Abstract
CSV consists of a very complex of molecules and demonstrates significant cellular activities capable of stimulating immune functions in vivo. The purpose of this study was to analyze the effects of CSV on sex, weight, route of injection and the balance of pro- and anti-inflammatory cytokines in mice. The susceptibility and route of injection were analyzed by lethal (LD50) determination. The effects of CSV were also analyzed in blood from immunized mice using detection by means of antibodies and mediators production. Several functional bioassays were employed: TNF activity was assayed by measuring its cytotoxic activity in L929 cells, and other cytokines were assayed by enzyme-linked immunosorbent assay, whereas nitric oxide levels were detected by Griess colorimetric reactions in sera from BALB/c mice. After injecting subcutaneously, the LD50 presented an increase of the CSV correlation and similar levels of susceptibility were obtained for female and male from BALB/c mice. Significant differences were observed in the time-course of cytokine levels. The balance of pro- and anti-inflammatory cytokines TNF/IL-10 and IL-6/IL-10 ratios were significantly higher in injected mice group when compared with those obtained for non-injected group. The CSV is poor in antigenic composition and it is difficult to get antibodies specific to neutralizing the lethal factor. The effect of immunization with 0.5 LD50 of CSV on the balance of pro- and anti-inflammatory cytokines was measured. The maximum levels of TNF and IL-6, IFN-γ and NO were observed on days 7 and 21 after immunization, respectively. IL-10 levels peaked between days 21 and 28 after immunization with CSV. With respect to balance of pro- and anti-inflammatory cytokines it was possible to observe that negative correlation between serum levels of IL-6/IL-10 and TNF/IL-10 exists. These ratios may possibly reflect the balance of pro- and anti-inflammatory cytokines in serum, which may by manifested in the inflammatory status during the envenoming processes. In conclusion, an increase in the serum levels of TNF and IL-6 may be a useful marker for scorpion envenomation.
INTRODUCTION
Scorpion Centruroides noxius is considered one of the most dangerous species to humans in Mexico. It is well established that the predominant lethal action of scorpion venom exerts a variety of effects on excitable tissues. These venoms exert a variety of effects on excitable tissues and exhibit enormous variety according to the species. Victims of scorpion stings presented perturbation of the nervous, cardiovascular, and respiratory systems [1]. The specific signs are directly related to the venom components, the patients stung by scorpions may develop a systemic inflammatory response syndrome, and the balance of cytokines released may also play a major role in the pathogenesis [2–4].
A successful immune response agent on constructed using some of their structural components is dependent on the activation of an appropriate set of immune effectors function. The two arms of this response, cell and antibody-mediated immune responses, though both are dependent on a proper antigen presentation by antigen-presenting cells, are regulated by distinct subsets of CD4+ helper T cells that are divided in two main subsets, Th1 and Th2 [5]. These two subsets of the Th cells differ in the effectors functions and mainly in the repertoire of cytokines that they secrete in response to antigenic stimulation. Th1 cells exclusively secrete interferon-gamma (IFN-γ), interleukin-12 (IL-12), and IL-2, whereas Th2 cells secrete IL-4, IL-5, IL-6, IL-10, and IL-13. There are also some cytokines such as IL-3, tumor necrosis factor (TNF-α), which is common to both subsets [5]. Th1 cells protect against intracellular microorganisms, induce the transient production of IgG2a opsonizing antibodies, and promote delayed-type hypersensitivity responses. In contrast, Th2 cells protect against extracellular pathogens, which are mainly eradicated by neutralizing antibodies that include the induction of IgE and the IgA. Cytokines are direct mediators of inflammation and influence the progress and direction of many immunological reactions [6]. They may be divided into proinflammatory cytokines such as TNF, IL-1, and IL-8, that include the mobilizing immune system cells to proliferate and produce more cytokines creating an inflammatory cascade, and as anti-inflammatory cytokines, such as IL-10 whose function is to dampen or control the inflammatory response.
At a local site of injury or infection and during the initial appearance of pro- and anti-inflammatory mediators in the circulation, the beneficial effects of these compounds counter balance their effects. The production of pro- and anti-inflammatory cytokines is strongly controlled by complex feedback mechanisms [5, 7]. Proinflammatory cytokines are primarily responsible for initiating an effect against exogenous pathogens. However, excessive production of these mediators may significantly contribute to shock, multiple organ failure, and death [5, 8, 9]. In contrast, anti-inflammatory cytokines are crucial for down regulating the incremented inflammatory process and maintaining homeostasis for the correct functionating of vital organs [10, 11]. A balanced ratio of pro- and anti-inflammatory cytokines is important for appropriate immune response; excessive inflammation or hyporesponsiveness can lead to complications.
Many different cytokines have been described in severe envenomation and among these, TNF-α, IL-1, and IL-6, that seem to be the major proinflammatory cytokines and secondarily of anti-inflammatory cytokines, such as IL-10 [3]. TNF-α is one of the most important cytokines involved in the pathophysiology of envenomation. IL-1 and TNF-α are synergistic and share many biological effects in sepsis [12]. The role of IL-6 is controversial; it has both pro- and anti-inflammatory properties [13]. It down-regulates the synthesis of IL-1 and TNF and has little effect on the synthesis of IL-10 [14, 15]. IL-6 also inhibits the expression of the other proinflammatory cytokine, TNF-α, thus introducing a controlling step in the amplification of inflammation [16].
Cytokine production in severe envenomation has been studied, and it seems that two forces, pro- and anti-inflammatory cytokines, are elevated during these processes. However, their clinical significance and prognostic value have not been elucidated [3]. During envenomation, both pro- and anti-inflammatory cytokines response, although there is a shift toward increased anti-inflammatory and reduced proinflammatory cytokine production. It seems that a complex network of interactions between different cytokines and possibly other components of the immune response takes place during severe envenomation. The pathogenesis of envenomation is characterized by an imbalanced activation of Th1 and Th2 cells. IL-10 is the main biological function to limit and terminate the inflammatory responses blocking the Th1-derived cytokines. Inadequate concentrations of IL-10 can result in excess inflammation.
The purposes of the present study are (1) to analyze the influence of sex, weight, and route of Centruroides scorpion venom (CSV) injection, (2) to investigate the symptoms, antibody production, and mediators released following injection of whole CSV, and (3) to evaluate the inflammatory status during the envenoming processes.
MATERIALS AND METHODS
Materials
Actinomycin D, o-phenylenediamine (OPD), naphthylenediamine (NADPH), sulphonylamide (FAD), NO2 reductase, fosforic acid, antimouse IgG peroxidase conjugate were supplied by Sigma, St Louis, MO, USA. Fetal calf serum (FCS) was obtained from Equitech-Bio, Inc. Kerrville. Avidin-horseradish peroxidase, 2,2′-azino-bis(3-ethyl-benzthiazoline-6-sulfonic acid) (ABTS) were supplied by Sourthen Biotechnology Associates Inc., USA. Antimouse IL-6 (clones MP5-20F3 and MP5-32C11), rIL-6, antimouse IL-10 (clones JES5-2 A and SCX), rIL-10, antimouse IFN-γ (clones XMG1.2 and AN18), rIFN-γ were purchased from Pharmingen, Calif, USA. All other reagents were of analytical grade.
Animals
BALB/c female mice (12–20 g body weight) were supplied by Bioterio (Instituto de Biotecnología, UNAM, Mexico). Mice were maintained and used according the animal welfare international recommendation for animal welfare (Committee Members, International Society in Toxicology, 1992) [17].
Venom
Lyophilized crude venom from adult specimens of Centruroides noxius scorpion venom (CSV) are pooled from more than 20 adult specimens and referred to as CSV. Centruroides noxius scorpion venom was generously given by Dr Jorge Paniagua (Laboratorio Silanes, DF, Mexico).
Lethality
The lethal dose (LD50) was estimated by injecting intraperitoneally and/or subcutaneously with different doses of CSV into groups of five BALB/c female and male mice with different ages, sex, and weights. Deaths occurring during 24 h were recorded and LD50 was calculated by probit.
Effects of centruroides scorpion venom (CSV) on cytokine levels
Groups of mice were injected subcutaneously with different amounts of CSV, dissolved in 0.1 mL of saline solution. Control mice received 0.1 mL of saline solution. Since mortality was of a fraction of the injected animals, the number of mice per experimental group ranged between 5 and 15 in order to obtain blood samples from at least five mice for each time interval. Mice were bled at different times, and sera were separated and stored at −20°C until use.
Immunization of mice
The immunization and bleeding schedules were as follows. Group of 15 mice were subcutaneously immunized at weekly intervals for three times with saline solution, or CSV (0.5LD50 = 7.75 μg/mouse), respectively. After different time intervals, animals were bled from the retro-orbital to evaluate the immune response. Sera samples were stored frozen at −20°C until use. One week after the third immunization the control or immunized mice were challenged intraperitoneally with 15.5 μg (1 LD50) or 77.5 μg (5 LD50) per animal of CSV. The mortality was recorded at 24, 48, and 72 h after the challenge.
Antibody titration
Specific antibodies against antigens used for the immunization were estimated by standard ELISA, which was performed as described by Gebara et al [18]. In brief, ELISA plates were coated with 100 μL of the appropriate antigen CSV. The antigen were diluted to a concentration of 2 μg per well in 0.1 M NaHCO3, pH 8.2, and incubated at room temperature for 6 h. After this time, the wells were washed three times with PBS containing 0.05% Tween-20 (PBS/Tween), then blocked by addition of blocking buffer 10% defated milk and 2% fetal calf serum (FCS), and the plates were incubated at 4°C for a further period of 18 h. After washing the wells with PBS/Tween, the plates were incubated with serial dilutions of sera from mice non-injected and/or immunized for different times for 2 h at room temperature. After washing the wells, 50 μL of 1 : 1000 dilution of biotinylated goat antimouse immunoglobulin avidin-horseradish peroxidase-conjugated were added to each well. The plates were incubated 2 h at room temperature, the wells were washed five times with PBS/Tween, and 50 μL of the substrate buffer containing 0.2% o-phenylenediamine and 0.015% H2O2 was added to each well. The reaction was stopped with 4.5 M H2SO4, and the absorbance at 490 nm was measured in a microplate reader (BIO-RAD 3550-UV model). Sera samples were considered to be positive for antibody response if the absorbance value was greater than 0.1 and the ratio between sample optical density absorbance and control absorbance was 2 or more.
Cytokine assay
IL-6, IL-10, and IFN-γ were assayed by two-site sandwich enzyme-linked immunosorbent assay ELISA method as described by Schumacher et al [19]. In brief, the wells of ELISA plates were coated for 6 at room temperature with 100 μL of 0.1 M sodium carbonate buffer (pH 8.2) containing the appropriate dilutions of the first antibody rat antimouse IL-6 antibody MP5-20F3, rat antimouse IL-10 antibody JES5-2A or rat antimouse IFN-γ antibody XMG1.2. The wells were then washed with 0.1% phosphate-buffered saline (PBS-Tween-20) and blocked with 100 μL of 10% FCS in PBS for 2 h at room temperature. After washing, duplicate sera samples of 50 μL were added to the well. After 18 h of incubation at 4°C, the wells were washed and incubated with 100 μL (2 μg/mL) of biotinylated monoclonal antibodies rat antimouse IL-6 MP5.32C11 or antimouse IL-10 SCX or antimouse IFN-γ AN18, as second antibodies by 45 min at room temperature. After washing, 50 μL of avidin-horseradish peroxidase conjugate were added and the plates were incubated for a further 30 min period also at room temperature. After washing, 50 μL of substrate buffer containing 150 μg/mL of 2,2′-azino-bis(3 ethyl benzthiazoline-6-sulfonic acid) (ABTS) were added to each well. The reaction was stopped after 30 min with N,N-dimethyl formamide (DMF) plus sodium dodecyl sulfate (SDS) and the absorbance was measured at 405 nm in a microplate reader. Sera cytokine levels were determined using standard curve established with the appropriate recombinant cytokines (expressed in pg/mL). The minimum levels of each cytokine detectable in the conditions of the assays were 50 pg/mL for IL-6, IFN-γ, and IL-10.
TNF was assayed by evaluating its cytotoxic activity on the target fibroblast cell line L-929, as described by Ruff and Gifford [20]. To monolayer of L-929 cells in medium RPMI-1640 supplemented with 5% FCS (3–5 × 104 cells per well) 50 μL of serum samples serially diluted in RPMI-1640 containing 1.0 μg/mL of actinomycin D were added. After incubation for 18 h at 37°C in a humidified atmosphere of 5% CO2, the supernatants were removed and the remaining living cells were assessed after fixing and staining with crystal violet (0.2% in 20% methanol). The absorbance at 620 nm of each well was red in a microplate reader. Cytotoxic was calculated as follows: (Abscontrol – Abssample / Abscontrol)×100. TNF activity was expressed in nanograms per milliliter, using a standard curve prepared with recombinant TNF-α. The detection limit of this assay was 5 pg/mL.
Nitrite determination
The nitrite levels in female mice sera as an indication of NO production were determined as previously described [21]. In brief, 40 μL of each mice sera sample were incubated in a 96-well, flat-bottomed plate with 40 μL of the reduction solution (NADPH, 1.25 ng/mL; FAD 10.4 ng/mL; KH2PO4, 0.125 M) containing 0.5 U of NO2− reductase for 2 h at 37°C. After this time, 80 μL of Griess reagent (0.1% naphthylenediamine hydrochloride, 1% sulphonylamide, 3% H3PO4) were added to each well. The optical densities were measured at 540 nm in a microplate reader and NO2− concentrations were determined using a standard curve of NaNO3 ranging from 1.25 to 275 nM (expressed as nMol/mL).
Statistical analyses
Results are presented as the mean ± standard deviations (SD). Statistical analysis was performed by the Student's t-test, and the level of significance was set at P < .05.
RESULTS
Effect of CSV on female and male mice
Sex and route of injection
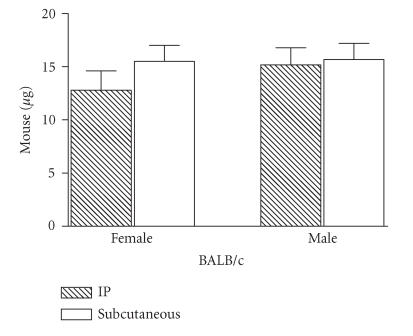
To verify whether the mouse sex and route of injection showed an effect on mortality, CSV was injected intraperitoneally or subcutaneously, at different concentrations, in female or male BALB/c strain mice to determine the median lethal doses (LD50). Figure 1 shows that the LD50 of CSV in BALB/c female mice by the intraperitoneally and subcutaneously routes were 12.8 and 15.5 μg/mice, respectively. When mice received an intraperitoneally and/or subcutaneously injection of one LD50 of CSV, a fraction of the injected animals died. The time-course of mortality differed between the routes. In CSV intraperitoneally injected female mice, the majority of deaths occurred within the first 2 h. In contrast, most of the deaths occurring after subcutaneously injection were observed at later time intervals of 4 and 5 h (results not shown). No deaths were observed in injected with saline solution. No different sensibilities for various amounts of venom were observed in both groups of animal injected intraperitoneally or subcutaneously (Figure 1).
Figure 1.
Effects of CSV toxicity according to mouse sex. Groups of female mice from different strains, 16–20 g of body weight, were injected intraperitoneally or subcutaneously with different amounts of CSV. The LD50 value was calculated by probit analysis of the death occurring within 24 h of venom injection. The experiment was repeated twice and each point represents the values of pooled sera from 5 animals ± SD.
Body weight
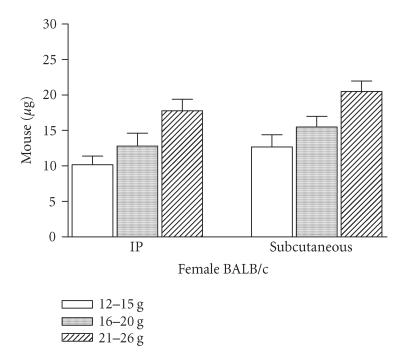
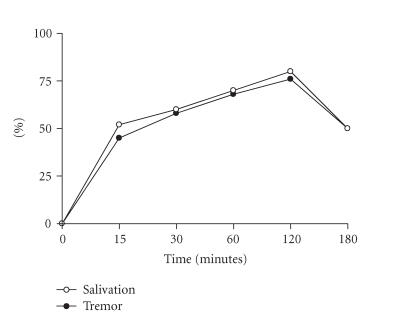
To verify whether body weight showed an effect on mortality, BALB/c female mice were injected intraperitoneally or subcutaneously with different CSV concentrations. These animals were distributed in three groups, with different body weights. As body weight increased, it was possible to observe a decrease in susceptibility for all groups (Figure 2). When mice received an intraperitoneally or subcutaneously injection of 1 LD50 of CSV, the time-course of mortality did not differ between the groups studied. In all groups, the majority of deaths occurred within first 2 and 4 h for intraperitoneally and subcutaneously routes, respectively. Death was usually preceded by certain signals or symptoms such as salivation and tremor. Groups of BALB/c female mice of 16–20 g of body weight were injected subcutaneously with 1 LD50 of CSV, and at different intervals of time specific signs were observed (Figure 3). The maximum number of animals that presented salivation and tremor was observed at 120 min post-injection, decaying thereafter.
Figure 2.
Effect of CSV on body weight. Groups of female mice from BALB/c strain, with different body weights, were injected intraperitoneally and/or subcutaneously with different amounts of CSV. Deaths occurring during 24 h were recorded and the LD50 value was calculated. The experiment was repeated twice and each point represents the values of pooled sera from 5 animals ± SD.
Figure 3.
Symptoms. Groups of 15 female mice from the BALB/c strain, 16–20 g of body weight, were injected subcutaneously with 1 LD50 of CSV. Each point represents the percent animal number with salivation and/or tremor. The experiment was repeated twice and each point represents the values of pooled sera from 5 animals ± SD.
Mediators production
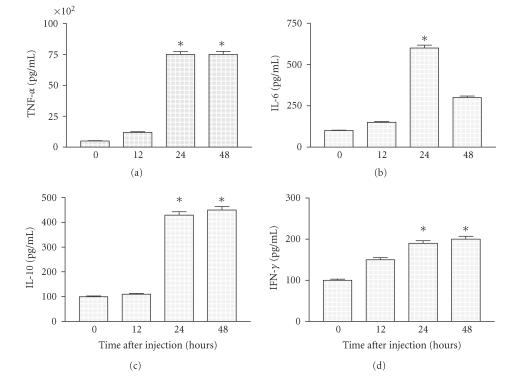
To determine mediators production, groups of BALB/c female mice with 16–20 g of body weight were injected subcutaneously with 1 LD50 of CSV and bled after different time intervals. Mice injected with saline solution had undetectable levels of all mediators assayed in the serum. These animals were divided into two groups: one that showed severe symptoms of envenomation, and the other that did show moderate symptoms. The peak sera IL-6, IFN-γ, and IL-10 levels were significantly elevated in all injected groups compared with those obtained for non-injected (P < .001) (data not shown). The maximum levels of cytokines in injected sera mice that showed severe symptoms of envenomation could be observed until 12 h after injecting, decaying thereafter (data not shown). Figure 4 shows that the maximum levels of IL-6 at 24 h decaying thereafter. The highest levels of TNF and IFN-γ were observed between 24–48 h (Figure 4). Interestingly, the peak maximum of IL-10 production was observed at 48 h after injection (Figure 4).
Figure 4.
Mediators production on mice with moderate symptoms. Groups of mice were injected with different amounts of CSV. Mice were bled at different time intervals after injection and sera were separated for cytokine studies determined as described in “Materials and Methods.” Experiments were repeated twice and each point represents the value of pooled sera from 5 animals ± SD. Statistical differences between the treatments were marked with asterisk (P < .001).
To evaluate the balance of pro- and anti-inflammatory cytokines TNF/IL-10 and IL-6/IL-10 ratios were determined. TNF/IL-10 ratios were significantly higher in injected mice group (P < .001), at 24 and 48 h when compared with those obtained for non-injected group (Table 1). In contrast, IL-6/IL-10 ratios were discreet incremented after 24 h of injection, decaying thereafter.
Table 1.
Balance of pro- and anti-inflammatory cytokines. Groups of mice were injected subcutaneously with 1 LD50 of CSV, noninjected group was treated with saline solution. Sera cytokine concentrations (pg/mL) in injected groups and/or noninjected group at 24 and 48 h of envenoming evaluation.
| TNF/IL-10 | IL-6/IL-10 | |
| 0 h | ||
| Injected groups | 0 | 0 |
| Non-injected groups | 0 | 0 |
| 24 h | ||
| Injected groups | 17.44(a) | 1.39(b) |
| Non-injected groups | 4.5 | 0.90 |
| 48 h | ||
| Injected groups | 16.66(a) | 0.96(b) |
| Non-injected groups | 4.5 | 0.90 |
(a)P < .001, (b)not significant.
Taking these results, it was possible to establish the optimal conditions for BALB/c mice exposure to CSV. Thus in the following set of experiments, the female groups were treated by subcutaneously route with 0.5 LD50.
Immune response induced in mice
Antibody production and protection
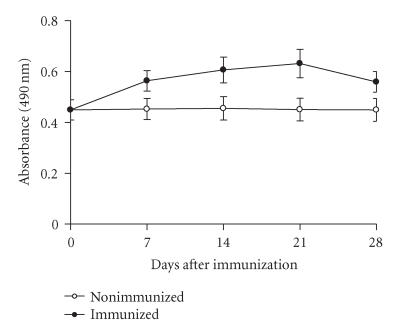
To verify if the different abilities of CSV to stimulate the mouse immune system were due to the kinetics of antibody and mediators production, groups of BALB/c female mice were weekly immunized subcutaneously with 0.5 LD50 of CSV each seven days. At days 7, 14, 21, or 28 after immunization, the animals from each corresponding group were bled, the sera pooled and used for antibody and mediators kinetic production. Figure 5 shows that IgG levels a discreet increase after immunization with CSV. The levels of antibodies in mice immunized with CSV increased up to 21 days, decaying thereafter (Figure 5).
Figure 5.
Antibody response in immunized mice. Immunizations were administered as described in Materials and Methods. Groups of mice immunized were bled on days 28 and the antiserum was tested on ELISA plates coated with CSV as described in “Materials and Methods.” The experiment was repeated twice and each point represents the value of pooled sera from 5 animals ± SD.
To test whether CSV the discreet the antibody production was also reflected in vivo upon challenge with CSV. Groups of mice immunized as described above were challenged with 1 or 5 LD50 of the CSV per animal, and the protection was evaluated. As is shown in Table 2 in control female mice the lethality of the venom was not neutralized at 50% and 0% for 1 and 5 LD50 of dose challenge, respectively. In contrast, for groups of mice previously immunized with CSV, the lethality percent neutralized were 60% and 20%, for 1 and 5 LD50 of dose challenge, respectively (Table 2). These results suggest that the CSV is poor in antigenic composition and it is difficult to get antibodies specific to neutralize the lethal factor.
Table 2.
Mortality ratio (number dead/number tested) of mice immunized with scorpion venom. Groups of mice were weekly subcutaneously immunized with 0.5 LD50 of CSV. One week after the third injection the animals were intraperitoneally challenged with 1 or 5 LD50 of CSV per animal. Nonimmunized mice were challenged with 1 or 5 LD50 per animal. The mortality was recorded at 24, 48, and 72 h.
| N°dead/N°tested | |||
| Group | Dose of challenge (% mortality) | ||
| 1 LD50 | 5 LD50 | ||
| Nonimmunized | 7/15 (50) | 15/15 (100) | |
| Immunized | 6/15 (40) | 12/15 (80) | |
None of the mice immunized showed any side-effects or, particularly, any toxicity symptoms during the immunization program. After challenge the toxic symptoms were observed in CSV groups. The evolution of clinical manifestations of envenoming in immunized groups was observed in minor of the animals with salivation and tremor after challenge. When comparing the number of animals with salivation and tremor between the nonimmunized groups and groups immunized with CSV, both challenged with 5 LD50 per animal of the venom, a lower number of animals presented this symptom observed in the latter group (data not shown).
Effect of immunization with CSV on mediator production
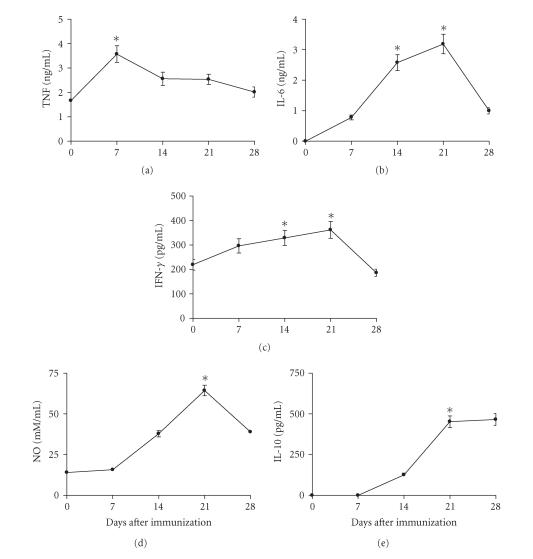
Sera from mice immunized subcutaneously with 0.5 LD50 of CSV were assayed for cytokines and NO on days 7, 14, 21, or 28 after immunization. Figure 6 shows that TNF production was significantly higher for sera from mice immunized with CSV when compared with those obtained from control group (P < .01). The highest levels of TNF produced in sera from mice immunized with CSV were observed on 7th day after immunization. The peak of IL-6 production was observed on day 21 after immunization for animals immunized with CSV (Figure 6). Figure 6 also shows that highest levels of IFN-γ were observed on days 14 and 21 after immunization. On day 21 after immunization, there was a significant increase in the production of IFN-γ in mice immunized with CSV (P < .001) when compared with those obtained from nonimmunized groups. The peak of IL-10 production was observed on days 21 and 28 after immunization with CSV (Figure 6). The IL-10 levels were significant higher for sera from mice immunized with CSV when compared with those obtained from control group (P < .001).
Figure 6.
Kinetics of pro- and anti-inflammatory cytokines. Groups of mice were immunized subcutaneously with 0.5 LD50. Sera samples were collected on days 0, 7, 14, 21, and 28 days after immunization. The cytokines levels were determined as described in “Materials and Methods.” The experiment was repeated twice and each point represents the values of pooled sera from 5 animals ± SD. Statistical differences between the treatments were marked with asterisk (P < .001 for IL-6, IL-10, IFN-γ, and NO) and (P < .01 for TNF).
The sera NO levels were assessed by determining the concentration of NO2−. At all time intervals of immunization, mice presented elevated levels when compared with those observed in group mice injected only with saline solution (Figure 6) (P < .001). The highest levels of NO2− production were observed on day 21 after immunization for animals immunized with CSV.
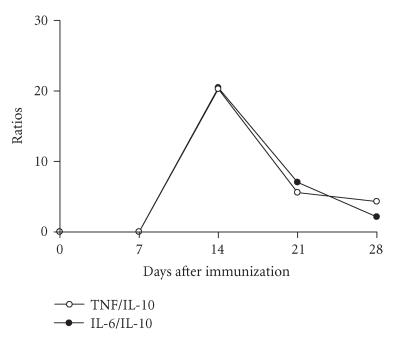
The balance of pro- and anti-inflammatory cytokines also was determined for the groups of animals immunized subcutaneously with 0.5 LD50 by different intervals of time. The immunization with CSV induced a marked increment in TNF/IL-10 and IL-6/IL-10 ratios sera levels, with an increase occurring within the first 14 days, decaying thereafter (Figure 7).
Figure 7.
Kinetics of balance of pro- and anti-inflammatory cytokines. Groups of mice were immunized subcutaneously with 0.5 LD50 of CSV as described above. The cytokines levels were determined as described in “Materials and Methods.” The TNF/IL-10 and IL-6/IL-10 ratios were calculated. The experiment was repeated twice and each point represents the values of pooled sera from 5 animals ± SD.
DISCUSSION
In Mexico, Centruroides noxius scorpion is responsible for an important number of accidents among humans. Scorpion venom consists of complex mixtures of several toxins that exhibit various biological activities. The already or experimentally injected animals may exhibit signs and symptoms which involve the central nervous systems, stimulation of autonomic system, and, occasionally, respiratory and heart failure, and even death [4, 22]. Various factors can contribute to the manifestation of specific signs and symptoms as reactions to stings with respect to the scorpion venom toxicity which may vary [23]. However it has been demonstrated that other factors may also be considered as clinical signs such as the age or size of the victims, for example, children are normally more severely affected, the site of the injection, and the individual's vulnerability to venom [4, 22, 24, 25].
The present study was designed to know the effects of CSV on mice with respect to the route and doses of venom administered, the severity of envenomation and the mediators' production were studied and discussed. The effect of CSV was studied to evaluate the susceptibility between female and male from BALB/c strain and the efficacy of the injection route. This study compared the effect of injecting CSV intraperitoneally or subcutaneously; and no different distribution of susceptibility was observed among analyzed animals. With respect to an increase in age and body weight, a different susceptibility was observed. The time-course of mortality did not differ between the groups studied.
The rapid absorption and distribution of scorpion venom toxins indicate that scorpion envenoming is an extreme emergence case. The victims may exhibit signs and symptoms involving the central nervous system stimulation of the autonomic nervous system, and occasionally respiratory and heart failure, even death [1, 4]. Specific signs and symptoms are usually manifested very soon after envenoming and develop into systemic inflammatory very soon after envenoming and develop into systemic inflammatory manifestations and organ failure. Salivation and tremor are rarely absent in scorpion envenomation. In this study the clinical manifestations of envenoming in animal groups injected with CSV showed that the symptom of salivation and tremor can be observed until 120 min after challenge. The presence of clinical signs in group mice injected with CSV was corroborated by a significant increase of mediators' levels. Increasing evidence from animal studies as well as clinical experience show that the involvement of the inflammatory cascade and release of cytokines play a major role in the pathogenesis of many envenoming syndromes.
Various studies have been revealed that most cytokines are a group of regulatory and immunomodulatory proteins involved in a number of physiological processes. The role of cytokines during inflammation is both initiation and fine-turning of the whole process: some cytokines initiate and amplify the response, others sustain or attenuate it, and some of them cause it to resolve.
The present study showed that in animal groups with severe symptoms of envenomating, the CSV is capable to induce the secretion of large amounts of TNF, IL-6, and IFN-γ. High levels of TNF-α and IL-6 are rapidly induced early in an envenomation and can be associated with a compromised prognosis for shock in animals. If they were over expressed, they may exacerbate the severity of an envenomation condition. In contrast, IL-10 inhibits the Th1-derived cytokines. Its main biological function is to limit and resolve the inflammatory responses.
Several studies have shown that some venom may induce a differential release of cytokines, with the theoretical potential to either accelerate or down regulate cytokine-induced organ dysfunction or shock. Snake and spider venoms seem to induce IFN-γ, TNF, and IL-6 release, [26–28]. Conversely, T serrulatus scorpion venom has been shown to down-regulate TNF-α production [25]. In a recent study, conducted with patients with different envenomation phases showed increases in TNF-α and IL-6 levels [2]. However, the pathogenesis of the envenomation is rather complex, involving a great variety of inflammatory molecules and coagulation factors, and therefore, the correlation between venom-induced release of cytokines and outcome is not clear. Envenomation is presumed to be driven by the release of proinflammatory cytokine, mainly TNF-α and IL-6, while many of compensatory anti-inflammatory response may be attributed to the biological effects of the anti-inflammatory cytokines, such as IL-10.
In order to evaluate the differential effects of CSV on the pro- and anti-inflammatory cytokine profile, this study was organized so as to enroll a homogeneous mice including control animal group. In the role of injected group, there were an increment increase in the inflammatory response and a parallel decrease in the anti-inflammatory response, as characterized by an increase in the TNF/IL-10 and IL-6/IL-10 ratios 24 h after venom injection. No such differences were noted in the control group. The anti-inflammatory effect of CSV was corroborated with the increase of IL-10 levels.
Most importantly, in the study of the serum baseline, TNF-α levels detected a very significant increase in the TNF-α/IL-10 ratio until 24 h, due to a modest decrease in serum TNF-α levels and an increase in serum IL-10 levels at 48 h after the venom injection. In contrast, the IL-6/IL-10 ratio and the cytokine levels were unchanged at all time points, while IL-6 levels were significantly higher, and serum IL-10 levels and the IL-6/IL-10 ratio were significantly lower than those in the non-injected group. It seems, therefore that there is a later (at 48 h of injection) no immunomodulatory effect of CSV in severe envenomation, especially if there is a massive production of IL-6, which is rapidly reversed, but not for TNF-α. It is accepted that the envenomation has a bimodal nature and is characterized by a primary rather brief proinflammatory phase, followed by a sustained anti-inflammatory phase.
This study was designed in order to know the inflammatory mediators following CSV immunization, which play roles in the amplification of both humoral and cell-mediated immune responses; and subsequently several strategies for applying injections were tested.
With respect to the antibodies production, the CSV is poor in antigenic composition and it is difficult to get antibodies specific to neutralize the lethal factor results shown in Table 2. As well as the humoral immune response, the cytokines were also evaluated.
The immunization with CSV was capable to stimulate the production of cytokines that may be directly involved and contribute to pathophysiologic changes in envenoming. The present study showed that mice immunized subcutaneously with 0.5 LD50 of CSV showed the levels of TNF increasing until 7 days after venom. TNF-α plays a role in the regulation of many biological responses in vivo, and has been implicated in a wild range of pathological conditions, including the host response to sepsis, cachexia and the acute phase response to infection, trauma, and death. In patients with sepsis, the levels of TNF in the circulation increase proportionally with the degree of hypotension and organ failure [26, 29, 30].
This study also showed that the levels of IL-6 and IFN-γ increased between 14 and 21 days. It is an inflammatory cytokine with multiple effects including the ability to stimulate or to enhance the differentiation and proliferation of B and T cells, respectively [31, 32], the production of acute phase proteins by hepatocytes [33] and the toxicity of neutrophils [34]. IL-6 levels are also elevated in animals and humans correlate with the degree of disease severity [30]. In some experimental model, the amount of IL-6 appears to be under the control of IL-1 and TNF [35–37]. The observation that highest elevations of IL-6 preceded the decrease of TNF, obtained at 14 days, suggests a role as a negative modulation TNF production in this model. Different studies have shown that the specific IFN-γ is expressed in response to venom administration and regulated by IL-10 in vivo [26–28]. In agreement with this, high IFN-γ levels were also observed in mice injected with CSV.
One cytokine that has been shown to affect the modulating activity for the production of other cytokines is IL-10, as this cytokine can down-regulate the expression of TNF-α and other inflammatory cytokines [38] and probably plays such role in CSV envenomation. Specifically the highest IL-10 levels in immunized mice were associated with low levels of IFN-γ. In this study, as well as other, in agreement with that the time-dependent increase in IL-10 after injection with CSV. These results, obtained and combined with the observation that higher levels of TNF and IL-6 are regulated by IL-10, would suggest a direct correlation cytokine balance. Interestingly, IL-10 is expressed in elevated concentrations during sepsis [39]. The expression of IL-10 is more effective in less severe sepsis in regulating inflammatory cytokines. These data suggest that production of IL-6 and TNF is regulated by IL-10, which in turn is under the control of IFN-γ produced by activated T cells and NK cells [40]. This finding is not surprising, as other mediators have previously been shown to contribute to pathophysiologic changes in envenomation. All cytokines measured showed transient profile characterized by a rapid increase to peak levels, followed by a slower decline.
While some cytokines contribute to the demise of the host, in contrast, others appear to protect the host. One must be cautious with such a strict classification because whether a cytokine is harmful or beneficial to the host is dependent on the dose and time of venom administration.
With respect to the proinflammatory cytokines, they induce local and systemic inflammatory manifestations which include fever, an acute-phase response, and the induction of system shock in severe inflammatory response. A considerable body of evidence indicates that together with an important pro- and anti-inflammatory response contributes. The balance of inflammation by these cytokines and cytokine inhibitors is complicated by the fact that the immune system has redundant pathways with multiple elements having similar physiologic effects.
The hypothesis is that envenomation is better distinguished by the balance of inflammatory mediators rather than by the concentration of a single mediator alone. This study shows that severity of envenoming is associated with an altered balance of inflammatory cytokines, and this altered balance has a significant impact on severity of envenoming. Single expression alone such as early TNF expression at the primary site of inflammatory did not distinguish between different doses of venom. Thus, there is a close link between the severity of envenomated and the balance of inflammatory cytokines. Various studies have repeatedly shown that TNF and IL-1 play a central role in the progression of the sepsis cascade [41]. In the generation of this cascade, have been implicated multiple peptide mediators, that leading to the recruit must and activation of a variety of inflammatory cells [42, 43].
The present study shows that the reactions to envenoming were associated with age, body weight, and route of injection and that they are particularly prominent in the activation of IL-6, TNF, and IL-10, which were significantly elevated at 48 h compared with the levels in those sera who were subsequently shown to be controlled. To date, the interaction between pro- and anti-inflammatory cytokines in response to envenomation remains a controversial subject. Most evidence, including in the present findings points to the operation of a feedback or counter-regulatory mechanism. IL-6 and TNF-α are potent proinflammatory cytokines and are responsible for eliciting a strong inflammatory reaction, which, if left uncontrolled, may lead to severe hypotension multiple organ dysfunction, and death [5]. This proinflammatory state of acute phase response to envenomation will ultimately trigger a compensatory anti-inflammatory reaction involving antagonist mediators such as IL-10. van der Poll et al [44] reported that in humans, the release of IL-10 by Th2 cells, B cells, and macrophages has been shown to be upregulated by circulating TNF-α.
ACKNOWLEDGMENTS
This work was supported by Secretaria de Educación Publica (SEP-PROMEP), Mexico P/PROMEP: UAEMOR-PTC-02-01. The author thanks Dr Carlos Peña for critical review of the manuscript.
References
- 1.Ismail M. The treatment of the scorpion envenoming syndrome: the Saudi experience with serotherapy. Toxicon. 1994;32(9):1019–1026. doi: 10.1016/0041-0101(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 2.Magalhães MM, Pereira MES, Amaral CFS, et al. Serum levels of cytokines in patients envenomed by Tityus serrulatus scorpion sting. Toxicon. 1999;37(8):1155–1164. doi: 10.1016/s0041-0101(98)00251-7. [DOI] [PubMed] [Google Scholar]
- 3.Petricevich VL. Cytokine and nitric oxide production following severe envenomation. Current Drug Targets: Inflammation and Allergy. 2004;3(3):325–332. doi: 10.2174/1568010043343642. [DOI] [PubMed] [Google Scholar]
- 4.Sofer S. Scorpion envenomation. Intensive Care Medicine. 1995;21(8):626–628. doi: 10.1007/BF01711538. [DOI] [PubMed] [Google Scholar]
- 5.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8(3):275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani S. Lymphokine production by human T cells in disease states. Annual Review of Immunology. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 7.van Dissel JT, van Langevelde P, Westendorp RGJ, Kwappenberg K, Frölich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351(9107):950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 8.Jo T, Terada N, Takauchi Y, et al. Cytotoxic actions of cytokines on cultured mouse luteal cells are independent of nitric oxide. Journal of Steroid Biochemistry and Molecular Biology. 1995;55(3-4):291–296. doi: 10.1016/0960-0760(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 9.van der Meide PH, Schellekens H. Cytokines and the immune response. Biotherapy. 1996;8(3-4):243–249. doi: 10.1007/BF01877210. [DOI] [PubMed] [Google Scholar]
- 10.Gerard C, Bruyns C, Marchant A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. Journal of Experimental Medicine. 1993;177(2):547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. Journal of Experimental Medicine. 1993;177(4):1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbertson MJ, Werner HA, Goddard CM, et al. Anti-tumor necrosis factor-α prevents decreased ventricular contractility in endotoxemic pigs. American Journal of Respiratory and Critical Care Medicine. 1995;152(2):480–488. doi: 10.1164/ajrccm.152.2.7633696. [DOI] [PubMed] [Google Scholar]
- 13.Barton BE. IL-6: insights into novel biological activities. Clinical Immunology and Immunopathology. 1997;85(1):16–20. doi: 10.1006/clin.1997.4420. [DOI] [PubMed] [Google Scholar]
- 14.Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA. Characterization of early cytokine responses and an interleukin (IL)-6- dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. Journal of Experimental Medicine. 1997;185(7):1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. Journal of Clinical Investigation. 1998;101(2):311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceciliani F, Giordano A, Spagnolo V. The systemic reaction during inflammation: the acute-phase proteins. Protein and Peptide Letters. 2002;9(3):211–223. doi: 10.2174/0929866023408779. [DOI] [PubMed] [Google Scholar]
- 17.Committe Members. International Society on Toxicology. Toxicon. 1992;30:1–12. [Google Scholar]
- 18.Gebara VCBC, Petricevich VL, Raw I, Dias da Silva W. Effect of saponin from Quillaja saponaria (molina) on antibody, tumour necrosis factor and interferon-γ production. Biotechnology and Applied Biochemistry. 1995;22(pt 1):31–37. [PubMed] [Google Scholar]
- 19.Schumacher JR, O'Garra A, Schrader P, et al. Characterization of 4 monoclonal antibodies to mouse interleukin-5 and development of mouse and human IL-5 ELISA assays. Journal of Immunology. 1995;141(5):1575–1581. [Google Scholar]
- 20.Ruff MR, Gifford GE. Purification and physico-chemical characterization of rabbit tumor necrosis factor. Journal of Immunology. 1980;125(4):1671–1677. [PubMed] [Google Scholar]
- 21.Schmidt HHHW, Seifert R, Bohme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Letters. 1989;244(2):357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- 22.Ismail M. The scorpion envenoming syndrome. Toxicon. 1995;33(7):825–858. doi: 10.1016/0041-0101(95)00005-7. [DOI] [PubMed] [Google Scholar]
- 23.Kalapothakis E, Chávez-Olórtegui C. Venom variability among several Tityus serrulatus specimens. Toxicon. 1997;35(10):1523–1529. doi: 10.1016/s0041-0101(97)00017-2. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman D. Insect venom allergy, immunology, and immunotherapy. In: Tu AT, editor. Handbook of Neural Toxins: Insect Poisons, Allergens, and Other Invertebrate Venoms. Vol 2. New York, NY: Marcel Dekker; 1984. pp. 187–224. [Google Scholar]
- 25.Petricevich VL, Peña CF. The dynamics of cytokine d nitric oxide secretion in mice injected with Tityus serrulatus scorpion venom. Mediators of Inflammation. 2002;11(3):173–180. doi: 10.1080/09622935020138811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barros SF, Friedlanskaia I, Petricevich VL, Kipnis TL. Local inflammation, lethality and cytokine release in mice injected with Bothrops atrox venom. Mediators of Inflammation. 1998;7(5):339–346. doi: 10.1080/09629359890866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petricevich VL, Teixeira CFP, Tambourgi DV, Gutiérrez JM. Increments in serum cytokine and nitric oxide levels in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon. 2000;38(9):1253–1266. doi: 10.1016/s0041-0101(99)00227-5. [DOI] [PubMed] [Google Scholar]
- 28.Tambourgi DV, Petricevich VL, Magnoli FC, Assaf SLMR, Jancar S, Dias da Silva W. Endotoxemic-like shock induced by Loxosceles spider venoms: pathological changes and putative cytokine mediators. Toxicon. 1998;36(2):391–403. doi: 10.1016/s0041-0101(97)00063-9. [DOI] [PubMed] [Google Scholar]
- 29.Lomonte B, Tarkowski A, Hanson LA. Host response to Bothrops asper snake venom. Analysis of edema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation. 1993;17(2):93–105. doi: 10.1007/BF00916097. [DOI] [PubMed] [Google Scholar]
- 30.De Rezende NA, Dias MB, Campolina D, Chavez-Olortegui C, Diniz CR, Amaral CFS. Efficacy of antivenom therapy for neutralizing circulating venom antigens in patients stung by Tityus serrulatus scorpions. American Journal of Tropical Medicine and Hygiene. 1995;52(3):277–280. doi: 10.4269/ajtmh.1995.52.277. [DOI] [PubMed] [Google Scholar]
- 31.Muraguchi A, Hirano T, Tang B, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. Journal of Experimental Medicine. 1988;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takai Y, Wong GG, Clark SC, Burakoff SJ, Herrmann SH. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. Journal of Immunology. 1988;140(2):508–512. [PubMed] [Google Scholar]
- 33.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon β 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borish L, Rosenbaum R, Albury L, Clark S. Activation of neutrophils by recombinant interleukin 6. Cellular Immunology. 1989;121(2):280–289. doi: 10.1016/0008-8749(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 35.Fong Y, Tracey KJ, Moldawer LL, et al. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1β and interleukin 6 appearance during lethal bacteremia. Journal of Experimental Medicine. 1989;170(5):1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haworth C, Brennan FM, Chantry D, Turner M, Maini RN, Feldmann M. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-α. European Journal of Immunology. 1991;21(10):2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- 37.Lepore L, Pennesi M, Saletta S, Perticarari S, Presani G, Prodan M. Study of IL-2, IL-6, TNFα, IFNγ and β in the serum and synovial fluid of patients with juvenile chronic arthritis. Clinical and Experimental Rheumatology. 1994;12(5):561–565. [PubMed] [Google Scholar]
- 38.Moore KW, O'Garra A, Malefyt RW, Vieira P, Mosmann TR. Interleukin-10. Annual Review of Immunology. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 39.Marchant A, Devière J, Byl B, De Groote D, Vincent J-L, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994;343(8899):707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- 40.Young HA, Ortaldo JR. One-signal requirement for inter-feron-γ production by human large granular lymphocytes. Journal of Immunology. 1987;139(3):724–727. [PubMed] [Google Scholar]
- 41.Dinarello CA, Cannon JG, Wolff SM. New concepts on the pathogenesis of fever. Reviews of Infectious Diseases. 1988;10(1):168–189. doi: 10.1093/clinids/10.1.168. [DOI] [PubMed] [Google Scholar]
- 42.Okusawa S, Gelfand JA, Ikejima T, Connolly RJ, Dinarello CA. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. Journal of Clinical Investigation. 1988;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remick DG, Strieter RM, Eskandari MK, et al. Role of tumor necrosis factor-α in lipopolysaccharide-induced pathologic alterations. American Journal of Pathology. 1990;136(1):49–60. [PMC free article] [PubMed] [Google Scholar]
- 44.van der Poll T, Jansen J, Levi M, ten Cate H, ten Cate JW, van Deventer SJH. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. Journal of Experimental Medicine. 1994;180(5):1985–1988. doi: 10.1084/jem.180.5.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]