Abstract
The quantitative analysis of VEGF using ELISA in various subtypes of grade I meningiomas reported higher VEGF contents in meningothelial (2.38 ± 0.62 pg/μg protein, n = 7), transitional (1.08 ± 0.21 pg/μg protein, n = 13), and microcystic meningiomas (1.98 ± 0.87 pg/μg protein, n = 5) as compared with fibrous ones (0.36 ± 0.09 pg/μg protein, n = 5). In contrast to VEGF, no difference in the concentrations of bFGF was detected. VEGF levels did not correlate with meningioma grade (1.47 ± 0.23 pg/μg versus 2.29 ± 0.58 pg/μg for 32 and 16 grade I and II, resp), vascularisation (1.53 ± 0.41 pg/μg versus 1.96 ± 0.28 pg/μg for 24 low and 24 high vascularisated tumours, resp), and brain invasion (2.32 ± 0.59 pg/μg versus 1.46 ± 0.27 pg/μg for 7 and 41 patients with and without invasion, resp). The ELISA procedure is, thus, an interesting tool to ensure VEGF and bFGF levels in meningiomas and to test putative correlations with clinical parameters. It is, thus, tempting to speculate that ELISA would also be valuable for the quantitative analysis of other angiogenic growth factors and cytokines in intracranial tumours.
Meningiomas are the second most common primary intracranial tumours. Meningiomas present clinically by causing focal or generalised seizure disorders, focal neurological deficits, or neuropsychological decline [1]. Angiogenesis consists of the sprouting of capillaries from pre-existing vessels [2]. Angiogenesis is mediated by a number of different growth factor and is vital for tumour growth. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are two potent angiogenic growth factors that stimulated vascular endothelial cell proliferation and are involved in the neoplastic angiogenesis of several types of tumours including meningiomas [3–5]. Authors usually investigated VEGF and bFGF protein expressions using immunohistochemistry or western blotting and VEGF and bFGF transcripts using reverse transcriptase polymerase chain reaction. However, several studies have reported that enzyme-linked immunosorbent assay (ELISA) is an efficient tool to evaluate tissue levels of these two angiogenic growth factors [6–8]. In the present study we performed quantitative analysis of VEGF and bFGF in meningiomas in order to test if this technical approach was also suitable for intracranial tumours.
The procedure of the present study followed the rules edited by the French National Ethics. Forty eight patients who underwent surgery for intracranial meningiomas were investigated. The presence or absence of a brain edema was assessed in 36 cases. Tumours were classified according to the WHO criteria [9]. There were 32 grade I meningiomas including 13 transitional (2 men, 11 women, mean age 59 years), 7 meningothelial (1 man, 6 women, mean age 59 years), 5 microcystic (1 man, 4 women, mean age 51 years), 5 fibroblastic (5 women, mean age 58), 1 angiomatous (1 woman, age 78 years), and 1 psammomatous (1 woman, age 27 years). Twelve tumours were grade II meningiomas: 10 atypical (5 men, 7 women, mean age 58 years) and 2 chordoid meningiomas (2 women, mean age 52 years). Four tumours were classified as anaplastic grade III meningiomas (4 men, mean age 51 years). The presence or the absence of brain invasion was determined. The intensity of the neovascularisation and of the chronic inflammatory response in the tumours was semiquantitatively assessed. Inflammatory infiltrates were usually mild and mainly composed of lymphocytes associated with some plasmocytes. In 3 cases, a dense lymphocytic infiltration was found. Tumour tissues were obtained during the surgical procedure and were frozen at −80°C until used. Tissue samples were homogenized in potassium phosphate buffer and VEGF and bFGF contents were evaluated by specific ELISA assays (DuoSet, R&D Systems Europe) according to the manufacture's recommendations [7, 8]. The detection limits for VEGF and bFGF were 10 pg/mL and 30 pg/mL, respectively. All samples were assayed in duplicate. Samples were diluted by 1/2 and 1/20 for determination of VEGF and bFGF, respectively. Proteinemia was determined by the BCA protein assay reagent (Pierce, Rockford, Ill). Results in picograms (pg) VEGF and bFGF per micrograms (μg) of proteins are reported as means ± SEM.
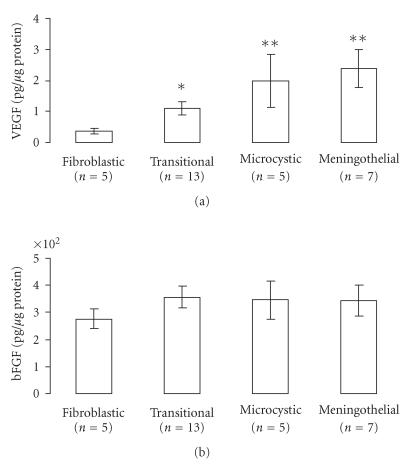
VEGF and bFGF proteins were detectable in all meningioma samples. As shown in Figure 1 (upper panel), among different subtypes of grade I meningiomas, VEGF contents were significantly elevated in transitional meningiomas (1.08 ± 0.21 pg/μg protein, n = 13), microcystic meningiomas (1.98 ± 0.87 pg/μg protein, n = 5), and meningothelial meningiomas (2.38 ± 0.62 pg/μg protein, n = 7) than in fibroblastic ones (0.36 ± 0.09 pg/μg protein, n = 5). In contrast to VEGF contents, no differences were found for bFGF contents (Figure 1, lower panel). As shown in Table 1, none of these two potent angiogenic growth factors showed any association in relation to tumour grade, the presence of inflammatory infiltrated cells, associated necrosis, edema, mitosis, brain invasion, or vascularisation (Table 1).
Figure 1.
VEGF and bFGF contents in various subtypes of grade I meningiomas VEGF (upper panel) and bFGF (lower panel) contents were determined by ELISA. Results (in pg/μg of protein) are reported as means ± SEM. n denotes the number of patients. For transitional meningiomas: VEGF range [0.25–9.85] and bFGF range [67.4–576.9]; for microcystic meningiomas: VEGF range [0.71–5.29] and bFGF range [164.3–520.0]; for meningothelial meningiomas: VEGF range [0.87–4.98] and bFGF range [183.6–573.7]; for fibroblastic meningiomas: VEGF range [0.10–1.69] and bFGF range [142.1–377.3]. *P < .04 and **P < .01 as compared with fibroblastic meningiomas. Statistical analysis was performed using the Mann-Whitney U test.
Table 1.
VEGF and bFGF values in meningiomas. VEGF and bFGF levels are expressed as pg per μg of protein. Results are reported as means ± SEM. n denotes the number of samples in each group [1]. Four or more mitoses per 10 high power fields [2]. Less than 4 mitoses per 10 high power fields. No statistical differences were observed between groups (Mann-Whitney U test).
| VEGF | bFGF | |
| Grade I (n = 32) | 1.47 ± 0.23 | 341.0 ± 22.9 |
| Grade II-III (n = 16) | 2.29 ± 0.58 | 364.3 ± 38.8 |
| With necrosis (n = 9) | 2.28 ± 0.53 | 299.7 ± 51.1 |
| Without necrosis (n = 39) | 1.62 ± 0.28 | 360.1 ± 0.25 |
| With brain invasion (n = 7) | 2.32 ± 0.59 | 364.8 ± 61.6 |
| Without brain invasion (n = 41) | 1.46 ± 0.27 | 346.0 ± 21.0 |
| With edema (n = 29) | 0.88 ± 0.26 | 357.8 ± 23.0 |
| Without edema (n = 7) | 1.61 ± 0.21 | 451.1 ± 54.8 |
| High mitotic index [1] (n = 8) | 1.53 ± 0.38 | 383.7 ± 66.2 |
| Low mitotic index [2] (n = 40) | 1.78 ± 0.29 | 349.4 ± 20.2 |
| Low vascularisation (n = 24) | 1.53 ± 0.41 | 333.1 ± 26.5 |
| High vascularisation (n = 24) | 1.96 ± 0.28 | 363.2 ± 29.4 |
| Mild infiltrate (n = 45) | 1.76 ± 0.26 | 344.9 ± 20.5 |
| Dense infiltrate (n = 3) | 1.49 ± 0.72 | 407.1 ± 80.6 |
The present study highlights that the ELISA method is a valuable method for the quantitative analysis of bFGF and VEGF contents in human meningiomas as previously reported for other human tumours [6–8]. Thus, VEGF and bFGF proteins were detectable in all meningioma samples. These results are in agreement with the presence of VEGF and bFGF mRNA transcripts in meningiomas [3, 4]. The quantitative analysis of VEGF levels in various subtypes of grade I meningiomas highlighted differences for their VEGF contents; fibroblastic meningiomas exhibiting the lower VEGF contents. The present results confirm a previous study reporting higher VEGF contents in meningothelial meningiomas as compared with fibrous ones [10]. We found no correlation between tumour VEGF content and meningioma grade, vascularisation, and brain invasion. Taken altogether these results suggest that VEGF could serve other functions than sole angiogenesis in meningiomas. For example, an autocrine VEGF stimulation of tumour cells would be suggested explaining differences of VEGF contents between subtypes of meningiomas. Confirming a previous study, [10] bFGF contents were not different in subtypes of grade I meningiomas and had no correlation with meningioma grade, vascularisation and brain invasion casting some doubts concerning the role of this growth factor in meningioma angiogenesis. These results confirm those of Samoto et al [4] showing no correlation between meningioma vascularity and bFGF transcripts. In conclusion, results obtained with the ELISA procedure corroborate those obtained by other investigators using other methods (such as immunohistochemical staining, in situ hybridization or western blotting) [5, 11]. It is, thus, tempting to speculate that the ELISA method would also be valuable for the quantitative analysis of other angiogenic growth factors and cytokines in intracranial tumours.
ACKNOWLEDGMENT
This work was supported by “La Ligue Nationale Française Contre le Cancer” (Comité de la Corrèze et de la Haute Vienne).
References
- 1.Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. The Lancet. 2004;363(9420):1535–1543. doi: 10.1016/S0140-6736(04)16153-9. [DOI] [PubMed] [Google Scholar]
- 2.Bussolino F, Albini A, Camussi G, et al. Role of soluble mediators in angiogenesis. European Journal of Cancer. 1996;32(14):2401–2412. doi: 10.1016/s0959-8049(96)00390-5. [DOI] [PubMed] [Google Scholar]
- 3.Nomura M, Yamagishi S, Harada S, Yamashima T, Yamashita J, Yamamoto H. Placenta growth factor (PlGF) mRNA expression in brain tumors. Journal of Neuro-Oncology. 1998;40(2):123–130. doi: 10.1023/a:1006198422718. [DOI] [PubMed] [Google Scholar]
- 4.Samoto K, Ikezaki K, Ono M, et al. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Research. 1995;55(5):1189–1193. [PubMed] [Google Scholar]
- 5.Pietsch T, Valter MM, Wolf HK, et al. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathologica. 1997;93(2):109–117. doi: 10.1007/s004010050591. [DOI] [PubMed] [Google Scholar]
- 6.Landriscina M, Cassano A, Ratto C, et al. Quantitative analysis of basic fibroblast growth factor and vascular endothelial growth factor in human colorectal cancer. British Journal of Cancer. 1998;78(6):765–770. doi: 10.1038/bjc.1998.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathonnet M, Descottes B, Valleix D, Labrousse F, Truffinet V, Denizot Y. Quantitative analysis using ELISA of vascular endothelial growth factor and basic fibroblast growth factor in human colorectal cancer, liver metastasis of colorectal cancer and hepatocellular carcinoma. World Journal of Gastroenterology. 2006;12(23):3782–3783. doi: 10.3748/wjg.v12.i23.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denizot Y, Chianéa T, Labrousse F, Truffinet V, Delage M, Mathonnet M. Platelet-activating factor and human thyroid cancer. European Journal of Endocrinology. 2005;153(1):31–40. doi: 10.1530/eje.1.01947. [DOI] [PubMed] [Google Scholar]
- 9.Kleihues P, Cavenee WK, editors. Pathology and Genetics of Tumours of the Nervous System. World Health Organization Classification of Tumours. Lyon, France: IARC Press; 2000. [Google Scholar]
- 10.Lamszus K, Lengler U, Schmidt NO, Stavrou D, Ergün S, Westphal M. Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery. 2000;46(4):938–948. doi: 10.1097/00006123-200004000-00033. [DOI] [PubMed] [Google Scholar]
- 11.Goldman CK, Bharara S, Palmer CA, et al. Brain edema in meningiomas is associated with increased vascular endothelial growth factor expression. Neurosurgery. 1997;40(6):1269–1277. doi: 10.1097/00006123-199706000-00029. [DOI] [PubMed] [Google Scholar]