B) Treatment with the zonulin inhibitor FZI/0 did not affect Rt. The effect of gliadin on Rt decrement was significantly inhibited when the tissue was pretreated with FZI/0.
DISCUSSION
The intestinal epithelium is the largest mucosal surface providing an interface between the external environment and the mammalian host. Under physiological circumstances the intestine represents the primary site for active transport of fluid and electrolytes from the gut lumen through the transcellular pathway. The paracellular pathway however serves as the predominant route for passive transepithelial solute flow. Healthy mature gut mucosa with its intact tj serves as the main barrier to the passage of macromolecules. Furthermore, the intestinal barrier functions as the major organ of defence against foreign antigens, toxins, and macromolecules entering the host via the oral/enteric route. During such healthy states, quantitatively small but immunologically significant fractions of antigens cross the defence barrier. These antigens are absorbed across the mucosa along two functional pathways. The vast majority of absorbed proteins (up to 90%) cross the intestinal barrier through the transcellular pathway, followed by lysosomal degradation that converts proteins into smaller non-immunogenic peptides. The remaining portion of proteins is transported as intact molecules, resulting in antigen specific immune responses. This latter phenomenon utilises the paracellular pathway that involves subtle but sophisticated regulation of intercellular tj that can contribute to antigen oral tolerance. When the integrity of the tj system is compromised such as during prematurity or exposure to radiation, chemotherapy, and/or toxins, an immune response to environmental antigens (including autoimmune diseases and food allergies) may develop.18 The specific cells that are important for the immune response lie in close proximity to the luminal antigens and account for up to 80% of all immunoglobulin producing cells in the body.19,20 Another important factor for intestinal immunological responsiveness is the major histocompatibility complex. HLA class I and class II genes are located in the major histocompatibility complex locus on chromosome 6. These genes code for glycoproteins, which bind and shuttle peptides from the cytoplasm to the cellular membrane making up the HLA-peptide complex, which is recognised by certain T cell receptors in the intestinal mucosa.21–23
Susceptibility to at least 50 diseases, including CD, has been associated with specific HLA class I or class II alleles. A common denominator of these diseases is the presence of several pre-existing conditions leading to an autoimmune process. The first component needed to develop an autoimmune process is a genetic susceptibility for the host immune system to recognise, and potentially misinterpret, an environmental antigen presented within the gastrointestinal tract. Secondly, the host must be exposed to the antigen. Finally, the antigen must be presented to the gastrointestinal mucosal immune system following its paracellular passage (normally prevented by the tj competency) from the intestinal lumen to the gut submucosa.24–26 In all cases, increased permeability appears to precede the disease and causes an abnormality in antigen delivery that triggers the multiorgan process leading to the autoimmune response.27
While our knowledge of tj ultrastructure and intracellular signalling events have significantly progressed during the past decade,11 relatively little is known about their pathophysiological regulation secondary to extracellular stimuli. Therefore, the intimate pathogenic mechanisms of diseases in which tj are affected have remained unexplored owing to our limited understanding of the extracellular signalling involved in tj regulation.13 The discovery of Vibrio cholerae derived Zot has shed some light on the intricate mechanisms involved in the modulation of the intestinal paracellular pathway11,14,19 and has allowed us to identify an intestinal mammalian analogue that participates in tj regulation.9,10,16 This analogue, that we have named zonulin, represents a novel eukaryotic protein that reversibly opens intestinal tj.9 We have recently demonstrated that zonulin expression is increased during the early stage of CD,10 suggesting that the reported opening of tj at the early stage of the disease28,29 could be mediated by zonulin.
The studies reported in this paper were aimed at establishing the link between enterocyte gliadin exposure and zonulin release. The results of our study indicate that gliadin activates the zonulin signalling pathway in normal intestinal epithelial cells in vitro. The cellular response observed only a few minutes after gliadin incubation was characterised by significant cytoskeleton reorganisation with a redistribution of actin filaments mainly in the intracellular subcortical compartment. Spectrofluorimetry experiments revealed that such cytoskeleton reorganisation was associated with an increment in F-actin secondary to an increased rate of intracellular actin polymerisation. We can exclude the fact that the gliadin induced increment in F-actin was due to new protein synthesis as it was not affected by preincubation of cells with cycloheximide, a potent inhibitor of protein synthesis. An endocytosis dependent polymerisation of the actin filaments was also ruled out by the experiments performed at low temperature. Instead, inhibition of the effect of gliadin that was observed after pretreatment with a PKC inhibitor suggested that actin polymerisation was dependent on PKC intracellular signalling. Moreover, the experiments performed in Ussing chambers showed that addition of gliadin peptides to the intestinal mucosa in vitro caused a significant reduction in Rt within a few minutes. We have previously demonstrated30 that large proteins can cross the intestinal barrier following changes in Rt of similar magnitude (∼20% decrement from baseline values) to that induced by gliadin (see fig 6 ▶). It is therefore possible to hypothesise that gliadin induced cytoskeleton reorganisation, as observed by fluorescence microscopy and spectrofluorimetry, acts on tj structural proteins causing changes in Rt and intestinal permeability to macromolecules, including gliadin.
Interestingly, the peak of actin polymerisation detected after only 60 minutes of gliadin incubation temporarily followed zonulin release by IEC-6 cells, suggesting that this event is secondary to gliadin dependent release of zonulin. We therefore elected to investigate whether the gliadin induced cytoskeleton effect was mediated by zonulin. We have previously demonstrated that following binding to its specific surface receptor, zonulin induces actin polymerisation, followed by cytoskeleton redistribution to the subcortical cell compartment.9 The cytoskeleton changes induced by zonulin are followed by tj disassembly, leading to increased intestinal paracellular permeability.9
The experiments performed with the synthetic peptide FZI/0, which can compete and block zonulin binding to its receptor,11 showed complete inhibition of the peak of F-actin increment induced by gliadin, as well as complete inhibition of the gliadin induced reduction in intestinal Rt in vitro. However, pretreatment with the synthetic peptide failed to inhibit gliadin induced zonulin release. These results suggest that FZI/0 exerts its inhibitory effect on gliadin induced actin polymerisation by blocking the zonulin receptor rather than affecting zonulin release.
At this stage we do not know whether gliadin dependent activation of the zonulin system requires interaction of gliadin with a specific enterocyte receptor(s) or is a consequence of an unspecific response. However, the observation that gliadin induces release of zonulin and changes in Rt only when added to the mucosal aspect of the enteric epithelial cells (data not shown) suggest that the gliadin polarised effect is dependent on interaction with a surface receptor present on the brush border of the enterocyte.
Considering the results of this study and our preliminary data generated using intestinal tissues from both coeliac patients in remission and healthy controls (S Drago, A Fasano, unpublished), we can hypothesise a possible gliadin mechanism of action leading to a zonulin mediated increase in actin polymerisation and intestinal permeability. Enterocytes exposed to gliadin physiologically react by secreting zonulin in the intestinal lumen. While in normal intestinal tissues this secretion is self limited in time (see fig 4 ▶), in CD gut tissues the zonulin system is chronically upregulated,10 leading to a sustained increase in intestinal permeability to macromolecules, including gliadin, from the lumen to the lamina propria. Here, gliadin is deamidated by tissue transglutaminase and then recognised by HLA-DQ2/DQ8 bearing antigen presenting cells, triggering the onset of the CD autoimmune reaction in genetically susceptible subjects. Experiments to challenge this hypothesis are currently in progress in our laboratories.
Acknowledgments
The work was partially supported by the National Institutes of Health grant DK-48373 (AF) and a grant from the Assessorato Igiene, Sanità e Assistenza Sociale, Regione Autonoma della Sardegna, Cagliari and from the Ministero dell’Università e della Ricerca Scientifica e Tecnologica (quota 40%), Roma, Italy (SDV).
Abbreviations
CD, coeliac disease
Rt, transepithelial electrical resistance
Zot, zonula occludens toxin
tj, tight junctions
PKC, protein kinase C
BSA, bovine serum albumin
PBS, phosphate buffered saline
CV, coefficient of variation
REFERENCES
- 1.Trier JS. Diagnosis of celiac sprue. Gastroenterology 1998;115:211–16. [DOI] [PubMed] [Google Scholar]
- 2.Schuppand D. Current concepts of celiac disease pathogenesis. Gastroenterology 2000;119:234–42. [DOI] [PubMed] [Google Scholar]
- 3.Molberg O, Mcadam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in coeliac disease. Nat Med 1998;4:713–17. [DOI] [PubMed] [Google Scholar]
- 4.Pender SL, Tickle SP, Docherty AJ, et al. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol 1997;158:1582–190. [PubMed] [Google Scholar]
- 5.Fasano A. Modulation of intestinal permeability: an innovative method of oral drug delivery for the treatment of inherited and acquired human diseases. Mol Genet Metab 1998;64:12–18. [DOI] [PubMed] [Google Scholar]
- 6.Cooper BT. Intestinal permeability in coeliac disease. Lancet 1983;1:658–9. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnason I, Peters TJ, Veall N. Intestinal permeability defect in coeliac disease. Lancet 1983;1:1284–5. [DOI] [PubMed] [Google Scholar]
- 8.Schulzke JD, Bentzel CJ, Schulzke I, et al. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res 1998;43:435–41. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Uzzau S, Goldblum SE, et al. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci 2000;113:(pt 24)4435–40. [DOI] [PubMed] [Google Scholar]
- 10.Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000;355:1518–19. [DOI] [PubMed] [Google Scholar]
- 11.Lu R, Wang W, Uzzau S, et al. Affinity purification and partial characterization of the zonulin/zonula occludens toxin (Zot) receptor from human brain. J Neurochem 2000;74:320–6. [DOI] [PubMed] [Google Scholar]
- 12.Mays RW, Beck Ka, Nelson WJ. Organization and function of the cytoskeleton in polarized epithelial cell as component of the protein sorting machinery. Curr Opin Cell Biol 1994;6:16–24. [DOI] [PubMed] [Google Scholar]
- 13.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. J Biol Chem 1999;274:35179–85. [DOI] [PubMed] [Google Scholar]
- 14.Fasano A, Fiorentini C, Donelli G, et al. Zonula occludens toxin (Zot) modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest 1995;96:710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwiatkowska K, Sobota A. Signaling pathways in phagocytosis. Bioessays 1999;21:422–31. [DOI] [PubMed] [Google Scholar]
- 16.Di Pierro M, Lu R, Uzzau S, et al. Zonula occludens toxin structure-function analysis: identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem 2001;276:19160–5. [DOI] [PubMed] [Google Scholar]
- 17.Uzzau S, Lu R, Wang W, et al. Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol Lett 2001;194:1–5. [DOI] [PubMed] [Google Scholar]
- 18.Fasano A. Intestinal zonulin: open sesame! Gut 2001;49:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandtzaeg P, Halstensen TS, Kett K, et al. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology 1989;97:1562–84. [DOI] [PubMed] [Google Scholar]
- 20.Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol 1989;146:13–25. [DOI] [PubMed] [Google Scholar]
- 21.Bjorkman PJ, Saper MA, Samraoui B, et al. Structure of the human class I histocompatibility antigen, HLA-A2. Nature 1987;329:506–12. [DOI] [PubMed] [Google Scholar]
- 22.Bjorkman PJ, Saper MA, Samraoui B, et al. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature 1987;329:512–18. [DOI] [PubMed] [Google Scholar]
- 23.Cuvelier C, Mielants H, De Vos M, et al. Major histocompatibility complex class II antigen (HLA-DR) expression by ileal epithelial cells in patients with seronegative spondylarthropathy. Gut 1990;31:545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wendling D. Role of the intestine in the physiopathology of inflammatory rheumatism. Rev Rhum Mal Osteoartic 1992;59:389–92. [PubMed] [Google Scholar]
- 25.Bjarnson I, Williams P, Smethurst P, et al. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut 1986;27:1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bjarnason I, Peters TJ, Levi AJ. Intestinal permeability: clinical correlates. Dig Dis 1986;4:83–92. [DOI] [PubMed] [Google Scholar]
- 27.Fasano A. Pathological and therapeutical implications of macromolecules passage through the tight junction. In: Cereijido M, Anderson J, eds. Tight junctions. CRC Press, 2001:697–722.
- 28.Madara JL, Trier JS.Structural abnormalities of jejunal epithelial cell membranes in celiac sprue. Lab Invest 1980; 43:254–61. [PubMed] [Google Scholar]
- 29.Schulzke JD, Bentzel CJ, Schulzke I, et al. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res 1998;43:435–41. [DOI] [PubMed] [Google Scholar]
- 30.Fasano A, Uzzau S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest 1997;99:1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]