Abstract
Sphingolipid signaling plays an important role in the regulation of central cellular processes, including cell growth, survival, and differentiation. Many of the essential pathways responsible for sphingolipid biogenesis, and key cellular responses to changes in sphingolipid balance, are conserved between mammalian and yeast cells. Here we demonstrate a novel function for the survival factor Svf1p in the yeast sphingolipid pathway and provide evidence that Svf1p regulates the generation of a specific subset of phytosphingosine. Genetic analyses suggest that Svf1p acts in concert with Lcb4p and Lcb3p to generate a localized pool of phytosphingosine distinct from phytosphingosine generated by Sur2p. This subset is implicated in cellular responses to stress, as loss of SVF1 is associated with defects in the diauxic shift and the oxidative stress response. A genetic interaction between SVF1 and SUR2 demonstrates that both factors are required for optimal growth and survival, and phenotypic similarities between svf1Δsur2Δ and ypk1Δ suggest that pathways controlled by Svf1p and Sur2p converge on a signaling cascade regulated by Ypk1p. Loss of YPK1 together with disruption of either SVF1 or SUR2 is lethal. Together, these data suggest that compartmentalized generation of distinct intracellular subsets of sphingoid bases may be critical for activation of signaling pathways that control cell growth and survival.
SPHINGOLIPIDS are important structural components of cellular membranes. The role of sphingolipid metabolites as key second messengers is becoming increasingly clear. Sphingoid base signaling has been shown to regulate diverse cellular processes, including cell proliferation, apoptosis, angiogenesis, and differentiation (for reviews, see Pyne and Pyne 2000; Hannun et al. 2001; Cuvillier 2002; Spiegel and Milstien 2003; Saba and Hla 2004). Since these processes play a central role in carcinogenesis, these observations have generated increasing interest in sphingolipid signaling pathways as offering a series of attractive targets for novel cancer therapies (Toman et al. 2001; Spiegel and Kolesnick 2002; Ogretmen and Hannun 2004). Evolutionary conservation of sphingolipid metabolic pathways emphasizes the importance of these lipids. Analysis of these pathways in Saccharomyces cerevisiae may provide insight into a central regulatory mechanism for cell death in mammalian systems.
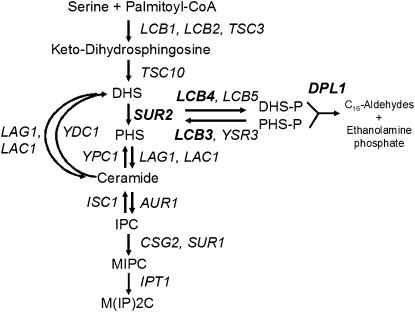
Generation of sphingoid bases begins with the condensation of serine and palmitoyl-CoA to yield 3-ketodihydrosphingosine, followed by conversion to sphinganine, commonly called dihydrosphingosine (DHS). In yeast, DHS can be used to generate dihydroceramide or can be converted to 4-hydroxysphinganine, also known as phytosphingosine (PHS), by the hydroxylase Sur2p. DHS and PHS are phosphorylated by the long-chain base kinases Lcb4p or Lcb5p. The phosphorylated bases can be dephosphorylated by the phosphatases Lcb3p or Ysr3p or broken down to generate C-16 aldehydes and ethanolamine phosphate by the lyase Dpl1p (Figure 1). While mammalian cells primarily generate sphingosine (SPH), S. cerevisiae lack the enzyme to make SPH and contain relatively high levels of PHS, which appears to function analogously to SPH. PHS and SPH can be converted to phytoceramide and ceramide, respectively. Ceramide species can be further metabolized to generate complex sphingolipids, including inositolphosphorylceramide (IPC), mannosylated IPC (MIPC), and mannosyl-diinositolphosphorylceramide [M(IP)2C]. Several recent reviews provide detailed summaries of sphingolipid metabolism (Dickson and Lester 1999, 2002; Obeid et al. 2002; Alvarez-Vasquez et al. 2005).
Figure 1.—
A schematic overview of yeast sphingolipid synthesis and metabolism. Deletion strains of the genes in boldface type were examined.
Several studies have demonstrated that efficient incorporation of the exogenous sphingoid bases DHS or PHS into ceramide and the complex sphingolipids requires both phosphorylation and subsequent dephosphorylation (Mao et al. 1997, 1999; Qie et al. 1997; Mandala et al. 1998; Funato et al. 2003). Yeast lacking the phosphatase Lcb3p do not convert 3H-DHS into ceramide or into the complex sphingolipids IPC, MIPC, and M(IP)2C (Mao et al. 1997, 1999; Qie et al. 1997; Mandala et al. 1998). Similarly, cells lacking the kinases Lcb4p and Lcb5p also demonstrate a deficiency in conversion of 3H-DHS to IPC and M(IP)2C (Funato et al. 2003). The apparent requirement for concerted action of the sphingoid base kinase and phosphatase for production of downstream lipids in the pathway has suggested a model in which sphingolipid species are differentially compartmentalized within the cell. With prolonged incubation, downstream sphingolipids in fact can be made at low levels in lcb4Δlcb5Δ cells but not in lcb3Δ cells (Funato et al. 2003). This implies the existence of an alternative bypass mechanism for inefficient conversion of exogenous bases that do not cycle through phosphorylation/dephosphorylation. The phosphorylation/dephosphorylation pathway may facilitate proper localization of exogenous lipids for metabolism by other proteins in the pathway.
Analyses of yeast mutants in the sphingolipid pathway have demonstrated that sphingoid base signaling in yeast regulates cellular processes, including the heat stress response (Dickson et al. 1997; Jenkins et al. 1997; Mao et al. 1999; Skrzypek et al. 1999; Jenkins and Hannun 2001; Ferguson-Yankey et al. 2002), nutrient uptake (Skrzypek et al. 1998; Chung et al. 2000, 2001; Hearn et al. 2003), endocytosis (Friant et al. 2000, 2001; Zanolari et al. 2000), and cytoskeletal organization (Friant et al. 2001; Balguerie et al. 2002). Most recently, it has been demonstrated that sphingoid bases activate the yeast kinases Pkh1p and Pkh2p (Friant et al. 2001). Pkh1/2p regulate several pathways, including a cell integrity pathway via activation of the AGC family of kinases that includes Pkc1p, Ypk1p, Ypk2p, and Sch9p (Roelants et al. 2002, 2004). These kinases regulate essential pathways, as cells lacking both PKH1 and PKH2 are nonviable (Casamayor et al. 1999). Sphingoid bases may also activate the downstream kinases Ypk1/2p and Sch9p directly. In vitro kinase assays show that activity of these kinases increases in the presence of PHS and is further enhanced through the addition of Pkh1p to the reaction (Liu et al. 2005b). The analogous pathway in mammalian cells is controlled by PDK1, and it has been shown that SPH can similarly activate PDK1 in mammalian cells (King et al. 2000).
We identified SVF1 in a screen for yeast factors regulating cell survival that could be partially complemented by expression of mammalian Bcl-xL (Vander Heiden et al. 2002). We have shown that while Svf1p and Bcl-xL have distinct functional properties, Svf1p functions to regulate survival under a variety of stress conditions. In particular, cells lacking SVF1 are defective in responding to the metabolic changes that occur during the diauxic shift (Vander Heiden et al. 2002) and are hypersensitive to cold stress, H2O2, menadione, and acetic acid, conditions that elevate production of, or exposure to, reactive oxidative species (Brace et al. 2005). The mechanism of action of Svf1p has not been defined. Here we demonstrate that Svf1p affects cellular survival in part via modulation of the sphingolipid metabolic pathway. Using a genetic approach, we place SVF1 in the sphingolipid pathway and suggest a model in which Svf1p regulates the generation of a localized pool of sphingoid bases affecting Ypk1p-dependent signaling pathways.
MATERIALS AND METHODS
Yeast strains and plasmids:
The S. cerevisiae W303 (MATa ade2-1 can1-100 his3-11.15 leu2-3.112 trp1-1 ura3-1) or BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) strain backgrounds were used as indicated in the text (see also Table 1). Strains were maintained on synthetic complete (SC) media: 0.17% yeast nitrogen base without ammonium sulfate, 1.0% ammonium sulfate (Fisher), amino acid dropout mixture, and 2% dextrose (Fisher), supplemented with 300 μm adenine hemi-sulfate. Selection media were used as necessary by using the appropriate amino acid dropout mixture. Counterselection of the URA3 containing plasmid pOW4 was achieved by plating to SC media containing 1 mg/ml 5-fluroorotic acid (FOA). Kanamycin selection was performed with YPD [2% peptone, 1% yeast extract (Fisher), 2% dextrose (Fisher), supplemented with 0.15 mg/ml l-tryptophan (Fisher), 2% agar] plates containing 0.2 mg/ml G418. Yeast media reagents were obtained from QBioGene, unless noted otherwise.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| Wild type | W303 MATaade2-1 can1-100 his3-11.15 leu2-3.112 trp1-1 ura3-1 | Vander Heiden et al. (2002) |
| svf1Δ | W303 asvf1Δ∷HIS3 | Vander Heiden et al. (2002) |
| sur2Δ | W303 α sur2Δ∷kanMX | This study |
| svf1Δ/+sur2Δ/+ | W303 a/α svf1Δ∷HIS3 sur2Δ∷kanMX | α sur2Δ∷kanMX × asvf1Δ∷kanMX |
| Wild type | BY4741 MATahis3Δ1 leu2Δ0 ura3Δ0 met15Δ0 | Research Genetics |
| svf1Δ | BY4741 asvf1Δ∷kanMX | Research Genetics |
| sur2Δ | BY4741 asur2Δ∷kanMX | Research Genetics |
| lcb3Δ | BY4741 alcb3Δ∷kanMX | Research Genetics |
| dpl1Δ | BY4741 adpl1Δ∷kanMX | Research Genetics |
| lcb4Δ | BY4741 alcb4Δ∷kanMX | Research Genetics |
| lcb5Δ | BY4741 alcb5Δ∷kanMX | Research Genetics |
| ysr3Δ | BY4741 aysr3Δ∷kanMX | Research Genetics |
| ypk1Δ/+ | BY4743 a/α ypk1Δ∷kanMX | Research Genetics |
| svf1Δ | BY4741 α svf1Δ∷kanMX | This study |
| svf1Δ | BY4741 α svf1Δ∷HIS3 | This study |
| sur2Δ | BY4741 α sur2Δ∷kanMX | This study |
| svf1Δ/+sur2Δ/+ | BY4741 a/α svf1Δ∷kanMX sur2Δ∷kanMX | α svf1Δ∷kanMX × asur2Δ∷kanMX |
| lcb3Δ/+sur2Δ/+ | BY4741 a/α lcb3Δ∷kanMX sur2Δ∷kanMX | α sur2Δ∷kanMX × alcb3Δ∷kanMX |
| lcb4Δ/+sur2Δ/+ | BY4741 a/α lcb4Δ∷kanMX sur2Δ∷kanMX | α sur2Δ∷kanMX × alcb4Δ∷kanMX |
| lcb5Δsur2Δ | BY4741 alcb5Δ∷kanMX sur2Δ∷kanMX | α sur2Δ∷kanMX × alcb5Δ∷kanMX |
| dpl1Δsur2Δ | BY4741 α dpl1Δ∷kanMX sur2Δ∷kanMX | α sur2Δ∷kanMX × adpl1Δ∷kanMX |
| lcb3Δsvf1Δ | BY4741 α lcb3Δ∷kanMX svf1Δ∷HIS3 | α svf1Δ∷HIS3 × alcb3Δ∷kanMX |
| lcb3Δsvf1Δ | BY4741 α lcb3Δ∷kanMX svf1Δ∷kanMX | α svf1Δ∷kanMX × alcb3Δ∷kanMX |
| lcb4Δsvf1Δ | BY4741 alcb4Δ∷kanMX svf1Δ∷kanMX | α svf1Δ∷kanMX × alcb4Δ∷kanMX |
| ypk1Δ/+svf1Δ/+ | BY4741 a/α ypk1Δ∷kanMX svf1Δ∷HIS3 | α svf1Δ∷HIS3 × aypk1Δ∷kanMX |
| ypk1Δ/+sur2Δ/+ | BY4741 a/α ypk1Δ∷kanMX sur2Δ∷kanMX | α sur2Δ∷kanMX × aypk1Δ∷kanMX |
| lcb3Δ/+dpl1Δ/+svf1Δ/+ | BY4741 a/α lcb3Δ∷kanMX dpl1Δ∷kanMX svf1Δ∷HIS3 | α lcb3Δ∷kanMX svf1Δ∷HIS3 × adpl1Δ∷kanMX |
| lcb3Δ/+dpl1Δ/+svf1Δ/+ | BY4741 a/α lcb3Δ∷kanMX dpl1Δ∷kanMX svf1Δ∷kan | α lcb3Δ∷kanMX svf1Δ∷kanMX × adpl1Δ∷kanMX |
| lcb3Δ/+dpl1Δ/+sur2Δ/+ | BY4741 a/α lcb3Δ∷kanMX dpl1Δ∷kanMX sur2Δ∷kanMX | α dpl1Δ∷kanMX sur2Δ∷kanMX × alcb3Δ∷kanMX |
Deletion strains of SVF1, LCB4, LCB5, LCB3, YSR3, SUR2, and DPL1 of the BY4741 background and YPK1 of the BY4743 background were obtained from the Research Genetics (Huntsville, AL) knockout collection (kanamycin resistance). An SVF1-null strain marked with the HIS3 gene was generated in the wild-type parental strain by homologous replacement using methods previously described (Bahler et al. 1998). In the W303 background, replacement of the coding region of SVF1 with the HIS3 and kanamycin resistance gene was performed as described (Bahler et al. 1998; Vander Heiden et al. 2002). All genomic deletions were confirmed by PCR to regions surrounding the deletion as well as phenotypic confirmation after tetrad dissection. Double and triple knockouts were generated through mating of single or double knockouts, selection of diploids, and tetrad dissection. All tetrad dissections were performed on YPD media with incubation at 30°. Marker segregation and PCR analysis were used to confirm the generation of double and triple knockouts. To avoid selection of suppressor mutations, strains with slow growth phenotypes were maintained as heterozygous diploids and were sporulated and dissected just prior to use in experiments.
SVF1, DPL1, and YPK1 amplified from yeast genomic DNA and human Bcl-xL cDNA were cloned into the single-copy high-expression vector pOW4 (uracil selection) and transformed into yeast using the standard lithium acetate method (Gietz et al. 1992).
Growth analysis:
In liquid culture, the indicated strains were grown overnight at 30° in SC media to saturation. Cells were normalized in SC media to an OD600 of 0.08. Normalized cultures were incubated with rotation at 20°, and samples were removed at the designated times to measure OD600. Three independent cultures were used for each time point and sample measurement.
For plate analysis, the indicated strains were normalized to ∼1 × 106 cells/ml (OD650 = 0.1 on a 96-well plate reader) and 5 μl of 10-fold serial dilutions were plated onto SC plates (or selection media as necessary) containing the indicated concentration of sphingosine, phytosphingosine, dihydrosphingosine, or ethanol control. Stock solutions of sphingosine, phytosphingosine, and dihydrosphingosine (BioMol, Plymouth Meeting, PA) were prepared in ethanol and added to plates at the concentration indicated. NP-40 was added to a final concentration of 0.0015% to facilitate dispersal of lipids in the media. Plates were incubated at 20° and were analyzed after 6 days.
For confirmation of synthetic lethality, the indicated diploid strains were transformed with a plasmid expressing the indicated gene. Upon tetrad dissection, each resulting genotype containing the plasmid was patched to YPD to allow for plasmid loss. Cells were then struck to plates lacking uracil as a control for viable growth in the presence of the indicated gene. Counterselection was performed on plates containing 1 mg/ml FOA and incubation at 30°. Nonviable strains were unable to grow on plates containing FOA.
HPLC measurement of sphingoid bases:
The indicated strains were grown at 20° in SC media, as described above, until midlog phase (∼22 hr). Where indicated, cells in midlog phase at 20° were treated with 30 μm C18-SPH (Biomol) for 1 hr prior to harvesting cells, and control cells were treated with an equal volume of ethanol. Trichloroacetic acid (TCA) was added to the cells (10 OD600 units) to a final concentration of 5%, and samples were incubated on ice for 5 min. Cells were harvested by centrifugation and washed once with 10 ml ice-cold 5% TCA, followed by two washes in 10 ml ice-cold water. Samples were frozen at −80° until further processing. The sphingoid bases were extracted and converted to fluorescent aminoquinoline derivatives, which were separated and analyzed by HPLC as described (Lester and Dickson 2001). C18-S1P (Biomol) was used as a standard to identify the peak corresponding to phosphorylation of the exogenously added SPH. Each sample was performed in duplicate. The analysis of wild-type and svf1Δ cells was performed in three independent experiments, each run in duplicate. The analysis of wild-type and svf1Δ cells expressing SVF1 and cells treated with SPH was performed once in duplicate. The analysis of wild type, svf1Δ, sur2Δ, lcb3Δ, and lcb4Δ was performed twice in duplicate, and the analysis of svf1Δlcb4Δ and svf1Δlcb3Δ was performed once in duplicate.
RESULTS
Cells lacking SVF1 are resistant to a growth inhibitory concentration of sphingosine:
SVF1 was initially identified in a screen for functional homologs of the mammalian survival protein Bcl-xL (Vander Heiden et al. 2002), and cells lacking SVF1 are hypersensitive to conditions associated with oxidative stress (Brace et al. 2005). Because of this association, we were interested in examining how SVF1 might influence other cellular pathways implicated in the regulation of cell survival in yeast. Sphingolipid signaling plays a key role in the regulation of mammalian cell death. We examined the response of yeast lacking SVF1 to a growth inhibitory concentration of SPH. To our surprise, we observed that svf1Δ cells demonstrate markedly increased resistance to SPH at concentrations as high as 50 μm (Figure 2A). This seemingly paradoxical observation suggested that Svf1p may have a specific function in regulating sphingolipid metabolic pathways.
Figure 2.—
SVF1-null cells are resistant to growth inhibitory concentrations of SPH. (A) W303 wild-type (WT) or svf1Δ cells were normalized and 10-fold serial dilutions were spotted onto plates containing ethanol (control) and 30 or 50 μm SPH. Plates were incubated at 20° for 6 days. (B) W303 WT or svf1Δ cells were transformed with a control vector, Bcl-xL, or SVF1. Ten-fold serial dilutions of normalized cells were plated onto selection plates containing 50 μm SPH or ethanol (control). Plates were incubated at 20° for 6 days. (C) BY4741 WT or svf1Δ cells were normalized and 10-fold serial dilutions were plated onto selection plates containing ethanol (control) and 30 or 50 μm SPH. Plates were incubated at 20° for 6 days.
We examined the ability of Bcl-xL to complement the SPH response phenotype of svf1Δ cells. Overexpression of Bcl-xL is unable to complement the SPH resistance of svf1Δ cells. Reintroduction of SVF1 to these cells, however, restores their sensitivity to SPH, demonstrating that this phenotype is specific for loss of SVF1 (Figure 2B). This observation supports our previous data that Svf1p and Bcl-xL have distinct roles in regulating cell survival (Brace et al. 2005).
To confirm the role of SVF1 in the response to elevated levels of SPH, we obtained an SVF1-null strain in an alternative yeast background. Similar to the result seen in the W303 background, BY4741 svf1Δ cells are resistant to growth on SPH (Figure 2C). Together, these data confirm a role for SVF1 in regulating growth and survival in the presence of elevated levels of SPH.
SVF1 demonstrates a genetic interaction with the dihydrosphingosine hydroxylase gene SUR2:
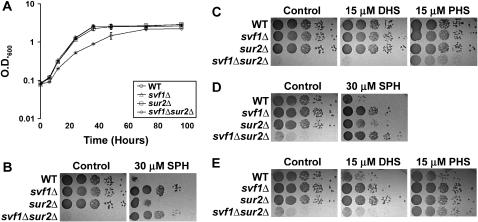
To better understand the function of SVF1 in the sphingolipid pathway, we generated double knockouts of SVF1 and genes regulating key steps in the metabolism of sphingoid bases, including LCB4, LCB3, SUR2, and DPL1. We identified a genetic interaction between SVF1 and the dihydrosphingosine hydroxylase SUR2. Yeast deficient in both SVF1 and SUR2 exhibit a slow growth phenotype (Figure 3A). A similar growth defect was observed in the W303 background (data not shown). These data demonstrate that SVF1 and SUR2 together are required for optimal cell proliferation, and their synthetic interaction suggests that they may act in parallel pathways to regulate growth and survival.
Figure 3.—
A genetic interaction is observed between SVF1 and SUR2. (A) Overnight cultures of BY4741 WT, svf1Δ, sur2Δ, and svf1Δsur2Δ cells were normalized to an OD600 of 0.08 and grown at 20°. At the indicated time points, samples were removed to measure OD600. Mean OD600 ± standard deviation of three independent cultures for each strain is shown. (B) BY4741WT, svf1Δ, sur2Δ, and svf1Δsur2Δ cells were normalized and 10-fold serial dilutions were spotted onto plates containing ethanol (control) or 30 μm SPH. Plates were incubated at 20° for 6 days. (C) BY4741WT, svf1Δ, sur2Δ, and svf1Δsur2Δ cells were normalized and 10-fold serial dilutions were spotted onto plates containing ethanol (control), 15 μm DHS, or 15 μm PHS. Plates were incubated at 20° for 6 days. (D) W303WT, svf1Δ, sur2Δ, and svf1Δsur2Δ cells were normalized and 10-fold serial dilutions were spotted onto plates containing ethanol (control) or 30 μm SPH. Plates were incubated at 20° for 6 days. (E) W303 WT, svf1Δ, sur2Δ, and svf1Δsur2Δ cells were normalized and 10-fold serial dilutions were spotted onto plates containing ethanol (control), 15 μm DHS, or 15 μm PHS. Plates were incubated at 20° for 6 days.
To determine if SUR2 is necessary for the SPH resistance we observed in svf1Δ cells, we examined growth of svf1Δsur2Δ cells in the presence of 30 μm SPH. Interestingly, not only were these cells resistant to SPH, but also the addition of SPH improved colony growth and density (Figure 3B). One hypothesis to explain these data is that the slow growth phenotype of svf1Δsur2Δ cells in the absence of SPH may be due to a relative deficiency in generation of essential sphingoid bases. Yeast cells generate DHS and PHS and do not produce SPH. To determine if the endogenous sphingoid bases DHS or PHS could restore growth, we plated cells in the presence or absence of PHS and DHS. In the presence of 15 μm PHS, but not of 15 μm DHS, growth was restored to svf1Δsur2Δ yeast (Figure 3C). Similar phenotypes were observed in the W303 background (Figure 3, D and E). This suggests that SVF1 and SUR2 act upstream of PHS in the sphingolipid pathway.
Cells lacking LCB3 and SUR2 exhibit a phenotype similar to that of svf1Δsur2Δ cells:
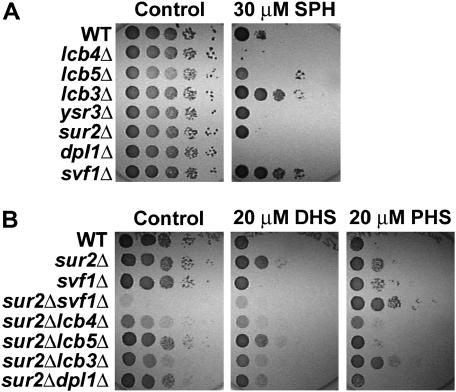
Examination of SPH resistance in strains deleted for genes encoding known regulators of the sphingolipid pathway may provide insight into Svf1p function and would allow epistatic placement of SVF1 in the sphingoid base pathway. We found that cells lacking the sphingosine-1-phosphate phosphatase LCB3, like svf1Δ cells, were resistant to SPH (Figure 4A). The lcb3Δsvf1Δ strain exhibits a similar phenotype to either single deletion strain (data not shown). We also generated a series of double knockouts with SUR2 and genes encoding known regulators of the sphingolipid pathway to determine if additional genetic interactions with SUR2 would provide insight into Svf1p function. We observed a growth defect in cells lacking SUR2 and LCB3 (Kim et al. 2000; Figure 4), as well as in cells lacking SUR2 and LCB4. Interestingly, like svf1Δsur2Δ cells, sur2Δlcb3Δ cells exhibit increased resistance to PHS (Figure 4B). The phenotypic similarities between Svf1p- and Lcb3p-deficient cells suggest that these factors may act in concert in the same pathway and/or may perform a similar function.
Figure 4.—
Cells lacking LCB3 exhibit a phenotype similar to svf1Δ. (A) BY4741 WT, lcb4Δ, lcb5Δ, lcb3Δ, ysr3Δ, sur2Δ, dpl1Δ, and svf1Δ cells were normalized and 10-fold serial dilutions were spotted onto plates containing ethanol (control) or 30 μm SPH. Plates were incubated at 20° for 6 days. (B) BY4741 WT, sur2Δ, svf1Δ, sur2Δ svf1Δ, sur2Δlcb4Δ, sur2Δlcb5Δ, sur2Δlcb3Δ, and sur2Δdpl1Δ cells were normalized and 10-fold dilutions were spotted onto plates containing ethanol (control), 20 μm DHS, or 20 μm PHS. Plates were incubated at 20° for 6 days.
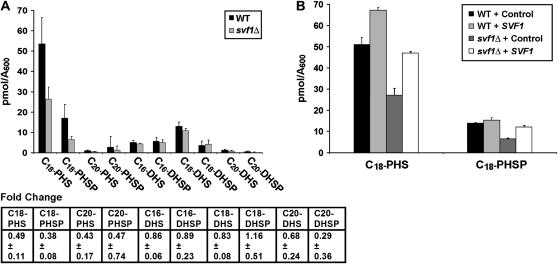
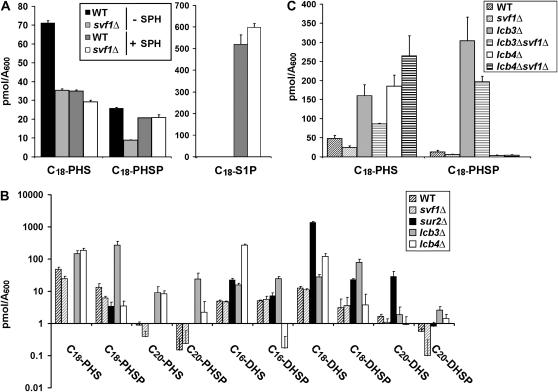
To determine if Svf1p has a biological function similar to that of the lipid phosphatase Lcb3p, we used HPLC to directly measure levels of various sphingoid bases in svf1Δ and wild-type cells. We observed a significant decrease in the amount of C18-PHS and C18-phytosphingosine phosphate (PHSP) in svf1Δ cells (Figure 5A). To confirm that this decrease in C18-PHS and C18-PHSP was due to loss of SVF1, we overexpressed SVF1 in these cells and measured sphingoid base levels. SVF1-null cells expressing SVF1 exhibit C18-PHS and C18-PHSP levels similar to wild-type cells. Wild-type cells overexpressing SVF1 generate higher levels of these lipids than wild-type cells containing a control vector (Figure 5B). Together, these data demonstrate that Svf1p regulates cellular levels of C18-PHS and C18-PHSP. While we observe a slight reduction of other lipids—specifically, C20-PHS and C-20 PHSP—in the svf1Δ strain, these lipids either were not consistently reduced or were not restored to wild-type levels by reintroduction of SVF1 (data not shown).
Figure 5.—
Sphingoid base measurement in svf1Δ cells demonstrates a reduction of C18-PHS and C18-PHSP in the mutant. (A) Overnight cultures of WT and svf1Δ cells were normalized to an OD600 of 0.08 and grown at 20°. Samples were collected in midlog phase and sphingoid base levels were determined by HPLC. The average picomoles/A600 of the indicated sphingoid base ± standard deviation of six independent samples is indicated for each strain. The fold change in svf1Δ cells compared to wild type for each sphingoid base is shown below the graph. (B) WT and svf1Δ cells were transformed with a control vector or SVF1. Overnight cultures were grown in selection media, normalized to an OD600 of 0.08, and incubated at 20°. Samples were collected in midlog phase and sphingoid base levels were determined by HPLC. The average picomoles/A600 of the indicated sphingoid base ± standard deviation of two independent samples is indicated for each sample.
Cells lacking SVF1 demonstrate improved growth in the presence of SPH. To determine how addition of SPH alters sphingoid base generation, we added 30 μm SPH to growing wild-type or svf1Δ cells and measured sphingoid base levels by HPLC. SPH suppresses the generation of C18-PHS in wild-type cells to a level comparable to that seen in svf1Δ cells and increases the production of C18-PHSP in svf1Δ cells to that of wild-type cells (Figure 6A). Therefore, addition of SPH appears to normalize the levels of these sphingoid bases in wild-type and svf1Δ cells. Wild-type and svf1Δ cells treated with SPH also generate similar levels of sphingosine-1-phosphate, S1P (Figure 6A). These data demonstrate that cells lacking SVF1 are not resistant to SPH through deficiencies in uptake of the sphingoid base from the media nor through an inability to phosphorylate SPH and confirm that treatment of cells with SPH can alter endogenous lipid levels. These data further suggest that the relative deficiencies of C18-PHS and C18-PHSP in svf1Δ cells may contribute to the growth defect of this strain: addition of SPH leads to both improved survival and relative normalization of levels of these lipids.
Figure 6.—
Sphingoid base measurement in deletion strains supports a role for Svf1p in the Lcb3p and Lcb4p pathway. (A) Overnight cultures of WT and svf1Δ cells were grown to midlog phase at 20°. SPH, or an equal volume of ethanol as a control, was added to a final concentration of 30 μm. Samples were collected after 1 hr and sphingoid base levels were determined by HPLC. The average picomoles/A600 of the indicated sphingoid base ± standard deviation of duplicate samples is indicated for each strain. (B) Overnight cultures of WT, svf1Δ, sur2Δ, lcb3Δ, and lcb4Δ were normalized to an OD600 of 0.08 and incubated at 20°. Samples were collected in midlog phase and sphingoid base levels were determined by HPLC. The average picomoles/A600 of the indicated sphingoid base ± standard deviation of four independent samples is indicated. (C) Overnight cultures of WT, svf1Δ, lcb3Δ, lcb3Δsvf1Δ, lcb4Δ, and lcb4Δsvf1Δ were normalized to an OD600 of 0.08 and incubated at 20°. Samples were collected in midlog phase and sphingoid base levels were determined by HPLC. The average picomoles/A600 of the indicated sphingoid base ± standard deviation of duplicate samples is indicated.
The sphingolipid profile of svf1Δ cells does not resemble that of sur2Δ, lcb4Δ, or lcb3Δ cells (Figure 6B). Sur2p is the only known dihydrosphingosine hydroxylase in the yeast genome, and SUR2-null cells manifest a striking reduction of PHS and elevation of DHS. Consistent with the known functions of Lcb4p and Lcb3p as a lipid kinase and phosphatase, respectively, LCB4- and LCB3-null cells accumulate markedly elevated levels of unphosphorylated and phosphorylated sphingoid bases, respectively. While loss of SUR2, LCB4, or LCB3 alters ratios of phosphorylated-to-unphosphorylated sphingoid bases dramatically, in svf1Δ cells these ratios are maintained in a range similar to that of wild-type cells. Taken together, these data suggest that unlike Sur2p, Lcb4p, and Lcb3p, Svf1p may not have a primary enzymatic function in the sphingolipid pathway, but may influence the production of a discrete subset of PHS and PHSP.
Generation of ceramide from exogenous sphingoid bases requires phosphorylation by Lcb4p followed by dephosphorylation by Lcb3p (Mao et al. 1997, 1999; Qie et al. 1997; Mandala et al. 1998; Funato et al. 2003). While these experiments test the exogenous addition of sphingoid bases, we hypothesize that this coupled reaction occurs on lipids generated intracellularly. As previously suggested, this coupling may serve to localize sphingolipids to specific intracellular compartments (Funato et al. 2003). Similar genetic interactions between LCB3 or LCB4 and SUR2 suggest that disruption of this coupling and loss of PHS are detrimental to cell growth. The genetic interaction observed between SVF1 and SUR2 suggests that Svf1p may play a role in the phosphorylation/dephosphorylation pathway, and we hypothesize that Svf1p might regulate this pathway to generate a subset of PHS. To explore this hypothesis, we examined sphingoid base profiles of cells lacking LCB3 or LCB4 in combination with loss of SVF1. We observe, as expected, that cells lacking LCB4 accumulate unphosphorylated sphingoid bases (Figure 6B). While svf1Δ cells exhibit reduced generation of C18-PHS, lcb4Δ and lcb4Δsvf1Δ cells accumulate similar levels of C18-PHS (Figure 6C). This suggests that Svf1p does not prevent generation of C18-PHS upstream of Lcb4p. In contrast, while cells lacking LCB3 exhibit increased levels of both C18-PHSP and C18-PHS, cells additionally lacking SVF1 do not accumulate these lipids to the same extent (Figure 6C). This suggests that Svf1p acts on, or upstream of, Lcb3p to generate PHSP and PHS. Together, these data support a model in which Svf1p regulates the concerted action of Lcb4p and Lcb3p to generate a subset of PHS and PHSP.
Loss of SVF1 but not SUR2 suppresses the lethality of the lcb3Δdpl1Δ strain:
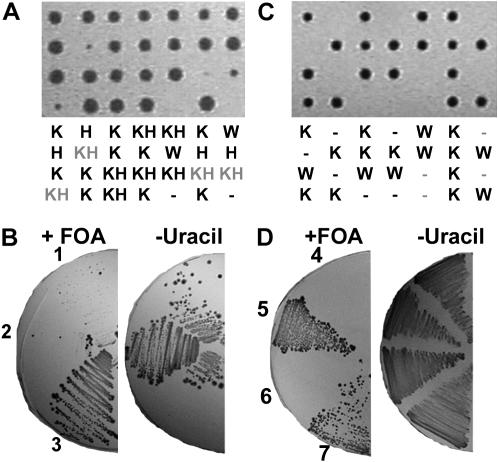
If Svf1p regulates the generation of a subset of sphingolipids, loss of SVF1 might be expected to suppress the growth defect of a strain that accumulates excessive amounts of this subset. One such mutant might be the lcb3Δdpl1Δ strain. Lcb3p and Dpl1p regulate different metabolic pathways reducing PHSP. Yeast lacking both LCB3 and DPL1 are nonviable, suggesting that although the analogous sphingolipid S1P is generally considered to be a prosurvival factor in mammalian cells, excessive PHSP in yeast can be toxic (Kim et al. 2000; Zhang et al. 2001). That this lethality is due to accumulation of phosphorylated bases is further supported by the observation that viability can be restored by deletion of the kinase LCB4 (Kim et al. 2000; Zhang et al. 2001). We hypothesized that if loss of LCB4 restores viability by preventing excessive generation of a discrete subset of phosphorylated sphingoid bases dependent on Svf1p, then loss of SVF1 might exhibit a similar phenotype. Triple knockouts were generated through mating of lcb3Δsvf1Δ with dpl1Δ. While in the control crosses of lcb3Δ and dpl1Δ, lcb3Δdpl1Δ cells were not recovered (data not shown), we were able to consistently obtain triple lcb3Δdpl1Δsvf1Δ cells (Figure 7A). The triple knockout cells demonstrate a slow growth phenotype, but were recovered at the expected ratios. To confirm this lethal interaction, diploid strains were transformed with a vector containing DPL1. Upon tetrad dissection, viable colonies expressing DPL1 were obtained. Cells were grown in the absence of selection to allow for plasmid loss and then plated onto counterselection plates containing FOA or selection media as a control. In the absence of DPL1 expression (FOA-containing plates), lcb3Δdpl1Δ cells were unable to grow, while, although growth was slow, lcb3Δdpl1Δsvf1Δ cells could form colonies (Figure 7B). This suggests that reduced generation of a subset of sphingoid bases dependent on Svf1p can partially suppress lethality of the dpl1Δlcb3Δ strain.
Figure 7.—
Loss of SVF1, but not SUR2, suppresses the lethality of lcb3Δdpl1Δ. (A) Diploid lcb3Δ/+dpl1Δ/+svf1Δ/+ cells were dissected onto YPD plates and incubated at 30°. A representative dissection plate with phenotypes (K, kanamycin resistance; H, histidine prototrophic; W, wild type) is shown. The triple knockout is shaded. (B) Diploid lcb3Δ/+dpl1Δ/+svf1Δ/+ cells were transformed with a vector expressing DPL1 and dissected onto YPD plates at 30°. lcb3Δdpl1Δsvf1Δ (1), lcb3Δdpl1Δ (2), and WT (3) cells expressing DPL1 were patched to YPD twice to allow for plasmid loss. Viable cells in the absence of DPL1 grew on counterselection media containing FOA. Cells were plated to −uracil as a control. Knockouts were confirmed by PCR. (C) Diploid lcb3Δ/+dpl1Δ/+sur2Δ/+ cells were dissected onto YPD plates and incubated at 30°. A representative dissection plate with phenotypes (K, kanamycin resistance; H, histidine prototrophic;W, wild type) is shown. The expected triple knockout is shaded. (D) Diploid lcb3Δ/+dpl1Δ/+sur2Δ/+ cells were transformed with a vector expressing DPL1 and dissected onto YPD plates at 30°. WT (5, 7) and lcb3Δdpl1Δsur2Δ (4, 6) cells expressing DPL1 were patched to YPD twice to allow for plasmid loss. Viable cells in the absence of DPL1 grew on counterselection media containing FOA. Cells were plated to −uracil as a control. Knockouts were confirmed by PCR.
It has been observed that the ability to obtain viable lcb3Δdpl1Δ mutants depends on the auxotrophic markers of the background strain. While prototrophic double mutants are viable (Skrzypek et al. 1999; Birchwood et al. 2001), double knockouts generated in strains auxotrophic for some amino acids are lethal (Kim et al. 2000; Zhang et al. 2001). This may be a consequence of the fact that sphingolipids play a role in nutrient uptake from the media (Chung et al. 2000, 2001; Hearn et al. 2003). Since we are using the HIS3 gene to replace the SVF1 gene, we wanted to confirm that the isolation of viable lcb3Δdpl1Δsvf1Δ cells was due to loss of SVF1, not to addition of the HIS3 gene. Therefore, we generated a strain in which the SVF1 gene was replaced with the kanamycin resistance gene. Using this strain, we were still able to isolate lcb3Δdpl1Δsvf1Δ triple knockouts (data not shown), demonstrating that the loss of SVF1 and not the incorporation of HIS3, is responsible for suppressing the lethality of lcb3Δdpl1Δ cells.
It has been suggested that PHS, not DHS, is the “active” sphingoid base in the cell and is responsible for growth inhibition (Chung et al. 2001; Friant et al. 2001). To determine if the reduced levels of PHS in the SVF1-null strain is responsible for suppressing lethality of the lcb3Δdpl1Δ double knockout, we attempted to generate a triple knockout with the enzyme responsible for the generation of PHS, SUR2. In this case, we were unable to recover any triple knockouts (Figure 7C). Again, diploid strains were transformed with a vector containing DPL1. Upon tetrad dissection, we obtained viable lcb3Δdpl1Δsur2Δ cells expressing DPL1. Cells were grown in the absence of selection to allow for plasmid loss and then plated onto counterselection plates containing FOA or selection media as a control. While wild-type cells could form colonies in the absence of DPL1 (FOA-containing plates), lcb3Δdpl1Δsur2Δ cells were unable to grow in the absence of DPL1 (Figure 7D). These data further indicate that Svf1p and Sur2p have distinct functions and support the hypothesis that Svf1p regulates the generation of a subset of sphingoid bases that controls survival.
Loss of YPK1 mimics the phenotypes observed in the svf1Δsur2Δ strain and demonstrates a synthetic interaction with SVF1 and SUR2:
We have suggested that SVF1 and SUR2 regulate the generation of separate subsets of sphingoid bases; however, this does not explain the growth defect seen in cells lacking these genes. It has been shown that PHS can activate the Pkh1/2p pathway (Friant et al. 2001). Since svf1Δsur2Δ cells are deficient in PHS, these cells may not properly activate this pathway. We therefore considered whether inadequate activation of downstream kinases in this pathway might contribute to the phenotypes observed in the svf1Δsur2Δ strain. A key downstream component of this signal transduction pathway is the kinase Ypk1p.
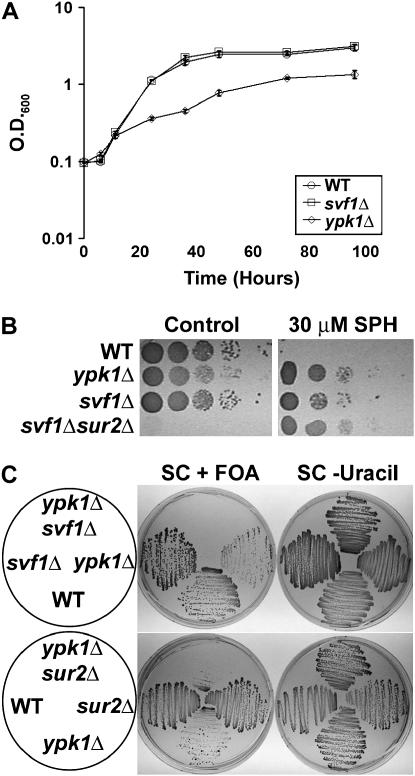
Similar to svf1Δsur2Δ cells, ypk1Δ cells exhibit a slow growth phenotype (Chen et al. 1993; Roelants et al. 2002; Figure 8A). Since svf1Δ and svf1Δsur2Δ cells are resistant to growth on SPH, we examined the growth of ypk1Δ cells on 30 μm SPH. Interestingly, similarly to svf1Δsur2Δ, not only are ypk1Δ cells highly resistant to what is typically a toxic dose of SPH, but also SPH markedly improves the growth of these cells (Figure 8B). These data suggest that YPK1 functions in the same pathway as SVF1 and SUR2.
Figure 8.—
Cells lacking YPK1 exhibit phenotypes similar to those of svf1Δsur2Δ. (A) Overnight cultures of WT, svf1Δ, and ypk1Δ cells were normalized to an OD600 of 0.08 and grown at 20°. At the indicated time points, samples were removed to measure OD600. Mean OD600 ± standard deviation of three independent cultures for each strain is shown. (B) WT, svf1Δ, ypk1Δ, and svf1Δsur2Δ cells were normalized and 10-fold serial dilutions were spotted onto plates containing ethanol (control) or 30 μm SPH. Plates were incubated at 20° for 6 days. (C) WT, ypk1Δ, svf1Δ, svf1Δypk1Δ, and sur2Δypk1Δ expressing YPK1 were patched to YPD twice to allow for plasmid loss. Viable cells in the absence of YPK1 grew on counterselection media containing FOA. Cells were struck to selection media as a control.
To further understand the epigenetic relationship among YPK1, SVF1, and SUR2, we attempted to generate double knockouts among these mutants. YPK1-null cells were mated to svf1Δ or sur2Δ cells. Upon tetrad dissection, we were able to obtain small colonies corresponding to ypk1Δ; however, we were unable to recover any svf1Δypk1Δ or sur2Δypk1Δ cells. To confirm this lethal interaction, diploid svf1Δ/+ypk1Δ/+ or sur2Δ/+ypk1Δ/+ strains were transformed with a vector containing YPK1. Upon tetrad dissection, viable colonies expressing YPK1 were obtained for each genotype. Cells were grown in the absence of selection to allow for plasmid loss and then plated onto counterselection plates containing FOA or selection media as a control. While wild-type, svf1Δ, sur2Δ, and ypk1Δ cells could form colonies on FOA-containing plates, svf1Δypk1Δ and sur2Δypk1Δ cells were unable to grow in the absence of YPK1 (Figure 8C). This confirms genetically the interaction between the generation of sphingoid bases and the activation of the YPK1 pathway and supports the hypothesis that SVF1 and SUR2 regulate the generation of different subsets of sphingoid bases that affect the activity of YPK1-dependent signaling pathways. This synthetic interaction also illustrates the importance of YPK1 activation and sphingoid base generation for growth and viability.
DISCUSSION
Cellular growth and survival are highly regulated processes that become disrupted in human diseases, including cancer. Understanding the function of proteins and pathways regulating growth and survival may provide insight into the process of oncogenesis. The genetic manipulability of the yeast S. cerevisiae and the conservation of survival pathways and enzymes between yeast and mammalian cells supports the use of S. cerevisiae as a model in which to study the processes of growth and survival with relevance to mammalian systems. We previously demonstrated that the yeast survival factor Svf1p plays a role in survival responses and is required for survival in the presence of reactive oxygen species (Vander Heiden et al. 2002; Brace et al. 2005). Here, we demonstrate a role for Svf1p in the sphingoid base pathway.
Exogenously added sphingoid bases require sequential phosphorylation and dephosphorylation for the cell to utilize these bases in the generation of downstream ceramide metabolites, and it is believed that these reactions serve to properly localize the sphingoid base to allow efficient action by downstream enzymes (Qie et al. 1997; Mao et al. 1997, 1999; Mandala et al. 1998; Funato et al. 2003). We hypothesize that these coupled phosphorylation/dephosphorylation reactions are required for the localized generation of a subset of PHS and PHSP regulated by Svf1p. We demonstrate through genetic manipulation and sphingoid base measurement that Svf1p functions to regulate the generation of C18-PHS and C18-PHSP and may do so through control of the concerted action of Lcb4p and Lcb3p. The suppression of the lethal dpl1Δlcb3Δ strain through additional loss of SVF1 suggests that preventing flow through this pathway can partially restore viability to a strain that accumulates this subset. The observation that loss of SUR2 does not suppress this lethality demonstrates that Svf1p and Sur2p have distinct functions and further supports the model that Svf1p regulates the generation of a subset of sphingoid bases distinct from Sur2p.
One possible function of Svf1p, and of the concerted action of Lcb3p and Lcb4p, may be to generate a localized pool of sphingoid bases to activate targets, including the Ypk1p pathway. Cells lacking SUR2 are unable to generate PHS (Figure 6B; Haak et al. 1997), yet they do not exhibit a growth defect and are able to generate complex sphingolipids with DHS as the sphingoid base backbone (Haak et al. 1997). In sur2Δ cells, Svf1p, Lcb3p, and Lcb4p may generate a localized pool of DHS that activates the Ypk1/2p pathway. DHS can stimulate Ypk1 activity, albeit with less efficiency than PHS (Liu et al. 2005a). However, loss of Sur2p in combination with the inability to localize sphingoid bases through the additional loss of Svf1p, Lcb3p, or Lcb4p may significantly reduce Ypk1p activation leading to growth arrest similar to that observed when YPK1 is deleted. Supporting this model, we do not observe growth defects in cells lacking Sur2p in combination with Lcb5p or Dpl1p, proteins not thought to be part of this coupling reaction (Figure 4).
These synthetic interactions with SUR2 support a model in which PHS generated by Sur2p and the localized generation of PHS by Svf1p, Lcb3p, and Lcb4p converge on activation of a pathway required for growth. Genetic evidence suggests that Svf1p and Sur2p influence activity of Ypk1p or a downstream pathway regulated by Ypk1p. We have demonstrated that YPK1-null cells exhibit a similar phenotype to that of sur2Δsvf1Δ, suggesting that lack of Ypk1p activity contributes to the phenotype of these cells. Alternatively, Svf1p and Sur2p may regulate the homologous kinase Ypk2p. YPK2 demonstrates a synthetic lethality with YPK1 (Chen et al. 1993), and SVF1 and SUR2 also exhibit lethality in combination with YPK1. These genetic interactions affecting viability are specific to YPK1: double knockouts between SVF1 and YPK2 or the upstream kinases PKH1/2 are viable and exhibit no growth defect (data not shown). Studies are ongoing to further understand the relationship between the Svf1p/Sur2p and the Ypk1/2 pathways.
High levels of SPH are toxic to wild-type cells, but the mechanism of cell death is not clear. The resistance to SPH seen in svf1Δ and the reversal of SPH toxicity seen in both sur2Δsvf1Δ and ypk1Δ strains suggest that cell viability is dependent on a common pathway regulated by Sur2p/Svf1p and Ypk1p. In vitro, it has been demonstrated that Ypk1p can be activated by SPH and that SPH is more potent than PHS at inducing Ypk1p activity (Liu et al. 2005a). In addition, it has been shown that constitutive overexpression of the catalytically active kinase domain of Ypk1p is growth inhibitory (Roelants et al. 2002). These data support a model in which overactivation of Ypk1p by SPH leads to growth arrest. One hypothesis is that SPH requires phosphorylation/dephosphorylation prior to activation of targets in the cell, and lack of SVF1 prevents this activation. This phosphorylation/dephosphorylation step may be required to properly localize SPH promoting Ypk1p activation. Specific localization of SPH and other sphingolipids by Svf1p may be required to recruit kinases, including Ypk1p, to specific cellular locations. Therefore, in the absence of Svf1p, SPH may be unable to activate targets that induce growth arrest. We demonstrate here that addition of SPH to wild-type cells reduces the intracellular level of C18-PHS, but has the opposite effect in svf1Δ cells. Adaptation of svf1Δ cells to survival with lower C18-PHS may contribute to the SPH resistance observed in this strain.
We present a model in which SVF1 regulates the localized generation of a pool of PHS. Previously observed phenotypes of svf1Δ suggest that a function of Svf1p may be to allow cells to adapt to rapid changes in environmental conditions, including the diauxic shift, temperature downshift, and oxidative stress (Vander Heiden et al. 2002; Brace et al. 2005). The hypothesized subset of PHS dependent on Svf1p may activate specific signaling pathways, including the Ypk1p pathway, that protect cells from these stresses. It has been suggested that Ypk1p plays a role in the cell wall integrity (CWI) pathway that is implicated in maintenance of viability under a variety of stress conditions. Activation of the CWI pathway in response to H2O2 and other oxidative stressors has been observed (Levin 2005; Vilella et al. 2005). The full complement of downstream components regulated by Ypk1p has not been defined, and other cellular processes may be regulated that influence survival. Genetic suppression of phenotypes observed in ypk1Δ or svf1Δsur2Δ strains may provide insight into these processes.
Although Svf1p affects PHS levels, Svf1p lacks sequence homology to domains conserved in hydroxylases, and it is unlikely that Svf1p generates PHS enzymatically. One possibility is that Svf1p may regulate the hydrolysis of ceramide or complex sphingolipids in the membrane, resulting in a localized production of PHS. Svf1p contains a predicted transmembrane domain by EMBOSS (Rice et al. 2000) and may reside in cellular membranes. Sphingoid bases, generated in the cytosol, might be recognized by the cell in the same way as exogenous addition. Evidence from mammalian cells suggests that SPH generated de novo and via hydrolysis of complex sphingolipids plays distinct roles in responding to cellular stress (Levade and Jaffrezou 1999; Sawai and Hannun 1999), and recent data suggest that a similar response exists in yeast (Cowart et al. 2006). In fact, the hydrolysis pathway in yeast appears to play a more important role in regulation of carbon source utilization and yeast reproduction. This is interesting, given that svf1Δ cells exhibit a defect in the diauxic shift.
Cellular localization of second messengers is important for proper activation of signaling pathways. Generation of PIP3 on mammalian cell membranes recruits kinases such as Akt to the membrane, where it can be acted on by its upstream kinase, PDK1. Localization is an important component regulating specificity in activation of signaling cascades. A similar situation may occur in yeast, where generation of PHS in defined intracellular locations could activate kinases in a specific manner and result in different outcomes on the basis of the location of the signal and the surrounding proteins. It has been demonstrated that phosphorylation and localization of Ypk1p is regulated by sphingoid bases, where addition of PHS recruits Ypk1p to the plasma membrane (Sun et al. 2000). A regulated subset of PHS dependent on Svf1p may influence Ypk1p localization, resulting in differential responses to stress. Further characterization of these pathways may shed light on the signaling properties of sphingoid bases in both mammalian and yeast cells.
Acknowledgments
We thank S. J. Kron and J. D. Boeke for yeast strains. C.M.R. is supported by the Flight Attendant Medical Research Institute and by the Burroughs Wellcome Fund. Work in R.C.D.'s laboratory was supported by a grant (GM41302) from the National Institutes of Health and by a grant (P20-RR020171) from the National Center for Research Resources.
References
- Alvarez-Vasquez, F., K. J. Sims, L. A. Cowart, Y. Okamoto, E. O. Voit et al., 2005. Simulation and validation of modelled sphingolipid metabolism in Saccharomyces cerevisiae. Nature 433: 425–430. [DOI] [PubMed] [Google Scholar]
- Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, III et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- Balguerie, A., M. Bagnat, M. Bonneu, M. Aigle and A. M. Breton, 2002. Rvs161p and sphingolipids are required for actin repolarization following salt stress. Eukaryot. Cell 1: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood, C. J., J. D. Saba, R. C. Dickson and K. W. Cunningham, 2001. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J. Biol. Chem. 276: 11712–11718. [DOI] [PubMed] [Google Scholar]
- Brace, J. L., D. J. Vanderweele and C. M. Rudin, 2005. Svf1 inhibits reactive oxygen species generation and promotes survival under conditions of oxidative stress in Saccharomyces cerevisiae. Yeast 22: 641–652. [DOI] [PubMed] [Google Scholar]
- Casamayor, A., P. D. Torrance, T. Kobayashi, J. Thorner and D. R. Alessi, 1999. Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr. Biol. 9: 186–197. [DOI] [PubMed] [Google Scholar]
- Chen, P., K. S. Lee and D. E. Levin, 1993. A pair of putative protein kinase genes (YPK1 and YPK2) is required for cell growth in Saccharomyces cerevisiae. Mol. Gen. Genet. 236: 443–447. [DOI] [PubMed] [Google Scholar]
- Chung, N., G. Jenkins, Y. A. Hannun, J. Heitman and L. M. Obeid, 2000. Sphingolipids signal heat stress-induced ubiquitin-dependent proteolysis. J. Biol. Chem. 275: 17229–17232. [DOI] [PubMed] [Google Scholar]
- Chung, N., C. Mao, J. Heitman, Y. A. Hannun and L. M. Obeid, 2001. Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae. J. Biol. Chem. 276: 35614–35621. [DOI] [PubMed] [Google Scholar]
- Cowart, L. A., Y. Okamoto, X. Lu and Y. A. Hannun, 2006. Distinct roles for de novo versus hydrolytic pathways of sphingolipid biosynthesis in Saccharomyces cerevisiae. Biochem. J. 393: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier, O., 2002. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta 1585: 153–162. [DOI] [PubMed] [Google Scholar]
- Dickson, R. C., and R. L. Lester, 1999. Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1438: 305–321. [DOI] [PubMed] [Google Scholar]
- Dickson, R. C., and R. L. Lester, 2002. Sphingolipid functions in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1583: 13–25. [DOI] [PubMed] [Google Scholar]
- Dickson, R. C., E. E. Nagiec, M. Skrzypek, P. Tillman, G. B. Wells et al., 1997. Sphingolipids are potential heat stress signals in Saccharomyces. J. Biol. Chem. 272: 30196–30200. [DOI] [PubMed] [Google Scholar]
- Ferguson-Yankey, S. R., M. S. Skrzypek, R. L. Lester and R. C. Dickson, 2002. Mutant analysis reveals complex regulation of sphingolipid long chain base phosphates and long chain bases during heat stress in yeast. Yeast 19: 573–586. [DOI] [PubMed] [Google Scholar]
- Friant, S., B. Zanolari and H. Riezman, 2000. Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. EMBO J. 19: 2834–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friant, S., R. Lombardi, T. Schmelzle, M. N. Hall and H. Riezman, 2001. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 20: 6783–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato, K., R. Lombardi, B. Vallee and H. Riezman, 2003. Lcb4p is a key regulator of ceramide synthesis from exogenous long chain sphingoid base in Saccharomyces cerevisiae. J. Biol. Chem. 278: 7325–7334. [DOI] [PubMed] [Google Scholar]
- Gietz, D., A. St. Jean, R. A. Woods and R. H. Schiestl, 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak, D., K. Gable, T. Beeler and T. Dunn, 1997. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 272: 29704–29710. [DOI] [PubMed] [Google Scholar]
- Hannun, Y. A., C. Luberto and K. M. Argraves, 2001. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry 40: 4893–4903. [DOI] [PubMed] [Google Scholar]
- Hearn, J. D., R. L. Lester and R. C. Dickson, 2003. The uracil transporter Fur4p associates with lipid rafts. J. Biol. Chem. 278: 3679–3686. [DOI] [PubMed] [Google Scholar]
- Jenkins, G. M., and Y. A. Hannun, 2001. Role for de novo sphingoid base biosynthesis in the heat-induced transient cell cycle arrest of Saccharomyces cerevisiae. J. Biol. Chem. 276: 8574–8581. [DOI] [PubMed] [Google Scholar]
- Jenkins, G. M., A. Richards, T. Wahl, C. Mao, L. Obeid et al., 1997. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J. Biol. Chem. 272: 32566–32572. [DOI] [PubMed] [Google Scholar]
- Kim, S., H. Fyrst and J. Saba, 2000. Accumulation of phosphorylated sphingoid long chain bases results in cell growth inhibition in Saccharomyces cerevisiae. Genetics 156: 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, C. C., F. T. Zenke, P. E. Dawson, E. M. Dutil, A. C. Newton et al., 2000. Sphingosine is a novel activator of 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 275: 18108–18113. [DOI] [PubMed] [Google Scholar]
- Lester, R. L., and R. C. Dickson, 2001. High-performance liquid chromatography analysis of molecular species of sphingolipid-related long chain bases and long chain base phosphates in Saccharomyces cerevisiae after derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate. Anal. Biochem. 298: 283–292. [DOI] [PubMed] [Google Scholar]
- Levade, T., and J. P. Jaffrezou, 1999. Signalling sphingomyelinases: Which, where, how and why? Biochim. Biophys. Acta 1438: 1–17. [DOI] [PubMed] [Google Scholar]
- Levin, D. E., 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69: 262–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K., X. Zhang, R. L. Lester and R. C. Dickson, 2005. a The sphingoid long chain base phytosphingosine activates AGC-type protein kinases in Saccharomyces cerevisiae including Ypk1, Ypk2, and Sch9. J. Biol. Chem. 280: 22679–22687. [DOI] [PubMed] [Google Scholar]
- Liu, K., X. Zhang, C. Sumanasekera, R. L. Lester and R. C. Dickson, 2005. b Signalling functions for sphingolipid long-chain bases in Saccharomyces cerevisiae. Biochem. Soc. Trans. 33: 1170–1173. [DOI] [PubMed] [Google Scholar]
- Mandala, S. M., R. Thornton, Z. Tu, M. B. Kurtz, J. Nickels et al., 1998. Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc. Natl. Acad. Sci. USA 95: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, C., M. Wadleigh, G. M. Jenkins, Y. A. Hannun and L. M. Obeid, 1997. Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J. Biol. Chem. 272: 28690–28694. [DOI] [PubMed] [Google Scholar]
- Mao, C., J. D. Saba and L. M. Obeid, 1999. The dihydrosphingosine-1-phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses. Biochem. J. 342 (Pt. 3): 667–675. [PMC free article] [PubMed] [Google Scholar]
- Obeid, L. M., Y. Okamoto and C. Mao, 2002. Yeast sphingolipids: metabolism and biology. Biochim. Biophys. Acta 1585: 163–171. [DOI] [PubMed] [Google Scholar]
- Ogretmen, B., and Y. A. Hannun, 2004. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer 4: 604–616. [DOI] [PubMed] [Google Scholar]
- Pyne, S., and N. J. Pyne, 2000. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 349: 385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qie, L., M. M. Nagiec, J. A. Baltisberger, R. L. Lester and R. C. Dickson, 1997. Identification of a Saccharomyces gene, LCB3, necessary for incorporation of exogenous long chain bases into sphingolipids. J. Biol. Chem. 272: 16110–16117. [DOI] [PubMed] [Google Scholar]
- Rice, P., I. Longden and A. Bleasby, 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16: 276–277. [DOI] [PubMed] [Google Scholar]
- Roelants, F. M., P. D. Torrance, N. Bezman and J. Thorner, 2002. Pkh1 and pkh2 differentially phosphorylate and activate ypk1 and ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol. Biol. Cell 13: 3005–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants, F. M., P. D. Torrance and J. Thorner, 2004. Differential roles of PDK1- and PDK2-phosphorylation sites in the yeast AGC kinases Ypk1, Pkc1 and Sch9. Microbiology 150: 3289–3304. [DOI] [PubMed] [Google Scholar]
- Saba, J. D., and T. Hla, 2004. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 94: 724–734. [DOI] [PubMed] [Google Scholar]
- Sawai, H., and Y. A. Hannun, 1999. Ceramide and sphingomyelinases in the regulation of stress responses. Chem. Phys. Lipids 102: 141–147. [DOI] [PubMed] [Google Scholar]
- Skrzypek, M. S., M. M. Nagiec, R. L. Lester and R. C. Dickson, 1998. Inhibition of amino acid transport by sphingoid long chain bases in Saccharomyces cerevisiae. J. Biol. Chem. 273: 2829–2834. [DOI] [PubMed] [Google Scholar]
- Skrzypek, M. S., M. M. Nagiec, R. L. Lester and R. C. Dickson, 1999. Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J. Bacteriol. 181: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel, S., and R. Kolesnick, 2002. Sphingosine 1-phosphate as a therapeutic agent. Leukemia 16: 1596–1602. [DOI] [PubMed] [Google Scholar]
- Spiegel, S., and S. Milstien, 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4: 397–407. [DOI] [PubMed] [Google Scholar]
- Sun, Y., R. Taniguchi, D. Tanoue, T. Yamaji, H. Takematsu et al., 2000. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol. Cell. Biol. 20: 4411–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toman, R. E., S. Milstien and S. Spiegel, 2001. Sphingosine-1-phosphate: an emerging therapeutic target. Expert Opin. Ther. Targets 5: 109–123. [DOI] [PubMed] [Google Scholar]
- Vander Heiden, M. G., J. S. Choy, D. J. VanderWeele, J. L. Brace, M. H. Harris et al., 2002. Bcl-x(L) complements Saccharomyces cerevisiae genes that facilitate the switch from glycolytic to oxidative metabolism. J. Biol. Chem. 277: 44870–44876. [DOI] [PubMed] [Google Scholar]
- Vilella, F., E. Herrero, J. Torres and M. A. de la Torre-Ruiz, 2005. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280: 9149–9159. [DOI] [PubMed] [Google Scholar]
- Zanolari, B., S. Friant, K. Funato, C. Sutterlin, B. J. Stevenson et al., 2000. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19: 2824–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., M. S. Skrzypek, R. L. Lester and R. C. Dickson, 2001. Elevation of endogenous sphingolipid long-chain base phosphates kills Saccharomyces cerevisiae cells. Curr. Genet. 40: 221–233. [DOI] [PubMed] [Google Scholar]