Abstract
Evolutionary maintenance of genetic sex-ratio variation is enigmatic since genes for biased sex ratios are disadvantageous in finite populations (the “Verner effect”). However, such variation could be maintained if a small number of nuclear sex-determining genes were responsible, although this has not been fully demonstrated experimentally. Brood sex ratios of the freshwater snail Pomacea canaliculata are highly variable among parents, but population sex ratios are near unity. In this study, the effect of each parent on the brood sex ratio was investigated by exchanging partners among mating pairs. There were positive correlations between sex ratios of half-sib broods of the common mother (r = 0.42) or of the common father (r = 0.47). Moreover, the correlation between full-sib broods was very high (r = 0.92). Thus, both parents contributed equally to the sex-ratio variation, which indicates that nuclear genes are involved and their effects are additive. Since the half-sib correlations were much stronger than the parent–offspring regressions previously obtained, the variation was caused by zygotic sex-determining genes rather than by parental sex-ratio genes. The number of relevant genes appears to be small.
THE genetics of sex-ratio variation are important in considering the adaptive sex ratios of organisms since they are direct products of sex-ratio evolution (Bull 1983; Werren and Beukeboom 1998) as well as possible constraints (West and Sheldon 2002). An important distinction among the genetic factors producing sex-ratio variation is whether they are cytoplasmic sex factors, nuclear sex-ratio genes, or nuclear sex-determining genes (Werren and Beukeboom 1998). Cytoplasmic sex factors are expressed either in the parent or in the zygote and are usually inherited only through the female lineage; this is demonstrated by the bacterium Wolbachia (Stouthamer et al. 2002). Sex-ratio genes are those expressed in the parent that affect offspring sex ratios, such as X chromosome drive genes in Drosophila spp. (Stouthamer et al. 2002). Sex-determining genes are those expressed in the zygote that determine the zygote's sex.
These genetic factors all affect the brood sex ratios, but they differ in their actions and responses to natural selection. Cytoplasmic sex factors often behave selfishly and distort the sex ratio toward females (Stouthamer et al. 2002; Dyson and Hurst 2004). Nuclear sex-ratio genes affect offspring sex ratios with little variance if not interacting with other genes (Fisher 1930; Verner 1965; Williams 1979). On the other hand, sex-determining genes are usually expressed in combination with other sex-determining genes to designate the zygote's sex. For instance, in the platyfish Xiphophorus spp., the sex is determined by three genetic factors: X, Y, and W (Bull 1983). Here the sex ratio is a trait of each brood containing individuals of different sex genotypes. Mating between a WX female and an XY male produces XY sons and XX, WX, or WY daughters, and their sex ratio (proportion of males in the offspring) is 0.25 (Bull 1983). On the other hand, mating between an XX female and a YY male produces all sons (XY males). Thus, sex-determining genes may affect brood sex ratios in a more complicated manner than sex-ratio genes. It is possible to consider the average brood sex ratio for an X factor once gene frequencies are given, but in reality the offspring sex ratio varies greatly among parents with an X, depending on the other sex-determining genes that they possess and on the genotypes of their mating partners. Note that this argument applies when the number of sex-determining genes is small. If the number of genes is large (i.e., sex-determining polygenes), the effect of each gene is more predictable under quantitative genetics for a threshold trait (Falconer 1989).
In infinite populations with evolutionarily stable population sex ratios, genes for any sex ratios are equally adaptive, which allows sex-ratio variation (Kolman 1960; Williams 1979). However, in finite populations where the sex ratios will fluctuate, genes for biased sex ratios are selected against, because individuals with such genes gain less by becoming members of the minor sex and lose more in the major sex (the “Verner effect”; Verner 1965; Williams 1979). Thus, the presence of large genetic variation in the sex ratio is enigmatic in organisms with small and/or viscous populations. However, genetic sex-ratio variation occurs in several animals, such as the mussel Mytilus galloprovincialis (Saavedra et al. 1997), the polychaete worm Ophryotrocha labronica (Premoli et al. 1996), the copepode Tigriopus californicus (Voordouw and Anholt 2002), and the parasitoid wasps Nasonia vitripennis (Orzack and Gladstone 1994) and Heterospilus prosopidis (Kobayashi et al. 2003). Although sex-ratio genes or sex-determining polygenes have traditionally been thought to be the cause of such variation (Fisher 1930; Verner 1965; Bulmer and Bull 1982), these genes tend to be selected against by the Verner effect. Instead, a possible cause of the sex-ratio variation is a small number of sex-determining genes (“multiple factors” in Bull 1983). This is because, as already stated, sex-ratio variation is not attributable to each sex-determining gene but to the combination of genes, if the number of relevant genes is small. However, sex-ratio variation caused by a small number of sex-determining genes has seldom been demonstrated experimentally, except for some simple systems like the platyfish's three-gene system (Bull 1983) or the housefly's multiple-factor system (Bull 1983; Dübendorfer et al. 2002).
The apple snail Pomacea canaliculata (Ampullariidae) is a freshwater snail from South America. It has invaded many Asian countries, North America, and Hawaii (Cowie 2002) and is a serious pest to rice plants as well as an influential invasive species (Wada 1997; Cowie 2002; Carlsson et al. 2004). Control methods are insufficient especially outside paddy fields, and hence genetic control is required (Yusa 2004b, 2007). The females lay pinkish egg masses above water, and young snails hatch and fall into the water at 10–14 days. Brood sex ratios (i.e., proportions of male hatchlings in each brood) vary greatly among parents from almost all male to all female (Yusa and Suzuki 2003). Yet the primary population sex ratios were almost 0.5 in all populations so far studied (Yusa and Suzuki 2003; Yusa 2004b, 2006, 2007). The variation in the brood sex ratio is probably genetic, since no environmental factors affect the sex ratio (Yusa 2004b). However, previous studies could not eliminate maternal effects on the sex ratio that may not be genetic (Falconer 1989).
In a recent study (Yusa 2006), brood sex ratios were regressed on the sex ratios of their parents' siblings in P. canaliculata (“parent–offspring regression” in sex-ratio literature; Orzack and Gladstone 1994; Premoli et al. 1996; Voordouw and Anholt 2002). The regression coefficients were low: 0.28 for mother–offspring regression (P < 0.05) and 0.10 for father–offspring regression (P = 0.5). In addition, correlation coefficients in offspring sex ratios between two full sibs mated to unrelated partners were different between sexes: r = 0.41 (P < 0.05) for the correlation between sisters and r = −0.13 (P = 0.5) between brothers. Such “parent–offspring regressions” or “correlations between siblings” are direct ways to analyze the inheritance of sex-ratio genes since they are expressed in the parents. However, in the case of sex-determining genes, such analyses are inconclusive because the phenotypes (sexes) are characteristics of offspring and not of parents. In fact, the inconsistent results do not support the involvement of typical sex-ratio genes (Yusa 2006). In the case of sex-determining genes, a more direct way to study the genetics is by mating each parent to two or more partners and examining the differences in sex ratios between half-sib broods. Such an experiment has seldom been conducted, except in the mussel M. galloprovincialis (Saavedra et al. 1997).
In this study, the genetics of sex-ratio variation was investigated by exchanging either the mother or the father among mating pairs and comparing the sex ratios of their offspring before and after the exchange. Then, whenever possible, the parents were returned to the original pairing. In each case, the paternity was ascertained using genetic markers (Yusa 2004a). If nuclear sex-determining genes are involved, significant correlations between half-sibs of the common mother or of the common father would be expected; however, if sex-ratio genes are involved, the correlations should be as low as the results of “parent–offspring regressions” in this snail (Yusa 2006).
MATERIALS AND METHODS
Collection and rearing of snails:
Juvenile snails were collected from a field population in Kumamoto City, southern Japan (32°46′ N; 130°36′ E), in August and September 2001. To ensure that females had not copulated, the females <2.0 g (shell height of ∼20 mm) were used, well below the size at first copulation (on average 6–9 g, depending on food availability; Estoy et al. 2002). Since it does not matter if males had copulated or not, larger males were used (up to 2.5 g). Very small snails were reared in a 155-liter aquarium under laboratory conditions (see below) until they reached 0.6 g (14 mm in shell height), which is when males start developing testis, making sex identification possible (Yusa 2004a). To utilize body colors as a genetic marker, all females were selected from yellow albino snails, which represented ∼1% of snails in the population (Yusa 2004a). Half of the experimental males were yellow albino snails, and the remaining half were brown wild types. The body-color genes follow Mendelian inheritance (Yusa 2004a), which indicates that the genes are not linked with sex genes. Yellow is recessive to brown.
Each snail of 0.6–2.0 g (or up to 2.5 g for males) was paired with a heterosexual partner (N = 98 pairs). To identify the sex, the presence or absence of the testis was checked by sight through the thin shell at the apex. The pairs were reared in 2-liter aquaria under laboratory conditions of 25° with 14:10 hr light:dark photoperiods. Commercial carp food pellets were fed as required, and powdered oyster shell was added as a calcium supply. Water was changed at least once a week. Further details of rearing conditions are described elsewhere (Yusa and Suzuki 2003; Yusa 2004b, 2006).
When an egg mass was identified, it was removed after it had hardened (∼2 days), placed in a petri dish, and allowed to hatch. Up to 40 hatchlings were taken from each brood and reared in a 2-liter perforated aquarium placed in a large outdoor tank with running water. Culturing conditions, whether in the laboratory or outdoors, have no effect on the brood sex ratio (Yusa 2004b). Once the snails reached 0.6 g or 14 mm in shell height (which took a minimum of 50 days), their sex was identified by dissection and checking for the presence or absence of the testis.
Experimental design:
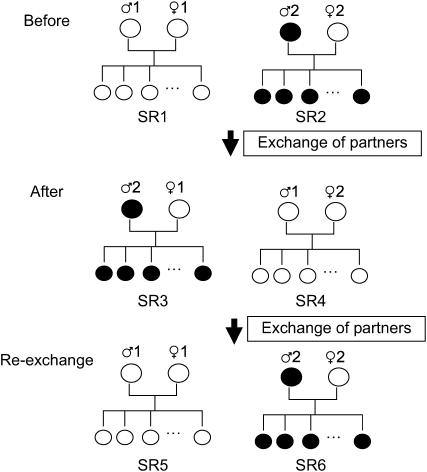
A brood was reared as explained above from each mating pair with either a yellow male (♂1 in Figure 1) or a brown male (♂2) mated to yellow females (♀1 and ♀2, respectively) to study their brood sex ratios (SR1 and SR2 in Figure 1). Then, one of the mating partners was exchanged with that of another pair with a male of a different body color (Figure 1). Partners were exchanged between two pairs in most cases, but sometimes three pairs were involved. The pairs for exchange were randomly chosen without reference to the brood sex ratio. In fact, the correlation in the brood sex ratio between these pairs was not significant before exchanging the partners (N = 47, r = 0.23, P = 0.13, after the data were m-transformed; see below for statistical method). To test possible effects of rearing conditions on mating pairs, either male (as shown in Figure 1) or female partners were exchanged among pairs, and the partners of the opposite sex were left unchanged.
Figure 1.—
Experimental design. See text for details of experimental procedure. Open circles indicate yellow snails and solid circles indicate brown snails. Comparison between half-sib broods of the common mother with different fathers was made by comparing SR1 and SR3 or SR2 and SR4. For the common father with different mothers, SR1 and SR4 or SR2 and SR3 were compared. Comparison was made between full-sib broods SR1 and SR5 or SR2 and SR6.
After exchange, egg masses of each pair were continuously taken and monitored for the body color of hatchlings, and when hatchlings sired by the second male appeared (those with the same body color as the male), up to 40 individuals were taken and reared in the same way as before to study their sex ratios (SR3 and SR4 in Figure 1). This procedure enabled comparisons of sex ratios for half-sib broods (see Figure 1 legend).
Then, whenever possible, partners were exchanged again and returned to their original pairing (Figure 1). Before re-exchanging, it was confirmed that the second male for two consecutive egg masses sired all hatchlings. Although the exact mechanism of sperm mixing in the female's seminal receptacle is unknown, this ensures complete sperm displacement by the male (Yusa 2004a). Then partners were exchanged again, egg masses were monitored, and up to 40 hatchlings sired by the original male were reared to identify their sex. This procedure enabled comparisons of sex ratios of full-sib broods (Figure 1).
In one case, hatchlings sired by a brown male contained both yellow and brown snails with the probability of ∼1:1 (yellow:brown = 54:46) before exchanging partners. Since the yellow gene (a) is recessive to the brown gene (A), the brown male was supposed to have the Aa genotype (Yusa 2004a). In this case, only brown hatchlings of the putative Aa male were used for the analysis, although there was no significant difference in the sex ratio between yellow hatchlings (29 males:1 female) and brown ones (40 males:0 female) before partner exchange (P = 0.43; Fisher's exact probability test).
Statistical analyses of sex ratios:
The sex ratio was expressed as the proportion of males in each brood. To minimize sampling errors, sex-ratio data were analyzed only for broods with at least 20 individuals at sex identification.
To test if the sex-ratio data deviate from the binomial distribution, a heterogeneity test was conducted. A generalized linear model with binomial errors was applied to the untransformed sex-ratio data to calculate the null deviance, and it was compared against the χ2 distribution (Wilson and Hardy 2002).
In this study, a threshold model for the sex ratio (Bulmer and Bull 1982; Voordouw and Anholt 2002; Kobayashi et al. 2003; Yusa 2006) was applied, in which the sex ratio is the proportion of individuals that exceed the threshold (individuals over the threshold become male). Then the sex ratio can be converted to the position of a normally distributed underlying trait (the “male tendency”; Voordouw and Anholt 2002) relative to the threshold set at 0. Thus, the sex ratio was transformed to m-value, which is the mean of the standardized normal curve of the underlying trait (Falconer 1989; Yusa 2006). For this analysis, the minimum sex ratio was set to 0.0125 (= 0.5/40) instead of 0, with the corresponding m-value being −2.24 (Yusa 2006). Pearson's r was used to study the correlation between brood sex ratios of full-sibs or half-sibs after the ratios were m-transformed. For the correlation between the sex ratio and mortality or hatchability, nonparametric Kendall's rank correlation was used because the mortality and hatchability were not normally distributed. In this case, these variables were not transformed.
RESULTS
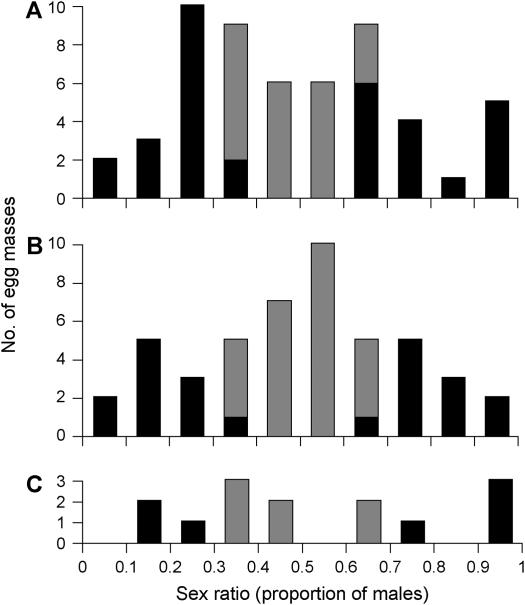
Among 98 mating pairs reared, data on brood sex ratios were obtained for 55 pairs before a parent was exchanged, 47 pairs after it, and 14 pairs after exchanging the parent again. In all cases, brood sex ratios varied greatly among pairs (Figure 2). The sex ratio varied from 0.05 (2 males:37 females) to 1.00 (40 males:0 females) before exchange, 0.08 (3 males:34 females) to 1.00 (40 males:0 females) after exchange, and 0.18 (7 males:31 females) to 1.00 (40 males:0 females) after re-exchange. The sex-ratio variations were significantly greater than expected under binomial distribution in all three cases (d.f. = 54, χ2 = 625.4, P < 0.001 before exchange; d.f. = 46, χ2 = 436.7, P < 0.001 after exchange; and d.f. = 13, χ2 = 195.4, P < 0.001 after re-exchange). Yet, the average sex ratio was almost 0.5 in all three cases (untransformed mean ± SD sex ratios = 0.50 ± 0.25 before exchange; 0.50 ± 0.23 after exchange; and 0.54 ± 0.29 after re-exchange).
Figure 2.—
Brood sex ratios (proportions of males) of P. canaliculata (A) before exchanging a parent, (B) after exchange, or (C) after re-exchanging to return to the original pairing. Solid areas denote egg masses with sex ratios significantly different from 0.5 by binomial test and shaded areas indicate those that are nonsignificant. Marginal sex-ratio values are included in the lower categories (e.g., the category 0.1–0.2 includes sex ratios <0.1 and ≥0.2).
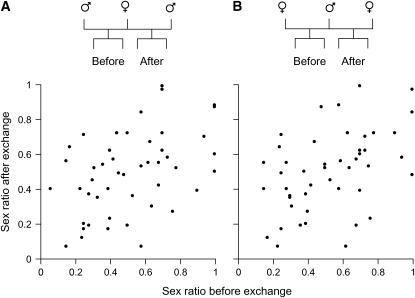
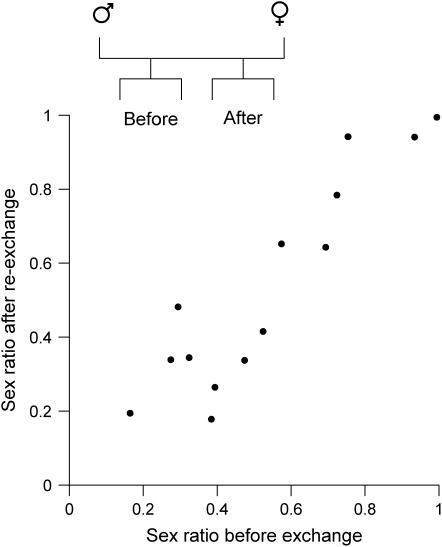
When sex ratios were compared between broods before and after exchanging a parent, there was a positive correlation between sex ratios of two half-sib broods of the common mother with different fathers (Figure 3A). The correlation coefficient was ∼0.5 (r = 0.42). Likewise, there was a positive correlation in the sex ratio between two half-sib broods of the common father with different mothers (Figure 3B). The correlation coefficient was also ∼0.5 (r = 0.47). Moreover, the correlation in the sex ratio between two full-sib broods before and after re-exchanging the parent was very high and almost 1.0 (r = 0.92; Figure 4).
Figure 3.—
Correlations in brood sex ratios between two half-sib broods before and after parental exchange. (A) Half-sibs of the common mother with different fathers (N = 47; r = 0.42; P < 0.01 after sex ratios were transformed to the underlying m-value). (B) Common father with different mothers (N = 47; r = 0.47; P < 0.001).
Figure 4.—
Correlations in brood sex ratios between two full-sib broods before and after the re-exchange of a parent to return to the original pairing (N = 14; r = 0.92; P < 0.001 after sex ratios were transformed to the m-value).
Other factors did not affect the brood sex ratio. First, the mortality of the hatchlings during experiments was low in all three experimental series, and the average ranged from 0.03 (both before and after exchanging a parent) to 0.06 (after re-exchanging). Thus, variation in sex-specific mortality among broods did not explain the variation of brood sex ratios. In fact, the correlations between mortalities and brood sex ratios were not significant in all three experimental series (τ = −0.14, P = 0.2 before exchange; τ = −0.16, P = 0.2 after exchange; and τ = −0.08, P = 0.7 after re-exchange; Kendall's rank correlation). Second, hatchability was not high, being 0.72 ± 0.20 (untransformed mean ± SD) before exchanging a partner, 0.70 ± 0.22 after exchange, and 0.66 ± 0.19 after re-exchange. However, correlations between hatchability and brood sex ratios were not significant in all three cases (τ = 0.07, P = 0.4 before exchange; τ = 0.03, P = 0.8 after exchange; τ = 0.26, P = 0.2 after re-exchange). Third, the effect of rearing conditions on each pair was investigated by exchanging either the father (N = 35 cases) or the mother (12 cases) among aquaria, while the other partners remained in the same aquaria. In accordance with the previous results, the sex ratio after exchange was positively related to the sex ratio before exchange in the case of half-sib broods of the mother (P < 0.01), but it was not affected by whether the mother was exchanged (N = 12) or remained (N = 35; P = 0.6; analysis of covariance). A similar result was obtained for half-sib broods of the father (regression: P < 0.01; effect of rearing condition: P = 0.6).
DISCUSSION
The high correlation coefficient between full-sib broods suggests that most variation in the sex ratio of P. canaliculata is genetic. Although the partners were not exchanged, high correlations (Kendall's τ = 0.70–0.81) between two full-sib broods were also obtained in a previous study (Yusa and Suzuki 2003). Direct manipulations of various environmental factors did not affect the brood sex ratio (Yusa 2004b). However, these results may have contained maternal effects such as various egg characteristics dependent on the nutritional conditions of the mother (Yusa and Suzuki 2003). This study showed that the sex-ratio variation was dependent on the father as well as the mother. Paternal effects are usually genetic (Falconer 1989), and hence at least half the sex-ratio variation is genetic. Additionally, an exchange of either the mother or the father among pairs eliminated the possible effects of rearing conditions. The high correlations between full-sib broods with no environmental effects suggest that virtually all sex-ratio variation is probably genetic.
This study also showed that both parents contributed equally to the sex-ratio variation. This pattern differs from results in the mussel M. galloprovincialis in which brood sex ratios are controlled by the mother and are almost independent of the father, suggesting that the genetic factors are cytoplasmic (Saavedra et al. 1997; Yusa 2007). The equal contribution of both parents in P. canaliculata indicates that the genetic factors that influence the sex ratio are nuclear genes inherited through both parents. The average sex ratio of 0.5 in this snail also favors the involvement of nuclear sex genes, as cytoplasmic factors often divert the sex ratio toward females (Stouthamer et al. 2002; Dyson and Hurst 2004).
The correlation coefficients in the sex ratios of half-sibs were ∼0.5 for both the common mother (r = 0.42) and the common father (r = 0.47). Such 0.5 coefficients in half-sib correlations are exactly what are expected when an individual is mated to two unrelated partners, and each individual contributes half the genetic sex factors to the offspring. Moreover, the correlation coefficient between full-sib broods was almost 1.0 (r = 0.92), which was approximately equal to the simple addition of the coefficients of half-sib correlations (0.89). This means that the brood sex ratios are determined by genes from both parents and that the genes act additively.
On the other hand, parent–offspring regressions in the sex ratio—i.e., regressions of the brood sex ratios on the sex ratios of their parents' siblings—were low in a previous study (slope = 0.28 for mother–offspring regression and 0.10 for father–offspring regression; Yusa 2006). A reanalysis of the data revealed that the correlation coefficient between the mother and offspring was r = 0.23 and that between the father and offspring was 0.07. In this study, the correlation coefficients between half-sibs were much higher (r = 0.42–0.47). This suggests that the sex-ratio variation depends not primarily on the parents but on the offspring. Therefore, the genetic factors are not parental sex-ratio genes but sex-determining genes expressed in the offspring.
The lower coefficients in parent–offspring regressions (or correlations) than in half-sib correlations also suggest that the number of sex-determining genes is small. If genes act additively as shown in this study and there are no environmental effects (Yusa 2004b), sex-determining polygenes should have nearly 0.5 coefficients in parent–offspring regressions (Yusa 2006). On the other hand, low coefficients in the regressions may be expected if the number of sex-determining genes is small, because each gene can produce various brood sex ratios in combination with other sex-determining genes.
The large sex-ratio variation in this snail also disfavors the involvement of sex-ratio genes or sex-determining polygenes. If such genes are involved, genes for biased sex ratios are likely to be selected against by the Verner effect in small populations (see Introduction). This snail normally inhabits relatively small water bodies such as ponds, ditches, or paddy fields in both original (Martin et al. 2001) and introduced areas (Wada 1997), suggesting that the populations are actually small. In addition, polygenic sex determination is unlikely to produce highly biased brood sex ratios even under a simple assumption of binomial distribution of sex phenotypes. Moreover, polygenic sex determination is often under environmental effects (Bull 1983), whereas no such effects have been detected in this snail (Yusa 2004b). These conditions suggest that the number of sex-determining genes is small.
Although the number of the relevant genes appears to be small, three genes (see Introduction for an example of platyfish) are insufficient to explain the continuous sex-ratio variation in this snail. Thus, at least four genes must be involved in sex determination and sex-ratio variation in P. canaliculata. Further evidence and the exact genetics of such “oligogenic” sex determination will be explored.
Acknowledgments
I thank Y. Suzuki and anonymous reviewers for comments on the manuscript; Y. Suzuki, K. Tanaka, and T. Wada for discussion and encouragement; and M. Hashimoto for help in rearing snails.
References
- Bull, J. J., 1983. Evolution of Sex Determining Mechanisms. Benjamin/Cummings, Menlo Park, CA.
- Bulmer, M. G., and J. J. Bull, 1982. Models of polygenic sex determination and sex ratio evolution. Evolution 36: 13–26. [DOI] [PubMed] [Google Scholar]
- Carlsson, N. O. L., C. Bronmark and L.-A. Hansson, 2004. Invading herbivory: the golden apple snail alters ecosystem functioning in Asian wetlands. Ecology 85: 1575–1580. [Google Scholar]
- Cowie, R. H., 2002. Apple snails as agricultural pests: their biology, impacts, and management, pp. 145–192 in Molluscs as Crop Pests, edited by G. M. Baker. CABI, Wallingford, UK.
- Dübendorfer, A., M. Hediger, G. Burghardt and D. Bopp, 2002. Musca domestica, a window on the evolution of sex-determining mechanisms in insects. Int. J. Dev. Biol. 46: 75–79. [PubMed] [Google Scholar]
- Dyson, E. A., and G. D. D. Hurst, 2004. Persistence of an extreme sex-ratio bias in a natural population. Proc. Natl. Acad. Sci. USA 101: 6520–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estoy, G. F., Jr., Y. Yusa, T. Wada, H. Sakurai and K. Tsuchida, 2002. Size and age at first copulation and spawning of the apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae). Appl. Entomol. Zool. 37: 199–205. [Google Scholar]
- Falconer, D. S., 1989. Introduction to Quantitative Genetics. Longman, New York.
- Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Clarendon, Oxford.
- Kobayashi, A., Y. Tanaka and M. Shimada, 2003. Genetic variation of sex allocation in the parasitoid wasp Heterospilus prosopidis. Evolution 57: 2659–2664. [DOI] [PubMed] [Google Scholar]
- Kolman, W. A., 1960. The mechanism of natural selection for the sex ratio. Am. Nat. 94: 373–377. [Google Scholar]
- Martin, P. R., A. L. Estebenet and N. J. Cazzaniga, 2001. Factors affecting the distribution of Pomacea canaliculata (Gastropoda: Ampullariidae) along its southernmost natural limit. Malacologia 43: 13–23. [Google Scholar]
- Orzack, S. H., and J. Gladstone, 1994. Quantitative genetics of sex ratio traits in the parasitic wasp, Nasonia vitripennis. Genetics 137: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli, M. C., G. Sella and G. P. Berra, 1996. Heritable variation of sex ratio in a polychaete worm. J. Evol. Biol. 9: 845–854. [Google Scholar]
- Saavedra, C., M. I. Reyero and E. Zouros, 1997. Male-dependent doubly uniparental inheritance of mitochondrial DNA and female-dependent sex-ratio in the mussel Mytilus galloprovincialis. Genetics 145: 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer, R., G. D. D. Hurst and J. A. J. Breeuwer, 2002. Sex ratio distorters and their detection, pp. 195–215 in Sex Ratios, Concepts and Research Methods, edited by I. C. W. Hardy. Cambridge University Press, Cambridge, UK.
- Verner, J., 1965. Selection for sex ratio. Am. Nat. 99: 419–421. [Google Scholar]
- Voordouw, M. J., and B. R. Anholt, 2002. Heritability of sex tendency in a harpacticoid copepod, Tigriopus californicus. Evolution 56: 1754–1763. [DOI] [PubMed] [Google Scholar]
- Wada, T., 1997. Introduction of the apple snail Pomacea canaliculata and its impact on rice agriculture, pp. 170–180 in Proceedings of International Workshop on Biological Invasions of Ecosystems by Pests and Beneficial Organisms. NIAES, Tsukuba, Japan.
- Werren, J. H., and L. W. Beukeboom, 1998. Sex determination, sex ratios, and genetic conflict. Annu. Rev. Ecol. Syst. 29: 233–261. [Google Scholar]
- West, S. A., and B. C. Sheldon, 2002. Constraints in the evolution of sex ratio adjustment. Science 295: 1685–1688. [DOI] [PubMed] [Google Scholar]
- Williams, G. C., 1979. The question of adaptive sex ratio in outcrossed vertebrates. Proc. R. Soc. Lond. Ser. B 205: 567–580. [DOI] [PubMed] [Google Scholar]
- Wilson, K., and I. C. W. Hardy, 2002. Statistical analysis of sex ratio: an introduction, pp. 48–92 in Sex Ratios, Concepts and Research Methods, edited by I. C. W. Hardy. Cambridge University Press, Cambridge, UK.
- Yusa, Y., 2004. a Inheritance of colour polymorphism and the pattern of sperm competition in the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae). J. Moll. Stud. 70: 43–48. [Google Scholar]
- Yusa, Y., 2004. b Brood sex ratio in the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae) is determined genetically and not by environmental factors. J. Moll. Stud. 70: 269–275. [Google Scholar]
- Yusa, Y., 2006. Genetics of sex-ratio variation inferred from parent-offspring regressions and sib correlations in the apple snail Pomacea canaliculata. Heredity 96: 100–105. [DOI] [PubMed] [Google Scholar]
- Yusa, Y., 2007. Causes of variation in sex ratio and modes of sex determination in the Mollusca—an overview. Am. Malacol. Bull. (in press).
- Yusa, Y., and Y. Suzuki, 2003. A snail with unbiased population sex ratios but highly biased brood sex ratios. Proc. R. Soc. Lond. Ser. B 270: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]