Abstract
To evaluate the effect of expression of latent membrane protein (LMP) 1 encoded by Epstein-Barr virus (EBV) on Cɛ mRNA expression, mRNA levels were examined by RT-PCR or Northern blot analysis upon transient transfection of LMP1 in the splenocytes derived from Brown-Norway rats with or without immunization with 2,4-dinitrophenyl-conjugated Ascaris suum antigen. Splenocytes were transfected with LMP1 expression vector, pSG5-LMP1, using lipofection method. Cɛ mRNA levels were considerably increased by transfection with pSG5-LMP1 in the splenocytes derived from the nonimmunized rats; however, Cɛ mRNA levels were decreased in the splenocytes derived from the immunized rats. Cɛ mRNA expression in IgE-producing cells are modulated by LMP1, which might depend on the differentiation status of B cells upon exposure to allergen.
INTRODUCTION
Epstein-Barr virus (EBV) is a member of the human herpesvirus family and latently infects more than 90% of the world's adult population. EBV infects the oropharynx tissues such as tonsils and salivary glands as its primary target tissues. Although EBV is ordinarily dormant in the host forever after primary infection, it sometimes allows B cells to acquire the unlimited growth potential. Therefore, lymphoma would be observed in the case of the weak host's immunoresponsiveness to the virus infection. EBV is also reportedly involved in allergic reactions. Increased levels of EBV antibodies are found both in children with asthma and atopic dermatitis [1, 2]. In in vitro studies, EBV-transformed B cells stimulated with IL-4 produce IgE in culture supernatants [3, 4], and the level of IgE secreted in culture supernatants correlates with the number of IgE-producing cells [4]. Transformation of B cells by EBV has been reported to increase the number of IgE-producing cells [5]. Clinical studies to clarify the correlation between EBV infection and serum levels of IgE, which play a pivotal role in allergic reaction, yielded however controversial and conflicting results [1, 2, 6, 7]. The mechanisms underlying the causal relationship between the EBV infections and the increase of the risk for allergic diseases remain to be fully elucidated. There are however two possible mechanisms underlying there. One of them is through activating antigen-presenting cells by secretion of several cytokines including tumor necrosis factor-α (TNFα), interferon-γ (IFN-γ), and interleukin-1α (IL-1α) from EBV-activated T cells [8]. Another one is through triggering T-cell independent class switch DNA recombination (CSR) by EBV-latent membrane protein-1 (LMP1) [9]. LMP1-induced CSR in B cells has been reported to be associated with transcriptional activation of germline Cγ, Cα, and Cɛ genes and to trigger the upregulation of activation-induced cytidine deaminase (AICD), a crucial component of the CSR machinery [9]. However, it is yet uncertain how the differentiation status of B cells, where EBV-LMP1 is expressed, is associated with their ability to produce IgE.
The latent gene products encoded by EBV are classified into four groups, and at least 9 proteins have been identified so far [10]. Of these latent proteins, the LMP1 is a cell surface protein, which is a receptor-like protein constitutively activated, leading to elicitation of several biological effects including B-cell transformation. LMP1, a 63-kDa integral membrane protein consisting of three domains, has within its C-terminus two activating regions referred to as C-terminal activating region-1 (CTAR1) and CTAR2 [11]. These CTARs are involved in activation of four different signaling pathways of LMP1, including the nuclear factor κB (NF-κB) pathway [11], which potentially activates immunoglobulin gene expression [12]. LMP1 can also protect the B cell from apoptosis by inducing antiapoptotic proteins such as BCL-2, MCL-1, and A20 [11]. Therefore, LMP1 can be regarded as the EBV-encoded protein with the highest potentiality to modulate the immune system involved in IgE synthesis.
Brown-Norway (BN) rat is a useful experimental animal for studies on transplantation immunity, heredity, reproduction, aging, and immunoallergy. The BN rat is especially available for a model of allergic inflammation such as allergic rhinitis, asthma, and food allergy, because it is a high responder to antigen (allergen) and exhibits high IgE production [13, 14].
In the present study, we examined whether or not LMP1 modulates IgE the production of splenocytes derived from BN rats with or without immunization with 2,4-dinitrophenyl-conjugated Ascaris suum antigen (DNP-As) and IgE-producing hybridoma FE3 cells.
MATERIALS AND METHODS
Animals
BN male rats weighing 200–240 g were purchased from Kyudo Co, Ltd (Kumamoto, Japan). We followed the Standards Relating to the Care and Management of Experimental Animals (Notification No 6, March 27, 1980, from the Prime Minister's office, Tokyo, Japan) for the care and use of the animals, together with the University of the Ryukyus guide for animal experiments. All animal studies were reviewed and approved by the Animal Care Committee at the University of the Ryukyus.
Conjugation of dinitrophenyl (DNP) groups to Ascaris suum (As) extracts
2,4-dinitrobenzene sulfonic acid sodium salt was purchased from Tokyo Kasei Inc, Ltd (Tokyo, Japan). Protein extraction from Ascaris suum and conjugation of the extracted proteins with DNP groups were carried out as described previously [13].
Immunization of BN rats with DNP-As
BN rats were injected with 1 mg of DNP-As together with killed Bordetella pertussis (1010 cells) (Nacalai Tesque, Kyoto, Japan), followed by a single booster injection of DNP-As (0.5 mg) 5 days after the first injection. Blood samples were taken from the tail veins of the rats at 6, 7, 8, and 10 days after the first injection.
Enzyme-linked immunosorbent assay for determination of serum IgE levels
IgE-capture ELISA was performed as described previously [13, 15]. In brief, the ELISA plate was coated with 5 μg/mL of rabbit antibodies against IgE FE-3 overnight at 4°C. After blocking with 1% gelatin-PBS, the wells were incubated with the rat serum appropriately diluted with 1% gelatin-PBS containing 0.05% Tween 20 (PBST) for 1 hour at room temperature. Following washing, the wells were incubated with 1 μg/mL of biotinylated DNP-As in 1% gelatin-PBST for 30 minutes at room temperature. After further washing, the wells were incubated with POD-conjugated streptavidin (ZYMED Laboratories, Calif, USA) in 1% gelatin-PBST for 30 minutes at room temperature, followed by final washing and addition of substrate solution (ABTS) (Sigma-Aldrich, Mo, USA). Spectrophotometric readings were then made using λ1 = 415 nm and λ2 = 492 nm wavelength filters of a dual wavelength microplate reader MTP-300 (CORONA Electric, Ibaraki, Japan).
Preparation of splenocytes
Nonimmunized and immunized BN rats were anesthetized with sodium pentobarbiturate, and the spleen was excised immediately after the rats had been killed by means of bleeding from the carotid artery. The immunized rats were employed for the experiments 7 days after the first immunization. The excised spleen was further torn to pieces, and then splenocytes were collected by centrifugation after removing these pieces.
Plasmid and transfection
pSG5-LMP1 was a gift of Dr Kieff (Harvard Medical School, Mass, USA). The vector was transfected into FE-3 cells or splenocytes derived from BN rats using DMRIE-C reagent (Invitrogen, Calif, USA) as follows. 0.5 mL of RPMI 1640 medium (Nissui Pharmaceutical Co, Tokyo, Japan) without serum (SFM) was added to each well of a 6-well plate. Then, 0.8 μL of DMRIE-C reagent was added to each well, and mixed gently by swirling the plate. Furthermore, 0.5 mL SFM containing 0.4 μg DNA was added to each well and then mixed by swirling plate. The plate was incubated at room temperature for 30 minutes to allow formation of the lipid-DNA complexes. 2 × 106 cells of FE-3 cells or 2 × 107 cells of rat splenocytes were placed in the each well in which 0.2 mL SFM had been prepared, and then incubated for 5 hours at 37°C in a CO2 incubator. Two mL growth mediums containing 15% FBS were then added to each well. In the case of splenocytes from nonimmunized rats, the cells were cultured in the presence or absence of 75 ng/mL rat IL-4 (Strathmann Biotec, Germany), because IL-4 is required for the process of IgE production by B cells [6, 8]. The cells were collected 24 hours after starting plasmid transfection. Irrespective of cell lineage, no significant difference in the cell viability was observed between cells transfected with pSG5 and cells transfected with pSG5-LMP1.
RNA isolation and Northern blot analysis
Total RNA (10 μg), prepared using PURESCRIPT RNA Isolation Kit (Gentra System, Minn, USA), was resolved by electrophoresis under denaturing conditions and transferred to a nylon membrane. Ribosomal RNA was stained on the membrane with methylene blue to assess RNA loading and transfer efficiency. The procedure of hybridization was followed by instruction manual directed by Clontech. The Cɛ and β-actin cDNAs for probe were prepared by RT-PCR. The LMP1 cDNA for probe was obtained as a restriction fragment of the pSG5-LMP1. The probes for Northern blot analysis were then labeled with (α−32P)dCTP using random oligo-primed reaction.
Reverse transcription of mRNA
Reverse transcriptions (RTs) were carried out according to the standard procedures. In brief, 1 μg of total RNA in 18.8 μL of reaction mixture (4 μL of 5x RT buffer, 4 μL of 2.5 mM dNTP mixture, 2 μL of 25 mM MgCl2, 2 μL of 0.1 M DTT, and RNase free water as the rest of mixture) and 0.2 μL of 50 μM synthetic oligo(dT) primer were mixed. After denaturing at 75°C for 3 minutes, the reaction was cooled down on ice and initiated by adding 1 μL of M-MLV RTase and incubated for 1 hour at 42°C.
Polymerase chain reaction (PCR)
The cDNAs for Cɛ, LMP1, and β-actin genes were amplified by the PCR method with Program Temp Control System PC-800 (Astec Inc, Fukuoka, Japan). The reaction was performed in a final volume of 20 μL of reaction mixture, which consisted of 20 mM Tris-HCl pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.5 μM of each primer, 1 μL of Taq DNA polymerase (Promega, WI, USA) (final concentrations), and 1 μL of sample. Primer sequences are as follows: Cɛ [16], forward, 5′CTTACCTGTCTGGTTTTGGACCTGG 3′, and reverse, 5′CGGAGGGAAGTGTTACCAGGC 3′; β-actin [16], forward, 5′GAGCTATGAGCTGCCTGACGG 3′, and reverse, 5′TTGCGGTGCACGATGGA 3′; and LMP1 [17], forward, 5′-TTTGTCTACTCCTACTGATGATCACC-3′, and reverse, 5′-AGTAGATCCAGAGACCTAAGACAAGT-3′. The PCR conditions were as follows: a single 5-minute at 94°C; 35 cycles (30 cycles for LMP1; 25 cycles for β-actin) of 94°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute; and a single 5-minute extension at 72°C. PCR products were run on a 2%-agarose gel, visualized with ethidium bromide and analyzed using the Gel Doc 1000 UV fluorescent gel documentation system (Bio-Rad Lab, Calif, USA) with the accompanying Multi-Analyst software (version 1.0).
Statistical analysis
Statistical analysis was performed by unpaired Student t test for between-group comparisons. Data are expressed as the means ± standard deviations (SD). When P < .05 was obtained, the means were considered to be significantly different.
RESULTS
Preparation of splenocytes from BN rats following immunization with DNP-As
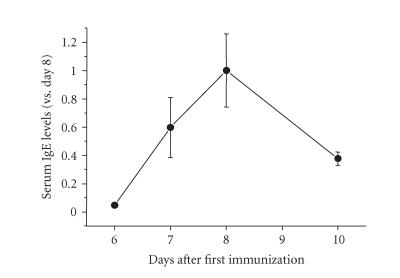
Before collecting splenocytes from the immunized rats, we firstly determined to when the splenocytes have to be collected following immunization, based on the changes of IgE levels determined by ELISA. IgE levels in the immunized rats began to rise at day 6, and reached a peak at day 8 as shown in Figure 1. The immunocompetent cells with diversity in their maturity would exist on the initial stage after booster injection. This stage is also predicted to be essential for B cells to be destined to become the IgE-producing plasmacytes. The splenocytes were therefore collected at the early stage, day 7, where IgE production was observed to start increasing in the rat.
Figure 1.
Changes of IgE levels in BN rats following immunization with DNP-As. The BN rats were injected with 1 mg of DNP-As together with killed Bordetella pertussis (1010 cells), followed by a single booster injection of DNP-As (0.5 mg) 5 days after the first injection. Blood samples were taken from the tail veins of the rats at 6, 7, 8, and 10 days after the first injection. Serum IgE levels in the rats were determined by IgE-capture ELISA as described in the “materials and methods.” The results were expressed as means ± SD of triplicate experiments.
Effect of LMP1 expression on Cɛ mRNA levels of splenocytes derived from BN rats without immunization with DNP-As
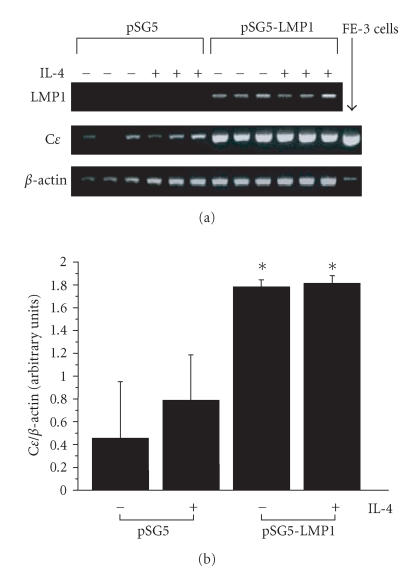
The splenocytes derived from nonimmunized rats were transfected with the expression vector pSG5-LMP1, and then cultured in the presence or absence of rat IL-4. Because Cɛ mRNA in the splenocytes derived from nonimmunized BN rats was not detected on Northern blot analysis, the effect of LMP1 expression on the Cɛ mRNA levels in the cells was examined by RT-PCR analysis. Since the primers for RT-PCR amplification of Cɛ mRNA can amplify both germline and mature Cɛ transcripts, the PCR bands represent the total amount of germline and mature Cɛ transcripts. The splenocytes even without treatment with IL-4 showed as high levels of Cɛ mRNA as the cells treated with IL-4, suggesting that the function of B cells was regulated by IL-4 derived from T-cell population in the splenocytes collected. Cɛ mRNA levels were higher in the cells transfected with pSG5-LMP1 than those in the control cells transfected with the empty vector pSG5 (P < .05, Figure 2).
Figure 2.
Effect of LMP1 expression on Cɛ mRNA levels of splenocytes derived from nonimmunized BN rats. The pSG5-LMP1 or empty vector pSG5 was transfected into the splenocytes derived from nonimmunized BN rats using lipofection method. (a) LMP1 and Cɛ mRNA expressions including both germline and mature Cɛ transcripts were determined by RT-PCR as described in the “materials and methods.” The results of three independent experiments are shown. (b) Cɛ mRNA levels are represented by the values normalized to the intensity of PCR products for β-actin. Data are expressed as means ± SD of six independent experiments. *P < .05 for with or without IL-4 treatment, pSG5-LMP1 versus pSG5.
Effect of LMP1 expression on Cɛ mRNA levels of splenocytes derived from BN rats immunized with DNP-As
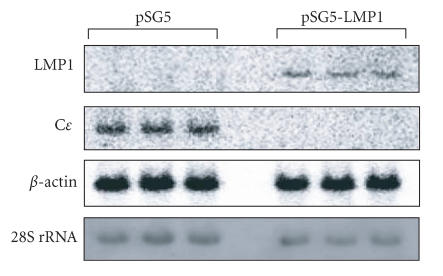
We next determined Cɛ mRNA levels in the splenocytes collected form the BN rats immunized with DNP-As on Northern blot analysis. Theoretically, (α−32P)-labeled probe for Cɛ transcripts can hybridize with both germline and mature Cɛ transcripts. The Cɛ mRNA band could be detected as a single band, which presumably represents mature Cɛ transcript based on its size of length. The results indicated that Cɛ mRNA levels were lower in the cells transfected with pSG5-LMP1 than those in the control cells transfected with pSG5 (Figure 3).
Figure 3.
Effect of LMP1 expression on Cɛ mRNA levels of splenocytes derived from BN rats immunized with DNP-As. The pSG5-LMP1 or empty vector was transfected into the splenocytes derived from BN rats immunized with DNP-As using lipofecton method. LMP1 and Cɛ mRNA expression were determined by Northern blot analysis as described in the “materials and methods.” The results of three independent experiments are shown.
Effect of LMP1 expression Cɛ mRNA levels of IgE-producing hybridoma, FE-3 cells
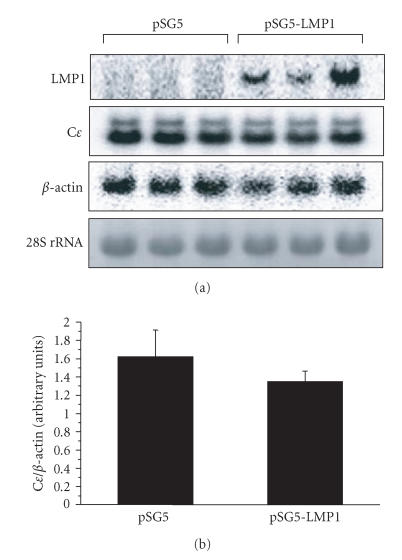
FE-3 cells are an IgE-producing hybridoma established by hybridizing mouse myeloma cells (SP2/0-Ag14/SF) with rat splenocytes that were isolated from BN rats immunized with DNP-As [13]. The cDNA sequence of the Cɛ3 and Cɛ4 domains of IgE FE-3 is identical to that of rat IgE IR162 [15], indicating that IgE obtained from the hybridoma cells is encoded in rat immunoglobulin locus. Our Southern blot analysis for the genomic DNA of the FE-3 cells indicates that switch recombination in the Cɛ gene locus is accomplished in the cells (our unpublished data). FE-3 cells are therefore expected to be available for various allergic experiments as a model of plasmacytes that constitutively produce IgE antibodies. As shown in Figure 4(a), two bands are seen in Northern blot. The faster migrating band corresponds to the mRNA for the secreted form of IgE, whereas the slower band for the membrane-bound form of IgE. Following the transfection of FE-3 cells with pSG5-LMP1 or pSG5 empty vector, there is no significant difference in IgE levels between the cells transfected with pSG5-LMP1 and the control cells transfected with pSG5 (Figure 4).
Figure 4.
Effect of LMP1 expression on Cɛ mRNA levels of IgE-producing hybridoma FE-3 cells. The pSG5-LMP1 or empty vector was transfected into IgE-producing hybridoma FE-3 cells using lipofection method. (a) LMP1 and Cɛ mRNA expressions were determined by Northern blot analysis as described in the “materials and methods.” The results of three independent experiments are shown. (b) Cɛ mRNA levels are represented by the values normalized to the intensity of PCR products for β-actin. Data are expressed as means ± SD of six independent experiments.
DISCUSSION
In the present study, we found that the effect of LMP1 overexpression on the Cɛ gene expression in the splenocytes was different depending on the immunization condition of BN rats. Although IL-4 failed to augment Cɛ mRNA expression, overexpression of LMP1 upregulated Cɛ mRNA expression in splenocytes derived from nonimmunized rats (Figure 2). This indicates that LMP1 upregulates Cɛ mRNA expression independently of stimulation by IL-4. Overexpression of LMP1, however, abrogated Cɛ mRNA expression in splenocytes derived from immunized rats (Figure 3). The underlying mechanism for negative effects of LMP1 on the B cells differentiating toward IgE-producing cells is yet unidentified. The positive effects of LMP1 expression on the B cells that have no definitive direction of differentiation, however, might be due to the promotion of germline Cɛ transcription and Cɛ gene rearrangement by LMP1 expression. In IgE-producing hybridoma FE-3 cells, which had accomplished gene rearrangement in Cɛ locus, Cɛ mRNA levels were not altered by overexpression of LMP1 (Figure 4).
Increased levels of EBV antibodies are found both in children with asthma and atopic dermatitis [1, 2], suggesting EBV infection might promote allergic disease. The correlation between EBV infection and serum IgE level is, however, controversial and still need to be clarified. Rystedt et al indicated that there was no correlation between EBV antibody titers and serum IgE levels [2]. Calvani et al reported that the prevalence of high IgE levels was more frequent in the EBV negative than in the positive subjects [7]. They also indicated that this higher prevalence in the EBV negative subjects was found only in the groups of five years old or less, whereas in the 6–19 years group the situation was reversed [7]. On the contrary, heterophil-positive infectious mononucleosis is found to be associated with high serum IgE level [6]. In addition, the increase in IgE level was observed very early in the course of the disease, mainly within the first week, rapidly followed by a significant drop below the preillness levels [6]. Based on these studies, it is anticipated that the host's immunoresponsiveness to virus infection, which varies in patient age and the passage of time from the primary infection, seems to influence the correlation between EBV infection and the serum IgE level.
The B-cell activating signal for IgE synthesis is derived through CD40 ligand and IL-4 or IL-13. Both IL-4 and IL-13 are capable of inducing germline Cɛ transcription in B cells, because IL-4 and IL-13 receptors share the IL-4Rα chain, which plays a pivotal role in the induction of germline Cɛ transcription, as the common component of both receptors [18, 19]. Target sites for several transcription factors have been identified in the Iɛ promoter, including STAT6, NF-κB, PU.1, BSAP, C/EBP (for human) [20–22], and AP-1 (for mouse) [23, 24]. IL-4 and IL-13 are known to induce activation of STAT6 [20, 22], which promotes RNA transcription at the Cɛ locus and is therefore regarded as the critical regulator for germline Cɛ transcription. CD40, which belongs to the TNF receptor family, is a membrane protein found on the surface of B cells and serves as the receptor for CD40 ligand expressing on the cell surface of T cells. CD40 mediates the activation of several transcription factors including NF-κB and STAT molecules [25], thereby leading to B-cell proliferation, antibody class switching, and modulation of apoptosis. Activation of CD40 signaling pathway in the presence of IL-4 or IL-13 leads to the expression of AICD [26]. This novel RNA-editing enzyme plays a key role upstream of the putative switch recombinase, leading to IgE isotype switching, mature Cɛ transcription, and IgE synthesis [12, 27]. Thus, IL-4Rα, which is involved in the activation of overlapping signaling pathways between IL-4 and IL-13, and CD40 signaling pathways are integrated to induce IgE isotype switching, followed by IgE synthesis. ɛ germline gene transcription, class switch recombination, and splicing of ɛ-chain gene transcript in the course of IgE synthesis are considered as phenomena synchronized with the differentiation process of B cell.
A strong correlation is noted between the IgE response and the number of circulating atypical lymphocytes in the heterophil-positive infectious mononucleosis [6]. In vitro experiments indicate that EBV is an enhancer of IgE production. EBV-transformed B cells stimulated with IL-4 are allowed to produce IgE in culture supernatants [3, 4], and the level of IgE secreted in culture supernatants correlates with the number of IgE-producing cells [4]. LMP1 is most likely the EBV-encoded protein with highest activity to modulate IgE production, because LMP1 is known to mimic CD40 through activating the overlapping signaling pathways between them. He et al reported that LMP1 triggered T-cell independent CSR [9]. LMP1-induced CSR is associated with transcriptional activation of germline Cγ, Cα, and Cɛ genes and triggers the upregulation of AICD [9].
The key molecules for the signaling by both LMP1 and CD40 are considered to be the intracellular TNF receptor-associated factor (TRAF) adapter proteins, which are recruited to the cytoplasmic C-terminus domains of both LMP1 and CD40 [28, 29]. LMP1 accomplishes its function by signaling through four different pathways: the NF-κB pathway, the c-Jun N-terminal kinase ((JNK)-AP-1) pathway, the p38/mitogen activated protein kinase (MAPK) pathway, and the Janus kinase ((JAK)-signal transducers and activators of transcription (STAT)) pathway [11]. Taken together, LMP1 is predicted to be involved in Cɛ gene expression by activating the NF-κB pathway and/or JAK-STAT pathway.
In conclusion, our findings suggest that Cɛ mRNA expression in IgE-producing cells are modulated by LMP1 gene expression and that this modulation leading to changes in expression levels of Cɛ mRNA might be dependent on the differentiation status of B cells upon exposure to allergen.
ACKNOWLEDGMENTS
We thank Dr Elliott D. Kieff and Dr Teruhito Yasui, Department of Microbiology and Molecular Genetics, Harvard Medical School, for providing pSG5-LMP1.
References
- 1.Strannegard IL, Strannegard O. Epstein-Barr virus antibodies in children with atopic disease. International Archives of Allergy and Applied Immunology. 1981;64(3):314–319. doi: 10.1159/000232709. [DOI] [PubMed] [Google Scholar]
- 2.Rystedt I, Strannegard I-L, Strannegard O. Increased serum levels of antibodies to Epstein-Barr virus in adults with history of atopic dermatitis. International Archives of Allergy and Applied Immunology. 1984;75(2):179–183. doi: 10.1159/000233610. [DOI] [PubMed] [Google Scholar]
- 3.Thyphronitis G, Tsokos GC, June CH, Levine AD, Finkelman FD. IgE secretion by Epstein-Barr virus-infected purified human B lymphocytes is stimulated by interleukin 4 and suppressed by interferon γ. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(14):5580–5584. doi: 10.1073/pnas.86.14.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CL, Thyphronitis G, Nutman TB. Enumeration of IgE secreting B cells. A filter spot-ELISA. Journal of Immunological Methods. 1990;132(1):37–43. doi: 10.1016/0022-1759(90)90395-c. [DOI] [PubMed] [Google Scholar]
- 5.Gudmundsson KO, Thorsteinsson L, Gudmundsson S, Haraldsson Á. Immunoglobulin-secreting cells in cord blood: effects of Epstein-Barr virus and interleukin-4. Scandinavian Journal of Immunology. 1999;50(1):21–24. doi: 10.1046/j.1365-3083.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 6.Bahna SL, Horwitz CA, Fiala M, Heiner DC. IgE response in heterophil-positive infectious mononucleosis. Journal of Allergy and Clinical Immunology. 1978;62(3):167–173. doi: 10.1016/0091-6749(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 7.Calvani M, Alessandri C, Paolone G, Rosengard L, Di Caro A, De Franco D. Correlation between Epstein Barr virus antibodies, serum IgE and atopic disease. Pediatric Allergy and Immunology. 1997;8(2):91–96. doi: 10.1111/j.1399-3038.1997.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 8.Lay J-D, Tsao C-J, Chen J-Y, Kadin ME, Su I-J. Upregulation of tumor necrosis factor-α gene by Epstein-Barr virus and activation of macrophages in Epstein-Barr virus-infected T cells in the pathogenesis of hemophagocytic syndrome. Journal of Clinical Investigation. 1997;100(8):1969–1979. doi: 10.1172/JCI119728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. Journal of Immunology. 2003;171(10):5215–5224. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young LS, Dawson CW, Eliopoulos AG. The expression and function of Epstein-Barr virus encoded latent genes. Journal of Clinical Pathology: Molecular Pathology. 2000;53(5):238–247. doi: 10.1136/mp.53.5.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumforth KR, Young LS, Flavell KJ, Constandinou C, Murray PG. The Epstein-Barr virus and its association with human cancers. Journal of Clinical Pathology: Molecular Pathology. 1999;52(6):307–322. doi: 10.1136/mp.52.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagihara Y. Regulatory mechanisms of human IgE synthesis. Allergology International. 2003;52(1):1–12. [Google Scholar]
- 13.Hanashiro K, Nakamura M, Tamaki N, Kosugi T. Production of a monoclonal dinitrophenyl-specific rat IgE and establishment of an IgE capture ELISA for estimating the concentration of rat IgE antibodies to dinitrophenyl-Ascaris suum. International Archives of Allergy and Immunology. 1996;110(4):371–379. doi: 10.1159/000237330. [DOI] [PubMed] [Google Scholar]
- 14.Tamaki N, Hanashiro K, Saitoh S, Nakamura M, Kosugi T. Evaluation of airway responses by measurement of the respiratory resistance in rats under nonanesthetized conditions. Journal of Japan Bronchoesophagology Society. 1995;46:464–475. [Google Scholar]
- 15.Nakasone T, Hanashiro K, Nakamura M, Sunakawa H, Kosugi T. An improved method for the detection of IgE FE-3 by ELISA using monoclonal anti-IgE FE-3 antibody. Ryukyu Medical Journal. 2001;20:129–135. [Google Scholar]
- 16.Tokeshi Y, Shimada S, Hanashiro K, Sunagawa M, Nakamura M, Kosugi T. The nucleotide sequence of dinitrophenyl-specific IgE and FcєRI α-subunit obtained from FE-3 hybridoma cells. Hybridoma and Hybridomics. 2001;20(5-6):361–368. doi: 10.1089/15368590152740761. [DOI] [PubMed] [Google Scholar]
- 17.Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(22):12250–12255. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilton DJ, Zhang J-G, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(1):497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DJ, Clark PA, Herbert J, et al. Function of the interleukin-2 (IL-2) receptor γ-chain in biologic responses of X-linked severe combined immunodeficient B cells to IL-2, IL-4, IL-13, and IL-15. Blood. 1995;85(1):38–42. [PubMed] [Google Scholar]
- 20.Thienes CP, De Monte L, Monticelli S, Busslinger M, Gould HJ, Vercelli D. The transcription factor B cell-specific activator protein (BSAP) enhances both IL-4- and CD40-mediated activation of the human є germline promoter. Journal of Immunology. 1997;158(12):5874–5882. [PubMed] [Google Scholar]
- 21.Mikita T, Kurama M, Schindler U. Synergistic activation of the germline є promoter mediated by Stat6 and C/EBPβ. Journal of Immunology. 1998;161(4):1822–1828. [PubMed] [Google Scholar]
- 22.Stutz AM, Woisetschlager M. Functional synergism of STAT6 with either NF-κB or PU.1 to mediate IL- 4-induced activation of IgE germline gene transcription. Journal of Immunology. 1999;163(8):4383–4391. [PubMed] [Google Scholar]
- 23.Mao C-S, Stavnezer J. Differential regulation of mouse germline Ig γ 1 and є promoters by IL-4 and CD40. Journal of Immunology. 2001;167(3):1522–1534. doi: 10.4049/jimmunol.167.3.1522. [DOI] [PubMed] [Google Scholar]
- 24.Shen C-H, Stavnezer J. Activation of the mouse Ig germline є promoter by IL-4 is dependent on AP-1 transcription factors. Journal of Immunology. 2001;166(1):411–423. doi: 10.4049/jimmunol.166.1.411. [DOI] [PubMed] [Google Scholar]
- 25.Hanissian SH, Geha RS. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997;6(4):379–387. doi: 10.1016/s1074-7613(00)80281-2. [DOI] [PubMed] [Google Scholar]
- 26.Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFκB. International Immunology. 2004;16(3):395–404. doi: 10.1093/intimm/dxh042. [DOI] [PubMed] [Google Scholar]
- 27.Oettgen HC, Geha RS. IgE regulation and roles in asthma pathogenesis. Journal of Allergy and Clinical Immunology. 2001;107(3):429–440. doi: 10.1067/mai.2001.113759. [DOI] [PubMed] [Google Scholar]
- 28.Sandberg M, Hammerschmidt W, Sugden B. Characterization of LMP-1's association with TRAF1, TRAF2, and TRAF3. Journal of Virology. 1997;71(6):4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pullen SS, Miller HG, Everdeen DS, Dang TTA, Crute JJ, Kehry MR. CD40-tumor necrosis factor receptor-associated factor (TRAF) interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry. 1998;37(34):11836–11845. doi: 10.1021/bi981067q. [DOI] [PubMed] [Google Scholar]