Abstract
Transient receptor potential (TRP) channel subunits form homotetramers that function in sensory transduction. Heteromeric channels also form, but their physiological subunit compositions and functions are largely unknown. We found a dominant-negative mutant of the C. elegans TRPV (vanilloid-type) subunit OCR-2 that apparently incorporates into and inactivates OCR-2 homomers as well as heteromers with the TRPV subunits OCR-1 and -4, resulting in a premature egg-laying defect. This defect is reproduced by knocking out all three OCR genes, but not by any single knockout. Thus a mixture of redundant heteromeric channels prevents premature egg laying. These channels, as well as the G-protein Gαo, function in neuroendocrine cells to promote release of neurotransmitters that block egg laying until eggs filling the uterus deform the neuroendocrine cells. The TRPV channel OSM-9, previously suggested to be an obligate heteromeric partner of OCR-2 in sensory neurons, is expressed in the neuroendocrine cells but has no detectable role in egg laying. Our results identify a specific set of heteromeric TRPV channels that redundantly regulate neuroendocrine function and show that a subunit combination that functions in sensory neurons is also present in neuroendocrine cells but has no detectable function in these cells.
TOUCH, hearing, taste, vision, smell, and temperature sensation may all rely on channels of the transient receptor potential (TRP) family to translate sensory stimuli into electrical signals (Montell 2005). These tetrameric cation channels can be homomers of identical subunits or heteromers of two or more different subunits. TRP channels have been widely studied by overexpressing homomeric channels in cultured cells or Xenopus oocytes. However, it remains unclear to what extent native TRP channels function as homomers vs. as heteromers, and what rules might govern the association of the various TRP subunits into functional heteromers.
Genetic studies can potentially reveal the physiological functions of TRP channels and whether homomers or heteromers carry out these functions. Four of the six mammalian TRPV (vanilloid-type) subunits have been knocked out in mice. TRPV1 knockouts have defects in responding to noxious stimuli (Caterina et al. 2000; Davis et al. 2000), in osmosensation by neurons of the supraoptic nucleus (Naeini et al. 2006), and in mechanosensation by urothelial cells (Birder et al. 2002). TRPV3 knockouts have a defect in thermosensation by the skin (Moqrich et al. 2005). TRPV4 knockouts have defects in sensing systemic osmotic pressure (Liedtke et al. 2000). Finally, TRPV5 knockouts have renal Ca2+-handling defects (Hoenderop et al. 2003a). It remains unclear whether these defects are due to loss of homomeric channels or due to the knockouts disrupting a more complex mixture of heteromers. In addition, TRPV subunits are expressed in overlapping patterns with other TRP subunits in tissues such as the inner ear, brain, and heart (Montell 2005), where their functions have not been revealed by the knockout studies. One reason for this could be that, as in Drosophila vision, coexpressed TRP subunits may compensate for the lack of one TRP subunit in single knockouts (Niemeyer et al. 1996; Reuss et al. 1997).
The functions of coexpressed TRPV subunits have been analyzed to some extent using genetically tractable invertebrates (Montell 2005; Kahn-Kirby and Bargmann 2006). The Caenorhabditis elegans TRPV subunit OSM-9 is found in the ciliated endings of neurons that sense touch to the nose (Colbert et al. 1997). Nose touch sensation requires both OSM-9 and a coexpressed TRPV subunit, OCR-2, which depend on each other for localization to cilia (Tobin et al. 2002). The Drosophila TRPV subunits IAV and NAN (similar to OSM-9 and OCR-2, respectively) similarly depend on each other for localization to chordotonal cilia (Kim et al. 2003; Gong et al. 2004), where they control the feedback gain during hearing (Gopfert et al. 2006). These observations led to the hypothesis that the coexpressed OSM-9/IAV and OCR-2/NAN subunits form obligate heteromers for proper localization to cilia and for function.
Here, we find that three coexpressed C. elegans TRPV subunits (OCR-1, -2, and -4) apparently form a complex mixture of functionally redundant homomeric and heteromeric TRPV channels to control neurotransmitter release from neuroendocrine cells. These channels can be inactivated by a dominant-negative OCR-2 subunit to reveal a defect in egg-laying behavior not seen in any of the single subunit knockouts. Further, the obligate partner of OCR-2 in sensory neurons, OSM-9, although coexpressed with the other channel subunits in the neuroendocrine cells, does not function with them in these cells.
MATERIALS AND METHODS
C. elegans strains:
All strains except those containing dpy-20(e1282ts) were grown at 20° and maintained using standard methods (Brenner 1974). Worms containing dpy-20(e1282ts) were grown at 25°. The strains used in this study included N2 wild type, LX671 ocr-2(vs29) IV, LX843 ocr-2(ak47) IV, CX10 osm-9(ky10) IV, LX844 ocr-1(ok132) V, LX979 ocr-1(ak46) V, LX950 ocr-4(vs137) IV, LX980 ocr-4(vs137) IV; ocr-1(ok132) V, LX981 ocr-2(ak47) ocr-4(vs137) IV, LX982 ocr-2(ak47) ocr-4(vs137) IV; ocr-1(ok132) V, LX842 ocr-2(vs29) osm-9(ky10) IV, LX748 ocr-2(ak47) osm-9(ky10) IV, LX983 ocr-2(ak47) osm-9(ky10) IV; ocr-1(ak46) V, LX984 ocr-4(vs137) osm-9(ky10) IV, MT13113 tdc-1(n3419) II, LX845 ocr-2(ak47) IV; ocr-1(ok132) V, LX669 unc-44(e362) ocr-2(vs29) dpy-20(e1282ts) IV, LX670 ocr-2(vs29) dpy-20(e1282ts) IV, LX725 ocr-2(ak47) dpy-20(e1282ts) IV, MT8189 lin-15(n765ts), and LX491 goa-1(n1134) I; lin-15(n765ts) X.
ocr-4 deletion mutant:
An ocr-4 deletion (vs137) was generated using standard C. elegans gene knockout methods (Hess et al. 2005). vs137 has a 688-bp deletion with 1 base inserted (uppercase “T” below). The resulting sequence around the deletion is: caacaacatattgcaaat … T … ttggaaaggtaggcttacactttt.
Behavioral assays:
Animals for behavioral assays were isolated as late L4 larvae and aged 11.5 hr at 20° (Figure 1C), 24 hr at 25° (Figure 2D), or 36 hr at 20° (all other figures) to obtain precisely staged adults. Numbers of unlaid or prematurely laid eggs were measured as in Chase and Koelle (2004) or Jose and Koelle (2005), respectively. Nose touch avoidance (Kaplan and Horvitz 1993) and osmotic avoidance (Hilliard et al. 2002) assays were adapted as described below. Worms were placed on agar plates with no bacteria and assayed 10–40 sec later. For nose touch avoidance, an eyelash was placed in the path of an advancing worm to cause head-on collisions and a response (stopping forward movement and starting a reversal within 3 sec) was scored. vs29 animals failed to reverse, but while ocr-2 null mutants moved over the eyelash, vs29 animals attempted to move under it. For osmotic avoidance, a 100-nl drop of 2 m fructose (Sigma, St. Louis) was placed in the path of an advancing worm and a response (stopping forward movement, starting a reversal within 3 sec, and moving away from the drop) was scored. ocr-2 null mutants briefly stopped forward movement and then advanced into the drop, whereas vs29 animals briefly stopped forward movement, initiated a short reversal, and then advanced into the drop. For transgenic experiments, animals that showed expression of the cotransformation marker were chosen from five independent transgenic lines, and 40 eggs laid by animals from each line were assayed. Student's t-test was used to calculate 95% confidence intervals for numbers of eggs shown in Figure 1, A–C. In all other assays, 95% confidence intervals for a single proportion were calculated using Wilson's estimates, and P-values for comparison of two proportions were calculated using the proportion of pooled values (Moore and McCabe 2003).
Figure 1.—
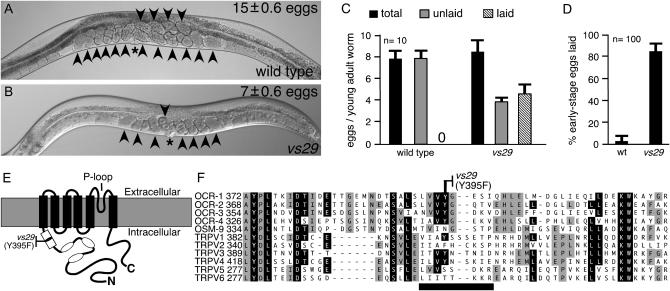
A missense mutation in the TRPV subunit OCR-2 causes eggs to be laid before the uterus is full. (A and B) vs29 animals accumulate fewer eggs than do wild-type animals. Representative wild-type (A) and vs29 (B) animals are shown. The average number of eggs accumulated by each strain, the vulva (asterisk), and eggs (arrowheads) are indicated. (C) vs29 animals begin to lay eggs earlier than do wild-type animals. Animals isolated at the late L4 stage were aged 11.5 hr. The total eggs produced during this time, the number laid, and the number retained in the uterus (unlaid) were counted. Averages for 10 animals/genotype are shown. Error bars indicate a 95% confidence interval of the mean. A 0 on the x-axis indicates that no laid eggs were seen for the wild type. (D) vs29 animals lay premature eggs. The developmental stages of freshly laid eggs (100/genotype) were determined. The percentage of eggs not yet developed beyond the eight-cell stage (early stage eggs) is indicated. Error bars indicate 95% confidence intervals. (E) TRPV channel subunit schematic. Functional channels are tetramers of such subunits. Ankyrin repeats (ovals), conserved region containing the vs29 mutation (open rectangle), transmembrane domains (solid rectangles), and pore (P)-loop are indicated. (F) vs29 mutation is in a region conserved in C. elegans and human TRPVs. Multiple sequence alignment of this region from C. elegans TRPV subunits (OCR-1–OCR-4 and OSM-9) and from human TRPV1–TRPV6. Amino acid residues identical in more than six or in five to six subunits are solid or shaded, respectively. The sequence change in vs29 is indicated and lies within a region (horizontal solid line) that is missing in the murine dominant-negative subunit TRPV1b (Wang et al. 2004).
Figure 2.—
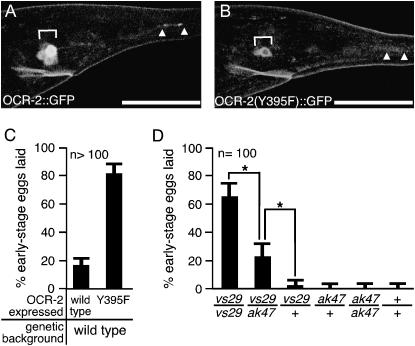
OCR-2(Y395F) is an unstable dominant-negative subunit whose effects are suppressed by wild-type OCR-2. (A and B) Expression of OCR-2∷GFP and OCR-2(Y395F)∷GFP. Fluorescence images showing expression of full-length OCR-2∷GFP (A) and full-length OCR-2(Y395F)∷GFP (B) in cell bodies (brackets) and cilia (arrowheads) of sensory neurons in the tail. OCR-2(Y395F)∷GFP is an unstable protein. Bars, 30 μm. (C) OCR-2(Y395F) dominantly causes premature egg-laying behavior. Wild-type OCR-2 or OCR-2(Y395F) was transgenically overexpressed under the control of the ocr-2 promoter and the 3′ regulatory region in a wild-type background. (D) Effect of varying dosage of ocr-2 alleles on egg laying. Animals carrying various combinations of ocr-2 alleles were assayed for premature egg laying. +, ak47, and vs29 denote wild-type, null, and vs29 alleles of ocr-2, respectively. Brackets with asterisks indicate significant differences (P < 0.05). Each strain analyzed was heterozygous for the marker mutation dpy-20(1282ts), which was necessary to verify some genotypes and was included in all others for consistency.
Transgenes:
Germline transformation was as described by Mello et al. (1991). Rescue of the egg-laying defect of vs29 was attempted using the cosmids C07G1 and T09A12 (i and ii, respectively, in supplemental Figure S1 at http://www.genetics.org/supplemental/), and a plasmid (iii in supplemental Figure S1 at http://www.genetics.org/supplemental/) with ocr-2 genomic DNA containing 2.4 kb upstream of the initiator ATG, the coding region, and 1.0 kb of downstream sequences. A total of 10 ng/μl of each clone and 10 ng/μl of myo-2∷gfp (co-injection marker, gift of A. Fire, Stanford University) was injected into vs29 animals. For OCR-2 overexpression experiments, cDNA encoding OCR-2 (gift from C. Bargmann, Rockefeller University) or encoding OCR-2(Y395F) was used to replace the ocr-2 coding region in supplemental Figure S1(iii) above. A toal of 50 ng/μl of overexpression plasmid and 10 ng/μl of myo-2∷gfp was injected into wild-type animals. To express full-length OCR-2∷GFP or OCR-2(Y395F)∷GFP, the gfp gene from pPD95.69 (gift of A. Fire) was fused to the wild-type cDNA construct above or the OCR-2(Y395F) cDNA to make fusion proteins with green fluorescent protein (GFP) precisely after the C-terminal residue of OCR-2 or OCR-2(Y395F), respectively. These were injected at 20 ng/μl with 50 ng/μl of the co-injection marker pL15EK (Moresco and Koelle 2004) into MT8189 animals. For overexpression of C. elegans TRPV genes, a genomic region for each was amplified (GeneAmp XL, Applied Biosystems, Foster City, CA), containing coding regions along with 5′ and 3′ regulatory regions extending to the neighboring genes. These were injected at 50 ng/μl with 10 ng/μl of myo-2∷gfp (co-injection marker) into vs29 animals.
For determining the expression patterns of the TRPVs, PCR fusion constructs were made on the basis of the method of Hobert (2002) as follows: 5′ and 3′ regulatory regions (extending to the neighboring genes) were amplified and fused by overlap extension PCR to the gfp gene amplified from pPD95.69. These were injected at 20 ng/μl along with the coinjection marker pL15EK (50 ng/μl) into MT8189 animals. We also made reporter constructs analogous to those of Tobin et al. (2002) lacking the 3′ untranslated region (UTR) of ocr-2: the 5′ regulatory region or the 5′ regulatory region plus the coding region through the third exon was fused to gfp coding sequences in pPD95.69 to make transcriptional (pAJ12) or translational (pAJ11) reporters, respectively. These GFP constructs were coinjected with pL15EK (50 ng/μl) into MT8189 animals. Injection of pAJ11 and pAJ12 at 200 ng/μl labeled the same cells as the PCR fusion products, but uv1 and a number of other cells were much more strongly labeled by the PCR fusion products injected at 20 ng/μl, apparently because the PCR fusion products contained the ocr-2 3′ UTR lacking in pAJ11 and pAJ12.
The coding sequences of ocr-2 rescuing construct iii (in supplemental Figure S1 at http://www.genetics.org/supplemental/) were replaced with coding sequences for the S1 subunit of pertussis toxin PTx to inactivate Gαo or with the light chain of tetanus toxin TTx to inactivate neurotransmitter release. A total of 10 ng/μl of toxin construct and 10 ng/μl of pAJ12 (co-injection marker) was injected into wild-type animals. Our TTx cDNA had a polymorphism that changes Ile 361 to Val. Although this change is not expected to make the toxin inactive, a reduction in its efficiency cannot be ruled out. A transgene (pMK376) expressing functional GOA-1∷GFP was made based on the work of Hughes et al. (2001) by inserting gfp coding sequences into goa-1 genomic DNA such that GFP was inserted into an internal loop of the G protein. Specifically, the sequences coding a flexible linker (SGGGGS), full-length GFP, and another linker (SGGGTS) were placed between goa-1 codons for T117 and E118. pMK376 was injected at 5 ng/μl with 50 ng/μl pL15EK (co-injection marker) into LX491 animals.
Sequence analysis:
Multiple sequence alignments of human and C. elegans TRPVs were performed using Clustal W and Megalign (Lasergene). The accession numbers of the sequences used are the following: OCR-1, REFSEQ: NP_505748; OCR-2, REFSEQ: NP_501380; OCR-3, REFSEQ: NP_510520; OCR-4, REFSEQ: NP_501172; OSM-9, REFSEQ: NP_500372; TRPV1, REFSEQ: NP_542437; TRPV2, REFSEQ: NP_057197; TRPV3, REFSEQ: NP_659505; TRPV4, SWISSPROT: Q9HBA0; TRPV5, REFSEQ: NP_062815; TRPV6, REFSEQ: NP_061116.
Imaging:
Confocal fluorescence images were obtained using an LSM-510 confocal microscope (Zeiss). We imaged two worms that expressed the co-injection marker from each of five independent transgenic lines expressing either OCR-2∷GFP or OCR-2(Y395F)∷GFP (a total of 10 worms/transgene). The fluorescence in the phasmid sensory neurons of the tail of each animal was measured (ImageJ).
Mosaic analysis:
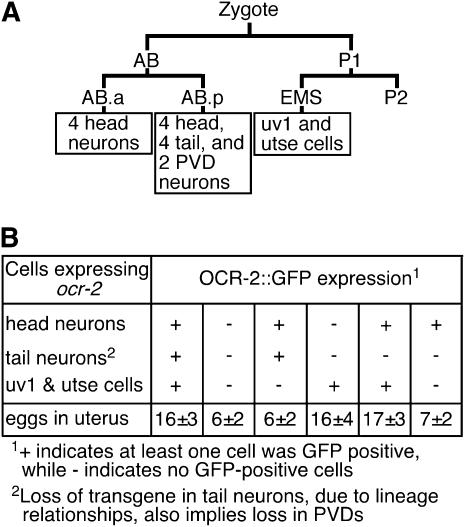
The OCR-2∷GFP construct (20 ng/μl) was injected along with the co-injection marker pL15EK (50 ng/μl) into ocr-2(vs29); lin-15(n765ts) animals to obtain a mosaic transgenic line. To determine the cells in which OCR-2 functions to control egg-laying behavior, rescue of the egg-laying defect in ocr-2(vs29) was correlated with OCR-2∷GFP expression (Figure 5). We counted the number of eggs in the uteri of animals grown at 15° to the L4 larval stage and then aged for 36 hr at 20°. Presence or absence of OCR-2∷GFP expression in head neurons (AWA, ASH, ADL, ADF, and two unidentified neurons), tail neurons (PHA and PHB), and the uv1/utse cells was scored for each animal. If OCR-2∷GFP was expressed even in one of the cells in the above three groups, the mosaic animal was scored as having OCR-2∷GFP expression in that group. More than 150 animals were screened for gfp expression to obtain 10 animals to age at 20° for each mosaic pattern.
Figure 5.—
OCR-2 functions in the uv1 and utse cells to control egg-laying behavior. (A) Schematic showing the lineal origins of cells that express OCR-2. The AB.a-derived neurons are ADLL, ADLR, ADFL, and ADFR (four head neurons). The AB.p-derived neurons are AWAL, AWAR, ASHL, and ASHR (four head neurons); PHAL, PHAR, PHBL, and PHBR (four tail neurons); and PVDL and PVDR (PVDs). Two additional unidentified head neurons that express OCR-2 are not shown, but are likely AB derived, since AB is the precursor of the vast majority of neurons. The lineal separation of uv1 and utse from all other OCR-2-expressing cells makes it convenient to isolate genetic mosaics to assess the function of OCR-2 in uv1 and utse. (B) OCR-2∷GFP expression in the uv1 and utse cells is necessary and sufficient to rescue the ocr-2(vs29) egg-laying defect. ocr-2(vs29) animals transformed with an extrachromosomal transgene that expresses full-length OCR-2 fused to GFP (OCR-2∷GFP) under the control of the ocr-2 promoter and the 3′ regulatory regions were scored for the presence of GFP fluorescence in individual cells and for the number of eggs held in utero. During development, the extrachromosomal transgene is lost in a mosaic manner, resulting in the expression of OCR-2∷GFP (as evidenced by GFP fluorescence) in all, none, or a subset of the cells that express OCR-2. Only animals that expressed OCR-2∷GFP in the uv1 and utse cells held a normal number of eggs in utero (16 ± 4 in animals with expression only in uv1 and utse cells vs. 15 ± 0.6 eggs in wild-type animals). Ten animals were scored for each mosaic pattern of OCR-2∷GFP expression. Errors indicate standard deviation.
RESULTS
A mutation in the TRPV subunit OCR-2 causes premature egg-laying behavior:
Several neurotransmitters signal to inhibit C. elegans egg-laying behavior (Bany et al. 2003; Moresco and Koelle 2004; Alkema et al. 2005). To identify molecules involved in such neurotransmission, we screened for mutants that exhibit increased egg-laying behavior (Bany 2004). In this study we present the analysis of one mutant, vs29, isolated in this screen.
The uterus of a wild-type animal on average accumulates 15 eggs due to a delay between egg production and egg laying (Figure 1A). In contrast, vs29 mutant animals on average accumulate only 7 eggs (Figure 1B). We found that wild-type adults assayed shortly after the larval-to-adult transition had made ∼8 eggs/worm, but retained all these eggs in the uterus, indicating that egg-laying behavior had not yet begun (Figure 1C). vs29 adults assayed at precisely the same developmental stage also had made 8 eggs/worm, but had already laid 4 of them (Figure 1C), indicating that egg-laying behavior had begun too early in vs29 animals. Thus wild-type animals inhibit egg laying until the uterus fills, while vs29 animals lay eggs before the uterus fills and thus fail to accumulate a normal number of eggs.
Fertilized C. elegans embryos (eggs) develop independently of whether they are retained in the uterus or laid. Since there is a delay between egg production and egg laying, the eggs of wild-type animals develop in the uterus such that all but 4% have progressed beyond the eight-cell stage by the time they are laid (Figure 1D). However, the eggs of vs29 animals are laid prematurely such that 85% are at the eight-cell stage or earlier when they are laid (Figure 1D). Thus, quantifying the percentage of early stage eggs laid provides a sensitive measure of premature egg-laying behavior.
We mapped the vs29 mutation to an 80-kb region on chromosome IV (supplemental Figure S1A at http://www.genetics.org/supplemental/). A multicopy transgene containing only the wild-type TRPV subunit gene ocr-2 from this region was able to rescue the premature egg-laying defect of vs29 mutants (supplemental Figure S1B at http://www.genetics.org/supplemental/). OCR-2, like all TRPV subunits, contains three ankyrin repeats in its intracellular N terminus, followed by six transmembrane domains (Figure 1E; Tobin et al. 2002). Sequencing the coding regions of the ocr-2 gene in vs29 revealed a point mutation that changes Tyr 395 to Phe. This Tyr residue lies in a region conserved in all TRPV subunits just N-terminal to the first transmembrane domain (open rectangle, Figure 1E) whose function has not been studied. The Tyr residue mutated in vs29 is conserved in most human and C. elegans TRPV subunits (Figure 1F).
A dominant-negative activity has recently been demonstrated for a minor splice variant of murine TRPV1 that lacks 10 amino acids (thick horizontal solid line, Figure 1F), including the tyrosine corresponding to Y395 of OCR-2. Coexpression of this minor splice variant (TRPV1b) with the major TRPV1 isoform (TRPV1a) in Xenopus oocytes strongly suppressed capsaicin-evoked currents that were seen when the major isoform was expressed alone (Wang et al. 2004). Further, TRPV1b was unstable and functioned as a dominant negative by heteromerizing with TRPV1a and destabilizing as well as inactivating the heteromer. These results suggest that OCR-2(Y395F) may function as a dominant negative by a similar mechanism.
OCR-2(Y395F) is an unstable protein that causes sensory defects seen in ocr-2 null mutants and an additional dominant premature egg-laying defect:
To test the hypothesis that OCR-2(Y395F) is an unstable protein that acts as a dominant-negative subunit by inactivating heteromers, we expressed both Y395F mutant and wild-type OCR-2 subunits in Xenopus oocytes. However, we failed to obtain current with wild-type OCR-2 using several stimuli known to open other TRPV channels (J. Chang, L. Heginbotham, A. M. Jose and M. R. Koelle, unpublished results), as had been seen previously by (Tobin et al. 2002), even upon coexpression of OSM-9, a subunit that functions with OCR-2 in sensory neurons. Thus, OCR-2 may require an as-yet-unknown gating stimulus or other components not present in Xenopus oocytes. We therefore examined OCR-2 and OCR-2(Y395F) stability and function in their native context.
To test if OCR-2(Y395F) is an unstable protein in vivo, we generated transgenic animals expressing fusions of full-length OCR-2 or OCR-2(Y395F) to GFP (Figure 2, A and B). The transgene expressing OCR-2∷GFP rescued the egg-laying defect seen in ocr-2(vs29) animals [transgenic animals had 16 ± 3 eggs held in utero vs. 7 ± 0.6 eggs in nontransgenic ocr-2(vs29) animals]. We examined five independent transgenic strains expressing each construct and measured the levels of the fusion proteins in tail sensory neurons, which had previously been shown to localize OCR-2∷GFP to the cell body and to sensory cilia (Tobin et al. 2002). Consistent with OCR-2(Y395F) being an unstable protein, we found that significantly less OCR-2(Y395F)∷GFP was present in tail sensory neurons compared to OCR-2∷GFP (12.1 ± 8.1 vs. 46.3 ± 8.2 fluorescence units/cell, P < 0.0002). OCR-2∷GFP was found in both the cell body and the sensory cilia, but OCR-2(Y395F) could be detected only in cell bodies.
To assess the function of OCR-2(Y395F) in vivo, we compared the behavioral defects in ocr-2(vs29) animals and ocr-2 null mutant animals. The ocr-2 null mutants have defects in multiple sensory behaviors, but have no egg-laying defects (Tobin et al. 2002). In an assay of mechanosensation, most wild-type animals reverse in response to nose touch, but both ocr-2 null mutants and ocr-2(vs29) animals failed to reverse (Table 1). In an assay of osmosensation, most wild-type animals moved away from a drop of high osmolarity solution, but both ocr-2 null mutants and ocr-2(vs29) animals failed to move away from the drop (Table 1). Thus sensory defects of ocr-2 null mutants were also present in ocr-2(vs29) animals. In contrast to the similarity of their sensory defects, ocr-2(vs29) animals were distinct from ocr-2 null mutants in their egg-laying behavior. While ocr-2(vs29) animals showed premature egg-laying behavior (laid 85% early stage eggs), ocr-2 null mutants, like wild-type animals, laid very few early stage eggs (Table 1). The fact that ocr-2(vs29) animals have a premature egg-laying defect not seen in the ocr-2 null mutant suggests that OCR-2(Y395F) does not simply lack all function, but rather actively causes the premature egg-laying defect.
TABLE 1.
ocr-2(vs29) animals are like ocr-2(ak47) mutants in sensory behaviors but not in egg-laying behavior
| % response to:
|
|||
|---|---|---|---|
| Genotype | Nose touch | Fructose | % early stage eggs |
| Wild type | 90 ± 6.1 | 88 ± 6.0 | 4 ± 4.5 |
| ocr-2(ak47) | 22 ± 8.1 | 10 ± 6.1 | 3 ± 4.1 |
| ocr-2(vs29) | 24 ± 8.3 | 24 ± 8.3 | 85 ± 7.1 |
Both ocr-2 null mutants [ocr-2(ak47)] and ocr-2(vs29) animals were defective in mechanosensation (response to nose touch) and in osmosensation (response to fructose). However, in contrast to ocr-2 null mutants, ocr-2(vs29) animals additionally laid a high percentage of early stage eggs. Errors indicate 95% confidence interval.
To test if OCR-2(Y395F) acts dominantly to cause the egg-laying defect, we transgenically overexpressed wild-type OCR-2 or OCR-2(Y395F) in wild-type animals (Figure 2C). Whereas the wild-type protein had little effect, OCR-2(Y395F) caused a dramatic premature egg-laying defect. Since some mechanosensitive channels (Chalfie and Wolinsky 1990) and TRP channels (Yoon et al. 2000) can be mutated to generate constitutively open channels that cause neurodegeneration, the observed effect of OCR-2(Y395F) may be caused by degeneration of cells required to control egg laying. We examined the morphology of cells that express OCR-2 by differential interference and fluorescence microscopy after labeling all of these cells with GFP or after labeling a subset with the vital dye DiO (data not shown). We saw no defects in these cells, indicating that no degeneration had occurred. To further investigate how the vs29 mutation affected ocr-2 function, we looked at the effects of varying the number of vs29 mutant and wild-type copies of the ocr-2 gene. We found that reducing the number of copies of vs29 from two to one reduced the severity of premature egg laying (compare vs29/vs29 vs. vs29/ak47, Figure 2D), further demonstrating that vs29 actively causes premature egg laying in a dosage-dependent manner. Furthermore, one copy of wild-type ocr-2 significantly suppressed the defect caused by one copy of vs29 (compare vs29/ak47 vs. vs29/+, Figure 2D). This suggests that wild-type OCR-2 subunits compete with the OCR-2(Y395F) subunits produced by ocr-2(vs29) to prevent the mutant subunits from causing premature egg laying. The fact that a single wild-type copy of ocr-2 outcompetes the dominant-negative ocr-2(vs29) to cause recessive inheritance (compare vs29/+ vs. +/+, Figure 2D) may be due to the fact that the vs29 mutation produces an unstable protein.
The above results show that OCR-2(Y395F) lacks normal OCR-2 function, but actively causes premature egg laying in a dosage-dependent manner, and that this active function can be suppressed by wild-type OCR-2. Since TRP subunits form homo- and heterotetramers (Xu et al. 2000; Kedei et al. 2001; Tobin et al. 2002; Hoenderop et al. 2003b; Strubing et al. 2003; Chubanov et al. 2004; Gong et al. 2004), one model consistent with all our results is that incorporation of a OCR-2(Y395F) subunit makes a TRP tetramer nonfunctional and that having a high ratio of wild-type to Y395F mutant subunits allows functional tetramers that exclude Y395F subunits to form. Using this model, the premature egg laying of the vs29 mutant can be explained if the OCR-2(Y395F) subunit, in addition to producing nonfunctional OCR-2 homomers, also incorporates into and makes nonfunctional tetramers containing other TRP subunits that regulate egg laying.
Multiple TRP subunits, including OCR-1, OCR-2, and OCR-4, control egg-laying behavior together:
For an in vivo test of our model that OCR-2(Y395F) makes TRP heterotetramers nonfunctional, we reasoned that overexpressing any TRP subunit that heteromerizes with OCR-2 should sequester Y395F subunits, allow formation of functional tetramers, and thus suppress the premature egg-laying defect of the ocr-2(vs29) mutant.
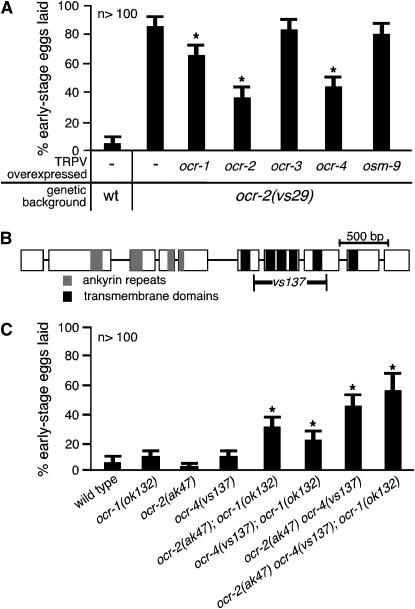
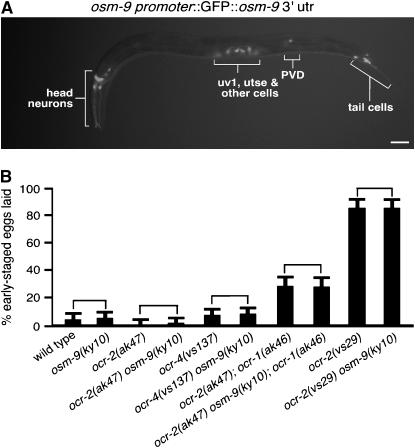
Accordingly, we transformed the ocr-2(vs29) mutant with genomic DNA for each C. elegans TRPV gene (ocr-1, -2, -3, -4, or osm-9) to generate high-copy transgenes that overexpressed individual subunits. We found that overexpression of wild-type OCR-1, -2, or -4 significantly suppressed the premature egg-laying defect of ocr-2(vs29) animals (Figure 3A), while OCR-3 and OSM-9 had no effect. This suggests that wild-type OCR-1, -2, and -4 subunits can heteromerize with and sequester OCR-2(Y395F) subunits.
Figure 3.—
OCR-2(Y395F) likely inactivates OCR-1, -2, and -4 to cause premature egg laying. (A) Overexpression of ocr-1, ocr-2, or ocr-4 can suppress the premature egg-laying defect of ocr-2(vs29) animals. Genomic DNAs for the C. elegans TRPV genes (ocr-1, -2, -3, -4, or osm-9) were transformed into ocr-2(vs29) animals as multicopy transgenes to overexpress individual TRPV subunits. Asterisks denote significant suppression (P < 0.05) compared to ocr-2(vs29) animals lacking transgenes. wt, wild type. (B) Schematic of the ocr-4 deletion mutation: ocr-4 exons (open boxes) and introns (lines connecting boxes), sequence-coding ankyrin repeats (shaded) and transmembrane domains (solid), and the extent of the deletion (vs137) are indicated. (C) OCR-1, -2, and -4 function partially redundantly to prevent premature egg laying. Asterisks denote significant defects (P < 0.05) compared to the wild-type control (leftmost bar). No single mutant showed a significant defect, but double and triple knockouts all showed significant defects.
Since our model hypothesizes that OCR-2(Y395F) causes premature egg laying by incorporating into heterotetramers with OCR-1, -2, and -4 subunits to make them nonfunctional, it predicts that knocking out the ocr-1, ocr-2, and ocr-4 genes together should also cause premature egg laying. We obtained the previously characterized ocr-1 and ocr-2 null mutants (Tobin et al. 2002) and isolated an ocr-4 deletion expected to be a null mutation as it results in a protein product lacking four of the six transmembrane domains as well as part of the pore region (Figure 3B). None of the single mutants showed significant premature egg laying (Figure 3C). We then generated every double- and triple-mutant combination of the ocr-1, ocr-2, and ocr-4 null mutants. In contrast to the single mutants, all double-mutant combinations, as well as the triple mutant, showed significant premature egg-laying behavior (Figure 3C). Since no single knockout shows a defect, but any combination of the knockouts does, these channel subunits act partially redundantly. We conclude that OCR-1, -2, and -4 can all heteromerize with OCR-2(Y395F) and that OCR-1, -2, and -4 likely assemble into a mixture of homo- and heterotetramers that function together to prevent premature egg laying. If any one of the subunits is absent, the remaining subunits can form channels that provide function.
We note that the premature egg-laying defect of the triple mutant (55 ± 10% early stage eggs laid) was significantly weaker than that of the ocr-2(vs29) single mutant (85 ± 7% early stage eggs laid). This suggests that OCR-2(Y395F) has an effect beyond inactivating the TRPV subunits OCR-1, -2, and -4. There could be additional TRP subunits that function with OCR-1, -2, and -4 in egg laying. There are 12 additional TRP subunits in C. elegans beyond the TRPV subfamily (Montell 2005) that could play a role, since TRP subunit interactions can occur between members of different subfamilies (Tsiokas et al. 1999).
Taken together, our results suggest that OCR-1, OCR-2, and OCR-4 function as partially redundant mixed heteromers to regulate egg-laying behavior. OCR-2(Y395F) likely inactivates these as well as heteromers containing other as-yet-unidentified TRP subunits.
All three TRPV subunits controlling egg-laying behavior are coexpressed only in a set of uterus-associated endocrine cells:
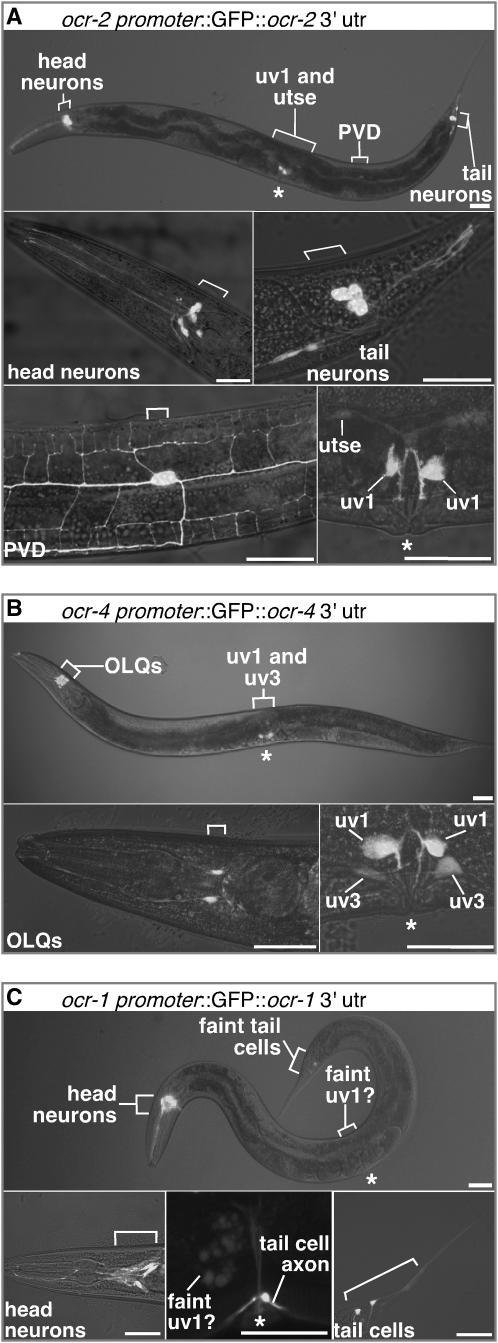
A requirement of the above model is that the OCR-1, -2, and -4 subunits are coexpressed in cell(s) that regulate egg laying. To examine the expression pattern of these three TRPV subunits, we made GFP reporter transgenes. GFP reporters for these genes have been described previously (Colbert et al. 1997; Tobin et al. 2002), but showed no expression in cells known to control egg laying. We constructed reporters that included additional regulatory sequences that did reveal such expression. We found OCR-2 reporter expression in sensory neurons, as had been seen by Tobin et al. (2002). These sensory neurons have no known role in egg laying. In addition, our OCR-2 reporter was expressed in cells of the egg-laying system (Figure 4A and supplemental Table S1 at http://www.genetics.org/supplemental/). These were the four uterus-associated uv1 cells attached to the ventral surface of the uterus, as well as the syncytial uv1-associated cell utse. In adults, OCR-2 reporter expression was much stronger in uv1 than in utse (Figure 4A, bottom right); in larval animals, it was the reverse (data not shown).
Figure 4.—
TRPV subunits that control egg-laying behavior are coexpressed only in uv1, the uterus-associated endocrine cells. (A–C) Expression patterns of GFP reporter constructs for TRPV subunits that control egg-laying behavior. Overlays of fluorescence and bright-field images of animals carrying GFP reporter transgenes for OCR-2 (A), OCR-4 (B), and OCR-1 (C). Bars, 30 μm. Fluorescent utse, uv1, and uv3 cells are labeled. Other cell bodies (brackets) and the vulva (*) are also indicated. Bottom right of A and B show expression in the two left uv1 cells, although the two right uv1 cells also expressed the reporter. For clarity, these and all subsequent confocal images of uv1 show only the left side.
Eggs are laid by passing from the uterus, where they are stored, through a ring formed by the utse, then through another ring formed by the four uv1 cells, and finally through the vulva (Newman and Sternberg 1996). The uv1 cells contain the neurotransmitter tyramine, which is known to inhibit egg laying (Alkema et al. 2005). They also contain the proteins synaptotagmin (Nonet et al. 1993), syntaxin (Saifee et al. 1998), and UNC-13 (J. Moresco, A. M. Jose, and M. R. Koelle, unpublished observations) that function in neurotransmitter release. As the uv1 cells do not make synapses (White et al. 1986), they appear to be endocrine cells that secrete tyramine and other neurotransmitters to inhibit egg laying.
The reporter construct for OCR-4 was expressed in adult uv1 cells (Figure 4B). In larvae, it was expressed in the precursor of the uv1 and utse, called VU, and its sister cell DU (Kimble and Hirsh 1979; Newman et al. 1996). One of the descendants of DU, uv3, retains detectable reporter expression in the adult (Figure 4B, bottom right). Our OCR-4 reporter was also expressed in the mechanosensory OLQ neurons (Kaplan and Horvitz 1993), previously shown to express a GFP reporter for OCR-4 by Tobin et al. (2002). Since the OCR-2 reporter was not expressed in OLQ neurons, the only site of overlap between expression of the OCR-2 and OCR-4 reporters was the uv1 endocrine cells (Figure 4; supplemental Table S1 at http://www.genetics.org/supplemental/).
The OCR-1 reporter was expressed in the head in AWA and ADL sensory neurons that also express the OCR-2 reporter, as had been seen by Tobin et al. (2002). Additionally, we saw expression in other neurons and in a few non-neuronal cells (Figure 4C; supplemental Table S1 at http://www.genetics.org/supplemental/). Significantly, we detected faint and occasional expression at the position of the uv1 cells (Figure 4C, bottom middle), although a definitive identification of the fluorescing cells was not possible due to the faintness of the signal.
The uterus-associated uv1 endocrine cells appear to be the only sites of coexpression of the OCR-1, -2, and -4 reporters. Further, the uv1 cells are also the only cells expressing any TRPV reporter that have been hypothesized to have a role in egg laying (Alkema et al. 2005).
OCR-2 functions in the uterus-associated endocrine cells to control egg laying:
To determine the cell(s) in which OCR-2 functions to control egg laying, we performed a mosaic analysis (Figure 5). We transformed ocr-2(vs29) animals with an extrachromosomal transgene that expresses full-length OCR-2 fused to GFP under the control of the ocr-2 promoter and 3′ regulatory regions. The transgene is lost in a mosaic manner during C. elegans development, and the pattern of mosaicism in individual animals was determined by scoring for the presence of GFP fluorescence in individual cells. We found that animals expressing OCR-2∷GFP in the uv1/utse cells were rescued for the premature egg-laying defect caused by ocr-2(vs29), while mosaic animals lacking expression in the uv1/utse cells but retaining expression in other cells were not rescued (Figure 5B). In contrast, presence of the transgene in other cells did not correlate with rescue of the ocr-2(vs29) premature egg-laying defect (Figure 5B). Thus OCR-2 appears to function exclusively in the uv1/utse cells to control egg laying. Our mosaic analysis for ocr-2, along with expression patterns described above, leads us to conclude that the uv1 and associated utse cells are the site of coexpression of OCR-1, -2, and -4 where these TRPV subunits form heteromeric channels that regulate egg-laying behavior.
Only a subset of TRPV channels in the uv1 endocrine cells function to control egg-laying behavior:
Although overexpression of OSM-9 failed to suppress the egg-laying defect of ocr-2(vs29), osm-9 was expressed in all cells that express ocr-2, including the uv1 and utse cells of the egg-laying system (Figure 6A; supplemental Table S1 at http://www.genetics.org/supplemental/). This is particularly surprising since OSM-9 and OCR-2 form obligate heteromers in sensory neurons, where they require each other for their mutual subcellular localization and function (Tobin et al. 2002). Since it is possible that the failure of OSM-9 to suppress the egg-laying defect of ocr-2(vs29) could be due to insufficient levels of osm-9 overexpression, we further examined the role of osm-9 in egg laying. We found that an osm-9 knockout caused no effect on premature egg laying, either alone or in combination with ocr-1, -2, and -4 mutations (Figure 6B). Taken together, our results suggest that although OSM-9 and OCR-2 function as obligate heteromers in sensory neurons, this heteromer either does not form or does not function in the uv1 endocrine cells to control egg laying.
Figure 6.—
OSM-9 is expressed in the uv1 cells but has no detectable effect on egg-laying behavior. (A) Overlay of fluorescence and bright-field image of an animal carrying a GFP reporter transgene for OSM-9. Bar, 30 μm. (B) osm-9 null mutations do not have any detectable effects on egg-laying behavior. Comparisons (brackets) of double- and triple-mutant combinations of the osm-9 null mutation with null mutations in other C. elegans TRPV subunits or with ocr-2(vs29) show no effect of OSM-9 on egg-laying behavior. Error bars indicate 95% confidence intervals.
uv1 endocrine cells are mechanically deformed by eggs and require neurotransmitter release as well as the G-protein Gαo to prevent premature egg laying:
We examined the uv1 cells to investigate how they might regulate egg laying. We used a GFP reporter that is expressed in uv1, utse, and some surrounding cells to visualize the anatomy of these cells in living animals (Figure 7, A and B). The appearance of the uv1 cells varied from animal to animal and from time to time within a single animal, depending on the exact positions of eggs in the uterus that mechanically deformed the uv1 cells. This can be visualized using three-dimensional reconstructions of confocal fluorescence images, which show that each uv1 cell was deformed if there was an egg in the underlying uterus, forcing the uv1 cell body to curve and follow the contour of the egg (supplemental Figure S2 at http://www.genetics.org/supplemental/). Thus uv1 cells have the potential to mechanically sense the presence of eggs in the uterus.
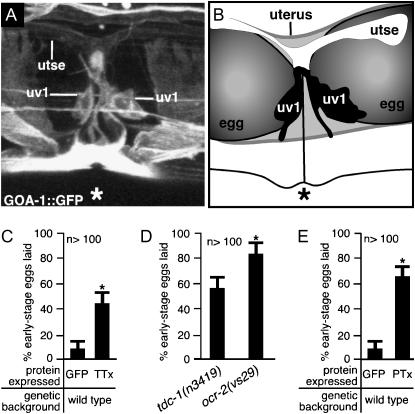
Figure 7.—
uv1 cells release neurotransmitters and require the G-protein GOA-1 to inhibit egg laying until the uv1 cells are mechanically deformed. (A and B) uv1 cells are deformed by eggs in the uterus. The uv1 cells, utse, and the vulva (*) are indicated. (A) A full-length GOA-1∷GFP fluorescent reporter is expressed strongly in uv1, weakly in utse, and in nearby cells, allowing visualization of anatomy in living animals. This projection of a three-dimensional confocal image is shown in various rotations in supplemental Figure S2 at http://www.genetics.org/supplemental/. (B) Tracing from the fluorescence image shown in A and a corresponding bright-field image showing the uv1 cells, utse, and eggs in the uterus. (C) Blocking neurotransmission in ocr-2-expressing cells causes premature egg laying. The ocr-2 promoter and the 3′ regulatory region were used either to express the light chain of tetanus toxin (TTx) to block neurotransmission or to express control GFP. Error bars indicate 95% confidence intervals, and asterisks denote significant differences (P < 0.05) in C–E. (D) ocr-2(vs29) animals have a more severe premature egg-laying defect than do animals lacking TDC-1, the biosynthetic enzyme for tyramine. (E) Inactivating the G-protein GOA-1 in ocr-2-expressing cells causes premature egg laying. The ocr-2 promoter and the 3′ regulatory region were used either to express the S1 subunit of pertussis toxin (PTx) to inactivate GOA-1 or to express control GFP (data replotted from C).
How might the uv1 endocrine cells control egg laying? While laser ablation is often used to assess cell function in C. elegans, the uv1 cells appear to be a structural component of the anatomy required for egg laying, and killing these cells is thus not a suitable method for assessing their role in regulating egg-laying behavior. So, we used a toxin to interfere with uv1 cell function and measured effects on egg laying. We used the ocr-2 promoter and 3′ regulatory region to express the light chain of tetanus toxin, a protease that cleaves synaptobrevin and prevents neurotransmitter release from neurons (Sweeney et al. 1995) and endocrine cells (Nemoz-Gaillard et al. 1998). The resulting transgenic animals exhibited premature egg-laying behavior (Figure 7C), suggesting that neurotransmitters released from uv1 or other cells that express ocr-2 inhibit egg laying. The neurotransmitter tyramine is synthesized in the uv1 (but in no other cells that express ocr-2) and inhibits egg-laying behavior (Alkema et al. 2005). Although null mutants of the biosynthetic enzyme that produces tyramine (TDC-1) have a strong premature egg-laying defect (Alkema et al. 2005), this defect is weaker than that of ocr-2(vs29) animals (Figure 7D), suggesting that uv1 cells release other neurotransmitters in addition to tyramine to inhibit egg laying. The uv1 cells also express the neuropeptide genes flp-11 and flp-22 (Kim and Li 2004), and these may play a role in egg-laying behavior. Thus, our results suggest that tyramine and other neurotransmitters released by the uv1 cells prevent premature egg laying and that in ocr-2(vs29) mutant animals this release is blocked. Neurotransmitter release can be triggered by Ca2+ entry through transiently expressed TRP channels (Obukhov and Nowycky 2002). Thus C. elegans TRPV channel activity might similarly promote release of neurotransmitters that inhibit egg laying.
What might gate TRPV activity in the uv1 cells? Since TRPV channels are known to mediate mechanosensation (O'Neil and Heller 2005), the mechanical deformation of the uv1 cells caused by eggs in the uterus may directly inactivate TRPVs in the uv1 cells to end the inhibition of egg laying. Alternatively, TRP channel function can also be regulated by heterotrimeric G-protein signaling (Montell 2005). In C. elegans, Tobin et al. (2002) showed that sensory neurons of the head require the TRPV channel OCR-2 and the Gα protein ODR-3 to respond to mechanical and osmotic stimuli. ODR-3 is expressed only in sensory neurons of the head and thus cannot function with TRPV channels in the egg-laying system. However, the C. elegans Gαo G-protein ortholog GOA-1 could play such a role, since goa-1 null mutants have a premature egg-laying defect (Mendel et al. 1995; Ségalat et al. 1995). To examine if GOA-1 is expressed in the uv1 cells, we made a GOA-1 reporter construct by inserting GFP-coding sequences in frame into the coding region of a goa-1 genomic clone (see materials and methods). The resulting transgene expresses a functional GOA-1∷GFP fusion protein that rescues the premature egg-laying defect of goa-1 mutants (J. Tanis and M. R. Koelle, data not shown). In addition to being expressed in most neurons and in the muscles of the egg-laying system, as seen with previous goa-1 reporters (Mendel et al. 1995; Ségalat et al. 1995), our functional reporter also showed expression in the uv1 and utse cells (Figure 7A). To test if GOA-1 inhibits egg laying by functioning in the cells that express ocr-2, we inactivated GOA-1 specifically in these cells. To do this, we used the ocr-2 promoter and 3′ regulatory region to express the S1 subunit of pertussis toxin, a potent inactivator of GOA-1 that, when expressed in all tissues, results in animals that are indistinguishable from goa-1 null mutants (Darby and Falkow 2001). The resulting transgenic animals showed a strong premature egg-laying defect (Figure 7E). Since the only ocr-2-expressing cells involved in egg laying appear to be the uv1 cells and perhaps their associated utse cell, GOA-1 appears to function along with TRPV channels in uv1/utse cells to inhibit egg laying.
GOA-1 was previously suggested to act in the HSN motor neurons to inhibit release of neurotransmitters that stimulate egg laying, thus inhibiting egg laying (Mendel et al. 1995; Ségalat et al. 1995; Shyn et al. 2003; Moresco and Koelle 2004). However, this work also showed that there must be an additional site outside of the HSNs where GOA-1 also acts to inhibit egg laying (Ségalat et al. 1995; Shyn et al. 2003). Our work now identifies such an additional site of action as the uv1 neuroendocrine cells. Curiously, GOA-1 acts in both the HSNs and uv1s to inhibit egg laying, but does so in one case (HSNs) by inhibiting neurotransmitter release and in the other (uv1s) by promoting neurotransmitter release. Further work is needed to understand this difference and to explore the possibility that neurotransmitters released from the HSNs might affect egg laying by acting on the uv1s, or vice versa.
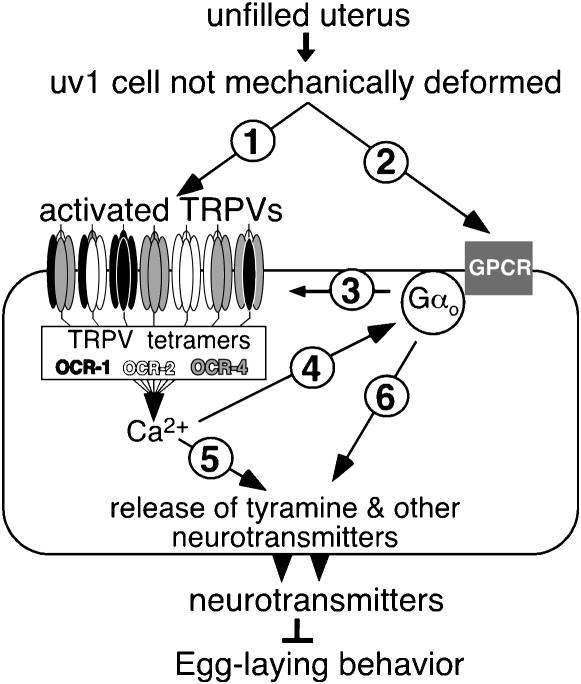
On the basis of all our results, we propose a model in which mechanical deformation of the uv1 cells by eggs in the uterus inactivates a set of partially redundant TRPV subunits (including OCR-2, OCR-4, and OCR-1) that form functional channels as mixed tetramers (Figure 8). When the uterus is not full, these cation channels are active, leading to Ca2+ influx that promotes the release of tyramine and other neurotransmitters to inhibit egg laying. The Gαo protein GOA-1 may activate the TRPV channels, as inactivating GOA-1 in TRPV-expressing cells had the same effect as inactivation of TRPV channels. Alternatively, GOA-1 may act in parallel or downstream of TRP channel activation to inhibit egg laying. In summary, inactivation of TRPV channels and/or the G-protein GOA-1, apparently in response to deformed uv1 endocrine cells, stops the release of neurotransmitters that inhibit egg laying, explaining the observation (Figure 1C) that egg laying is inhibited until eggs fill the uterus and deform the uv1 cells.
Figure 8.—
Model for inhibition of egg laying until the uterus is filled with eggs. uv1 neurosecretory cells release tyramine and other neurotransmitters to inhibit egg-laying behavior until they are mechanically deformed by eggs in the uterus, and both heteromeric TRPV channels and the G-protein Gαo are required in uv1 cells for this release. The relationship among mechanical deformation of uv1, TRPV activity, and Gαo activity remains undetermined, leaving several possibilities consistent with the data. uv1 deformation could directly mechanically gate TRPV channels (arrow 1), affect Gαo via a G-protein-coupled receptor (GPCR, arrow 2), or activation of both TRPV channels and Gαo could be required in parallel. If mechanical deformation acts via the G protein, Gαo could activate the TRPV channels to allow entry of Ca2+, a known activator of neurotransmitter vesicle release (arrows 3 and 5). Alternatively, if the TRPV channels are directly mechanically gated by uv1 deformation, a model that remains formally consistent with our analysis is that TRPV channels activate the G protein to in turn activate neurotransmitter release (arrows 4 and 6).
DISCUSSION
In every case in which TRP channel function has been studied genetically in Drosophila or C. elegans, it appears that function is carried out by multiple TRP subunits that can form heteromers. In three instances in which TRP function was studied in ciliated sensory neurons, two specific TRP subunits were required that apparently formed obligate heteromeric complexes such that knocking out either subunit caused a complete sensory defect (Barr et al. 2001; Tobin et al. 2002; Gong et al. 2004). In nonciliated cells, the situation is more complex in that there is partial redundancy between the coexpressed TRP subunits: knocking out a single subunit causes at most a partial defect. Drosophila vision depends on three TRPC subunits (TRP, TRPL, and TRPγ) expressed in photoreceptor neurons. Mutants of trp show strong sensory defects (Cosens and Manning 1969), whereas little or no defects are seen in trpl knockouts, except in a trp knockout background (Niemeyer et al. 1996). Finally, the contribution of trpγ can be revealed only in a dominant-negative trpγ mutant, which apparently inactivates heteromers of TRPγ and TRPL subunits (Xu et al. 2000). Mechanosensation in Drosophila by adult bristles or by larval nociceptors requires a TRPN subunit (NOMPC) or a TRPA subunit (Painless), respectively. However, nompC and painless mutants show only partial sensory defects, suggesting that other TRP channel subunits may also contribute to Drosophila mechanosensation (Walker et al. 2000; Tracey et al. 2003). Finally, our studies show that at least three TRPV subunits, OCR-1, -2, and -4, are inactivated in response to the mechanical deformation of uv1 endocrine cells by eggs in the underlying uterus. Knocking out any one subunit caused no detectable defect in this function, but double or triple knockouts did reveal significant defects. Even stronger defects were seen using a dominant-negative OCR-2 mutant, suggesting that there are additional TRP subunits that function together with OCR-1, -2, and -4. We note that there are at least 17 TRP subunit genes in C. elegans (Montell 2005). In summary, genetic studies of TRP subunits in invertebrates show that full TRP channel function generally cannot be revealed using single subunit knockouts due to functional redundancy among TRP subunits. The fact that strong defects can be revealed using dominant-negative subunit mutants in cases where single knockouts give weak or no defects further suggests that the redundant TRP subunits actually function in heteromeric complexes.
Similar redundant heteromerizing TRP subunits likely exist in mammals, but their physiological roles remain largely unexamined. Using heterologous pairwise expression of subunits or co-immunoprecipitation from native tissues, heteromers of TRPV5/6, TRPV1/3, TRPM6/7, TRPP2/TRPC1, and numerous TRPC subunit combinations have all been shown to occur (Tsiokas et al. 1999; Hofmann et al. 2002; Smith et al. 2002; Hoenderop et al. 2003b; Strubing et al. 2003; Chubanov et al. 2004; Hellwig et al. 2005). Are these heteromers actually functional in vivo? The role of OCR-2 was best revealed in a dominant-negative ocr-2 mutant that apparently inactivates a heteromerizing set of TRP channel subunits that function redundantly. We suggest that expression of dominant-negative TRP channel subunits in mammalian cells could similarly reveal the physiological functions of mammalian TRP heteromers.
We have described a dominant-negative mutation of the TRPV subunit OCR-2, in which Tyr 395 is changed to a Phe. This residue is present in a N-terminal juxtamembrane region conserved within the TRPV subfamily but of unknown function. A 10-amino-acid deletion within this same region in the TRPV1 splice form TRPV1b also produces a dominant-negative subunit that, like OCR-2(Y395F), is a destabilized protein that inactivates other channel subunits (Wang et al. 2004). This suggests that the juxtamembrane region is critical for controlling channel stability and function and that mutations in this region may generally be useful in producing dominant-negative channel subunits.
Our work reveals that the functional association between two TRPV subunits can be different in different cells. The TRPV subunits OSM-9 and OCR-2 apparently function as obligate heteromers in sensory neurons, since knocking out either subunit leads to both elimination of sensory function and mislocalization of the other subunit (Tobin et al. 2002). However, we found that OCR-2 and OSM-9, while both present in the endocrine cells that control egg-laying behavior, do not function together in these cells. Rather, OSM-9 has no detectable role in egg laying, and OCR-2 apparently functions in a mixture of heteromers with OCR-1 and -4 that function redundantly to control egg laying.
Our model (Figure 8) proposes that the Ca2+ entering through TRP channels in endocrine cells may directly trigger exocytosis of neurotransmitter vesicles until a mechanical stimulus deforms these cells. Consistent with this idea, Obukhov and Nowycky (2002) showed that TRPC4 overexpressed in cultured neuroendocrine cells provides sufficient Ca2+ to trigger exocytosis. We note that multiple TRP subunits are expressed in mammalian endocrine cells, but their function in these cells remains unknown, and it is unclear if they enable these cells to respond to sensory stimuli. For example, TRPV1 is expressed in rat pancreatic islet cells that secrete insulin (Akiba et al. 2004), and TRPC4 as well as TRPC7 are coexpressed in cultured insulin-secreting endocrine cells (Qian et al. 2002).
While it is possible that TRP channels transduce mechanical stimuli directly, our results show that both the Gα protein GOA-1 and a set of TRPV channels are required for function in mechanically deformed endocrine cells. This parallels the requirement for the Gα protein ODR-3 and OCR-2/OSM-9 TRPV channels in mechanosensation by C. elegans sensory neurons (Tobin et al. 2002). In these cases, the mechanical stimulus might be transduced by G-protein-coupled receptors that act through Gα proteins to regulate TRPV channel activity (Figure 8). Such an arrangement underlies mammalian taste (Zhang et al. 2003), Drosophila vision (Montell et al. 1985), and C. elegans olfaction (Colbert et al. 1997).
Acknowledgments
We thank the Caenorhabditis Genetics Center for strains, the Yale Center for Cell and Molecular Imaging for microscopes, H. Qin for microscopy advice, C. Bargmann for cDNA clones, J. Tanis for the PTx clone, P. De Camilli for the TTx clone, and S. Jordt for critical reading of the manuscript. This work was supported by National Institutes of Health grant NS03918.
References
- Akiba, Y., S. Kato, K. Katsube, M. Nakamura, K. Takeuchi et al., 2004. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem. Biophys. Res. Commun. 321: 219–225. [DOI] [PubMed] [Google Scholar]
- Alkema, M. J., M. Hunter-Ensor, N. Ringstad and H. R. Horvitz, 2005. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46: 247–260. [DOI] [PubMed] [Google Scholar]
- Bany, I. A., 2004. Genetic and cellular analysis of the inhibition of egg laying in Caenorhabditis elegans. Ph.D. Thesis, Yale University, New Haven, CT.
- Bany, I. A., M. Q. Dong and M. R. Koelle, 2003. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 23: 8060–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, M. M., J. DeModena, D. Braun, C. Q. Nguyen, D. H. Hall et al., 2001. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11: 1341–1346. [DOI] [PubMed] [Google Scholar]
- Birder, L. A., Y. Nakamura, S. Kiss, M. L. Nealen, S. Barrick et al., 2002. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 5: 856–860. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M. J., A. Leffler, A. B. Malmberg, W. J. Martin, J. Trafton et al., 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313. [DOI] [PubMed] [Google Scholar]
- Chalfie, M., and E. Wolinsky, 1990. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature 345: 410–416. [DOI] [PubMed] [Google Scholar]
- Chase, D. L., and M. R. Koelle, 2004. Genetic analysis of RGS protein function in Caenorhabditis elegans. Methods Enzymol. 389: 305–320. [DOI] [PubMed] [Google Scholar]
- Chubanov, V., S. Waldegger, M. Mederos y Schnitzler, H. Vitzthum, M. C. Sassen et al., 2004. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 101: 2894–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert, H. A., T. L. Smith and C. I. Bargmann, 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 17: 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosens, D. J., and A. Manning, 1969. Abnormal electroretinogram from a Drosophila mutant. Nature 224: 285–287. [DOI] [PubMed] [Google Scholar]
- Darby, C., and S. Falkow, 2001. Mimicry of a G protein mutation by pertussis toxin expression in transgenic Caenorhabditis elegans. Infect. Immun. 69: 6271–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. B., J. Gray, M. J. Gunthorpe, J. P. Hatcher, P. T. Davey et al., 2000. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405: 183–187. [DOI] [PubMed] [Google Scholar]
- Gong, Z., W. Son, Y. D. Chung, J. Kim, D. W. Shin et al., 2004. Two interdependent TRPV channel subunits, Inactive and Nanchung, mediate hearing in Drosophila. J. Neurosci. 24: 9059–9066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopfert, M. C., J. T. Albert, B. Nadrowski and A. Kamikouchi, 2006. Specification of auditory sensitivity by Drosophila TRP channels. Nat. Neurosci. 9: 999–1000. [DOI] [PubMed] [Google Scholar]
- Hellwig, N., N. Albrecht, C. Harteneck, G. Schultz and M. Schaefer, 2005. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 118: 917–928. [DOI] [PubMed] [Google Scholar]
- Hess, H., V. Reinke and M. Koelle, 2005. Construction and screening of deletion mutant libraries to generate C. elegans gene knockouts, in WormBook, edited by The C. elegans Research Community (doi/10.1895/wormbook.1.7.1, http://www.wormbook.org).
- Hilliard, M. A., C. I. Bargmann and P. Bazzicalupo, 2002. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr. Biol. 12: 730–734. [DOI] [PubMed] [Google Scholar]
- Hobert, O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Hoenderop, J. G., J. P. van Leeuwen, B. C. van der Eerden, F. F. Kersten, A. W. van der Kemp et al., 2003. a Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Invest. 112: 1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop, J. G., T. Voets, S. Hoefs, F. Weidema, J. Prenen et al., 2003. b Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 22: 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, T., M. Schaefer, G. Schultz and T. Gudermann, 2002. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 99: 7461–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, T. E., H. Zhang, D. E. Logothetis and C. H. Berlot, 2001. Visualization of a functional Gαq–green fluorescent protein fusion in living cells. J. Biol. Chem. 276: 4227–4235. [DOI] [PubMed] [Google Scholar]
- Jose, A. M., and M. R. Koelle, 2005. Domains, amino acid residues, and new isoforms of Caenorhabditis elegans diacylglycerol kinase 1 (DGK-1) important for terminating diacylglycerol signaling in vivo. J. Biol. Chem. 280: 2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn-Kirby, A. H., and C. I. Bargmann, 2006. TRP channels in C. elegans. Annu. Rev. Physiol. 68: 719–736. [DOI] [PubMed] [Google Scholar]
- Kaplan, J. M., and H. R. Horvitz, 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90: 2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedei, N., T. Szabo, J. D. Lile, J. J. Treanor, Z. Olah et al., 2001. Analysis of the native quaternary structure of the vanilloid receptor 1. J. Biol. Chem. 276: 28613–28619. [DOI] [PubMed] [Google Scholar]
- Kim, J., Y. D. Chung, D. Y. Park, S. Choi, D. W. Shin et al., 2003. A TRPV family ion channel required for hearing in Drosophila. Nature 424: 81–84. [DOI] [PubMed] [Google Scholar]
- Kim, K., and C. Li, 2004. Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J. Comp. Neurol. 475: 540–550. [DOI] [PubMed] [Google Scholar]
- Kimble, J., and D. Hirsh, 1979. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 70: 396–417. [DOI] [PubMed] [Google Scholar]
- Liedtke, W., Y. Choe, M. A. Marti-Renom, A. M. Bell, C. S. Denis et al., 2000. Vanilloid receptor-related osmotically active channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel, J. E., H. C. Korswagen, K. S. Liu, Y. M. Hajdu-Cronin, M. I. Simon et al., 1995. Participation of the protein Go in multiple aspects of behavior in C. elegans. Science 267: 1652–1655. [DOI] [PubMed] [Google Scholar]
- Montell, C., 2005. The TRP superfamily of cation channels. Sci. STKE 272: re3. [DOI] [PubMed]
- Montell, C., K. Jones, E. Hafen and G. Rubin, 1985. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230: 1040–1043. [DOI] [PubMed] [Google Scholar]
- Moore, D. S., and G. P. McCabe, 2003. Introduction to the Practice of Statistics, Ed. 4. W. H. Freeman, New York.
- Moqrich, A., S. W. Hwang, T. J. Earley, M. J. Petrus, A. N. Murray et al., 2005. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307: 1468–1472. [DOI] [PubMed] [Google Scholar]
- Moresco, J. J., and M. R. Koelle, 2004. Activation of EGL-47, a Gαo-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior. J. Neurosci. 24: 8522–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeini, R. S., M-F. Witty, P. Seguela and C. W. Bourque, 2006. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat. Neurosci. 9: 93–98. [DOI] [PubMed] [Google Scholar]
- Nemoz-Gaillard, E., A. Bosshard, R. Regazzi, C. Bernard, J. C. Cuber et al., 1998. Expression of SNARE proteins in enteroendocrine cell lines and functional role of tetanus toxin-sensitive proteins in cholecystokinin release. FEBS Lett. 425: 66–70. [DOI] [PubMed] [Google Scholar]
- Newman, A. P., and P. W. Sternberg, 1996. Coordinated morphogenesis of epithelia during development of the Caenorhabditis elegans uterine-vulval connection. Proc. Natl. Acad. Sci. USA 93: 9329–9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A. P., J. G. White and P. W. Sternberg, 1996. Morphogenesis of the C. elegans hermaphrodite uterus. Development 122: 3617–3626. [DOI] [PubMed] [Google Scholar]
- Niemeyer, B. A., E. Suzuki, K. Scott, K. Jalink and C. S. Zuker, 1996. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell 85: 651–659. [DOI] [PubMed] [Google Scholar]
- Nonet, M. L., K. Grundahl, B. J. Meyer and J. B. Rand, 1993. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell 73: 1291–1305. [DOI] [PubMed] [Google Scholar]
- Obukhov, A. G., and M. C. Nowycky, 2002. TRPC4 can be activated by G-protein-coupled receptors and provides sufficient Ca(2+) to trigger exocytosis in neuroendocrine cells. J. Biol. Chem. 277: 16172–16178. [DOI] [PubMed] [Google Scholar]
- O'Neil, R. G., and S. Heller, 2005. The mechanosensitive nature of TRPV channels. Pflugers Arch. 451: 193–203. [DOI] [PubMed] [Google Scholar]
- Qian, F., P. Huang, L. Ma, A. Kuznetsov, N. Tamarina et al., 2002. TRP genes: candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes 51 (S1): S183–S189. [DOI] [PubMed] [Google Scholar]
- Reuss, H., M. H. Mojet, S. Chyb and R. C. Hardie, 1997. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron 19: 1249–1259. [DOI] [PubMed] [Google Scholar]
- Saifee, O., L. Wei and M. L. Nonet, 1998. The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol. Biol. Cell 9: 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségalat, L., D. A. Elkes and J. M. Kaplan, 1995. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Nature 267: 1648–1651. [DOI] [PubMed] [Google Scholar]
- Shyn, S. I., R. Kerr and W. R. Schafer, 2003. Serotonin and Go modulate functional states of neurons and muscles controlling C. elegans egg-laying behavior. Curr. Biol. 13: 1910–1915. [DOI] [PubMed] [Google Scholar]
- Smith, G. D., M. J. Gunthorpe, R. E. Kelsell, P. D. Hayes, P. Reilly et al., 2002. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418: 186–190. [DOI] [PubMed] [Google Scholar]
- Strubing, C., G. Krapivinsky, L. Krapivinsky and D. E. Clapham, 2003. Formation of novel TRPC channel complex subunit interactions in embryonic brain. J. Biol. Chem. 278: 39014–39019. [DOI] [PubMed] [Google Scholar]
- Sweeney, S. T., K. Broadie, J. Keane, H. Niemann, C. J. O'Kane et al., 1995. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14: 341–351. [DOI] [PubMed] [Google Scholar]
- Tobin, D., D. Madsen, A. Kahn-Kirby, E. L. Peckol, G. Moulder et al., 2002. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35: 307–318. [DOI] [PubMed] [Google Scholar]
- Tracey, W. D., Jr., R. I. Wilson, G. Laurent and S. Benzer, 2003. painless, a Drosophila gene essential for nociception. Cell 113: 261–273. [DOI] [PubMed] [Google Scholar]
- Tsiokas, L., T. Arnould, C. Zhu, E. Kim, G. Walz et al., 1999. Specific association of the gene product of pkd2 with the TRPC1 channel. Proc. Natl. Acad. Sci. USA 96: 3934–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, R. G., A. T. Willingham and C. S. Zuker, 2000. A Drosophila mechanosensory transduction channel. Science 287: 2229–2234. [DOI] [PubMed] [Google Scholar]
- Wang, C., H. Z. Hu, C. K. Colton, J. D. Wood and M. X. Zhu, 2004. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J. Biol. Chem. 279: 37423–37430. [DOI] [PubMed] [Google Scholar]
- White, J. G., E. Southgate, J. N. Thomson and S. Brenner, 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Xu, X. Z., F. Chien, A. Butler, L. Salkoff and C. Montell, 2000. TRPgamma, a Drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron 26: 647–657. [DOI] [PubMed] [Google Scholar]
- Yoon, J., H. C. Ben-Ami, Y. S. Hong, S. Park, L. L. Strong et al., 2000. Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J. Neurosci. 20: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., M. A. Hoon, J. Chandrashekar, K. L. Mueller, B. Cook et al., 2003. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112: 293–301. [DOI] [PubMed] [Google Scholar]