Abstract
Background
After conventional resuscitation from hemorrhagic shock, splanchnic microvessels progressively constrict, leading to impairment of blood flow. This occurs despite restoration and maintenance of central hemodynamics. The authors’ recent studies have demonstrated that topical and continuous ex vivo exposure of the gut microvasculature to a glucose-based clinical peritoneal dialysis solution (Delflex), as a technique of direct peritoneal resuscitation (DPR), can prevent these postresuscitation events when initiated simultaneously with conventional resuscitation. This study aimed to determine whether DPR applied after conventional resuscitation reverses the established postresuscitation intestinal vasoconstriction and hypoperfusion.
Methods
Male Sprague–Dawley rats were bled to 50% of baseline mean arterial pressure and resuscitated intravenously over 30 minutes with the shed blood returned plus two times the shed blood volume of saline. Initiation of ex vivo, topical DPR was delayed to 2 hours (group 1, n = 8), or to 4 hours (group 2, n = 8), respectively, after conventional resuscitation. Intravital microscopy and Doppler velocimetry were used to measure terminal ileal microvascular diameters of inflow A1 and premucosal A3 (proximal pA3, distal dA3) arterioles and blood flow in the A1 arteriole, respectively. Maximum arteriolar dilation capacity was obtained from the topical application, in the tissue bath, of the endothelium-independent nitric oxide-donor sodium nitroprusside (10−4M).
Results
Hemorrhagic shock caused a selective vasoconstriction of A1 (− 24.1% ± 2.15%) arterioles from baseline, which was not seen in A3 vessels. This caused A1 blood flow to drop by −68.6% of the prehemorrhage value. Conventional resuscitation restored and maintained hemodynamics in all the animals without additional fluid therapy. In contrast, there was a generalized and progressive postresuscitation vasoconstriction of A1 (−21.7%), pA3 (−18.5%), and dA3 (−18.7%) vessels. The average postresuscitation A1 blood flow was −49.5% of the prehemorrhage value, indicating a persistent postresuscitation hypoperfusion. Direct peritoneal resuscitation reversed the postresuscitation vasoconstriction by 40.9% and enhanced A1 blood flow by 112.9% of the respective postresuscitation values.
Conclusions
Delayed DPR reverses the gut postresuscitation vasoconstriction and hypoperfusion regardless of the initiation time. This occurs without adverse effects on hemodynamics. Direct peritoneal resuscitation–mediated enhancement of tissue perfusion results from the local effects from the vasoactive components of the Delflex solution, which are hyperosmolality, lactate buffer anion, and, to a lesser extent, low pH. The molecular mechanism of this vasodilation effect needs further investigation.
Keywords: Hemorrhagic shock, Resuscitation, Trauma care, Peritoneal therapy, Hyperosmolality
Early management of trauma patients with massive blood loss involves control of bleeding and correction of vascular volume deficit. This initial definitive care remains the essential element for improving morbidity and mortality. Although the therapeutic end point of resuscitation from hemorrhagic shock is clinically derived, there is increasing evidence that with restoration and maintenance of central hemodynamics, urine output, and correction of lactic acidemia and base deficit, splanchnic organs, especially the gut, experience a progressive vasoconstriction and hypoperfusion.1,2 This gut hypoperfusion has been implicated in the pathogenesis of multiple-organ dysfunction via mechanisms related to tissue hypoxia and priming of circulating neutrophils, which provoke distal organ injury.3–6 Despite the significance of gut hypoperfusion in the morbidity and mortality of trauma and blood loss, few studies have attempted to normalize blood flow to the gut.7–10 However, in all these experimental studies, treatments were administered before the induction of shock in an effort to elucidate the mechanisms of gut hypoperfusion. Such preventive interventions are clearly not useful in the clinical arena.
In a recent intravital videomicroscopy study, the authors have shown that topical ex vivo exposure of a small segment of intestine to a clinical peritoneal dialysis solution prevents the microvascular vasoconstriction and hypoperfusion noted in that segment with conventional intravenous resuscitation from hemorrhagic shock.11 In addition, this technique of simulated direct peritoneal resuscitation (DPR), when initiated simultaneously as an adjunct to conventional resuscitation, produced an instantaneous and sustained vasodilation and hyperperfusion at all levels of the observed intestinal microvasculature in the segment exposed to the dialysis solution. In subsequent studies, the authors have shown that exposure of the whole peritoneal cavity to 30 mL of the same peritoneal dialysis solution at the time of resuscitation from hemorrhagic shock in rats results in a 30% increase in whole organ blood flow (measured with colored microspheres) in the gut, more than a 50% increase in spleen and pancreas flow, and more than a 100% increase in lung, psoas muscle, and diaphragm flow.12 Such hyperperfusion was not observed when a similar volume of saline (30 mL) was instilled into the peritoneal cavity. Furthermore, it was noted that this DPR-mediated splanchnic and distal hyperperfusion occurred without any change in central cardiovascular hemodynamics.
Initial intravenous fluid resuscitation with Ringer’s lactate solution infused at the trauma scene has been the standard treatment for civilian trauma casualties. The initiation of this intravenous volume replacement therapy in the management of army combatants is hindered by the absence of basic monitoring techniques on the battlefield, logistic constraints such as availability of large volumes of intravenous fluid, and prolonged evacuation times to definitive care facilities.13,14 In any event, the type of injury appears to determine the strategy for intervention. For penetrating injures, analogous to animal models of uncontrolled hemorrhagic shock, delayed or hypotensive resuscitation has yielded a better outcome.15 In contrast, for blunt trauma, resembling animal models of controlled hemorrhagic shock, early fluid resuscitation strategy appears to provide rapid reversal of hemodynamic and metabolic changes.16
Regardless of the injury type, compiled data on the available resuscitation regimens have shown that fluid resuscitation upregulates a systemic inflammatory response and exacerbates cellular injury.17 From a practical point of view, DPR requires the establishment of a peritoneal access under aseptic conditions. This limitation requires that DPR be initiated in a delayed fashion at a trauma facility after control of bleeding and stabilization of the patient with rapid early fluid resuscitation. Therefore, the purpose of the current study was to examine whether delayed simulation of DPR produces the same beneficial microvascular effects observed when a small intestinal segment is exposed to a peritoneal dialysis solution simultaneously with the initiation of conventional intravenous resuscitation, as in the authors’ previous study.11
METHODS
The study animals were maintained in a facility approved by the American Association for the Accreditation of Laboratory Animal Care. The research protocol was approved by the Institutional Animal Care and Use Committee and the Biohazard Safety Committee at the Louisville VA Medical Center. Male Sprague–Dawley rats (200–210 g) were used in the experiments. The animals were acclimated for 1 week before experimental use, during which time the animals received standard rat chow ad libitum.
Anesthesia was induced with intraperitoneal pentobarbital (50 mg/kg), and supplemental subcutaneous injections (25% the original dose) were given as needed to maintain a surgical plane of anesthesia throughout the experimental protocol. Before surgical preparation, all the animals received a subcutaneous injection of 2 mL normal saline to correct for body fluid loss during the 2-hour period of surgical preparation and equilibration. Body temperature was maintained at 37° ± 0.5°C with a rectal probe and a servo-controlled heating pad. Surgery was performed after loss of the blink and withdrawal reflexes. Tracheostomy was performed to reduce airway resistance, and the animal was allowed to breathe room air. The right femoral artery and vein were cannulated with PE-50 catheters for blood withdrawal and resuscitation, respectively. The carotid artery was cannulated for continuous monitoring and online recording of blood pressure on a pressure measurement system.
Hemorrhagic Shock Model
Hemorrhagic shock was achieved with blood withdrawal (1 mL/min) from the femoral artery in a syringe prerinsed with 0.02 mL heparin (1,000 U/mL). This was continued until 50% of mean arterial pressure (MAP) was attained. The 50% MAP was maintained for 60 minutes with further blood withdrawal or reinfusion as required. On the average, the total volume of blood withdrawn to maintain the target of 50% baseline MAP was 5.13 ± 0.18 mL. Conventional resuscitation was initiated with the intravenous return of the shed blood over 5 minutes. Normal saline equal to two times the volume of shed blood then was infused over the next 25 minutes.
Bathing Solutions
Solution A was a nonvasoactive modified Krebs’ solution that contained 6.92 g/L sodium chloride, 0.44 g/L potassium chloride, 0.37 g/L calcium chloride, and 2.1 g/L sodium bicarbonate at a pH of 7.4 and an osmolality of 286 mOsm/L. Solution B (DPR solution) was a clinical 2.5% dextrose-based dialysis solution (Delflex, Fresenius USA, INC. Ogden, UT) that contained 0.567 g/L sodium chloride, 0.392 g/L sodium lactate, 0.0257 g/L calcium chloride, and 0.0152 g/L magnesium chloride at a pH of 5.5 and an osmolality of 398 mOsm/L.
Intestinal Microvascular Preparation
The peritoneal cavity was exposed through a midline abdominal incision (~1.5 cm). Then a 2- to 3-cm segment of distal ileum was gently withdrawn from the peritoneal cavity with its neurovascular supply intact. The ileum was opened along the antimesenteric border with electrocautery. Enteric contents and mucus were gently removed from the mucosal surface. The animals were placed on a specially designed polyurethane board. The opened ileum then was suspended, serosal side up, over the viewing port while submerged in a tissue bath containing solution A with 4–0 silk sutures. The bathing solution was maintained at 37°C and bubbled with nitrogen and carbon dioxide to maintain the pH at 7.4 throughout the experiment. Isoproterenol was added to the bathing solution in a very dilute concentration (0.01 μg/mL) to retard peristalsis. This dose of isoproterenol was below the threshold that alters vascular smooth muscle tone.18
The animal was positioned on the stage of a trinocular microscope for direct in vivo intravital microscopy. Microvascular images were transmitted through the microscope to a photodiode array in an optical Doppler velocimeter (Microcirculation Research Institute, TX A & M University, College Station, TX) to measure centerline red blood cell velocity for the calculation of intestinal microvascular blood flow. The microvascular image then was transmitted to a digital camera (Hitachi Denshi, Models K-P-D51/D50) and a computer monitor. The microvascular digital images were stored as a streamline video on the computer hard drive for later measurement of microvascular diameter using calipers. Criteria for an acceptable preparation of the intestine for intravital microscopy included a baseline MAP greater than 90 mm Hg, a red blood cell velocity in a first-order arteriole exceeding 20 mm/second, and active vasomotion in the arteriolar system.
The standard nomenclature for intestinal microvessels as originally described by Bohlen and Gore was used.18 Briefly, first-order arterioles (A1) arise from a mesenteric arcade artery, traverse the mesenteric border of the bowel wall, and penetrate through the muscle layers to the submucosal layer. In the submucosal layer, second-order arterioles (A2) arise from the A1 and run along the longitudinal axis of the bowel. First- and second-order venules parallel the A1 and A2 arterioles. Third-order arterioles (A3) branch at right angles from A2 arterioles and continue on to terminate in the mucosa as a central villus arteriole. Along their course, A3 arterioles also give rise to smaller arterioles that supply the seromuscular layers of the bowel wall. Centerline red blood cell velocity in A1 arterioles was measured with optical Doppler velocimetry. The maximum velocity signal, displayed digitally, was used to calculate blood flow according to the formula V/1.6)(R2 × 0.001), where V is the centerline flow velocity, 1.6 is a correction factor that converts centerline velocity to average cross-sectional velocity, R is the intraluminal microvascular radius in μm, and 0.001 is a conversion factor to express flow in nL/second. This equation assumes a parabolic flow velocity and a circular conduit. Studies have identified 1.58 to 1.60 as the ideal correction factor for a wide range of microvessels.
Experimental Protocol
The time line for the experimental protocol is shown in Figure 1. After animal preparation, 60 minutes was allowed for animal equilibration and recovery from surgical stress. During that time and until the time for initiation of simulated DPR, the exteriorized ileum was continuously suffused with solution A in the tissue bath. Blood pressure, heart rate, rectal and bath temperatures, and bath pH were continuously monitored and recorded every 5 minutes (Digi-Med Signal Analyzers, Louisville, KY). Baseline microvascular measurements were taken during the last 10 minutes of the equilibration period when the variability in the measurements was less than 5%.
Fig. 1.
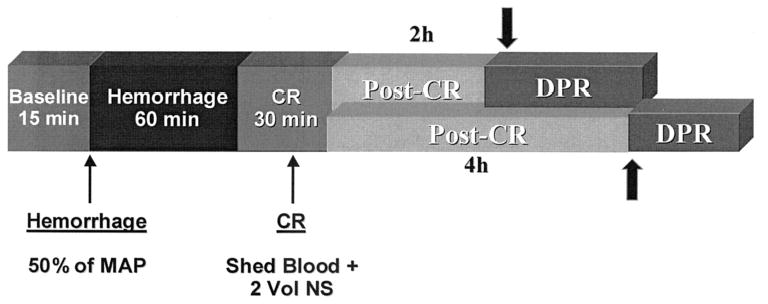
Time line of the experimental protocol. The arrow shows the timing for initiation of direct peritoneal resuscitation (DPR). MAP, mean arterial pressure; CR, conventional resuscitation
After baseline measurements, hemorrhagic shock was initiated and maintained according to protocol. Each time point measurement at the completion of shock and subsequently at 20-minute intervals consisted of mean arterial pressure, heart rate, rectal and bath temperatures, bath pH and diameters of the intestinal inflow A1 arteriole, and premucosal pA3 and dA3 arterioles, as well as the centerline red cell velocity in the A1 arteriole. After 60 minutes of hemorrhagic shock, the animals were resuscitated conventionally with shed blood and normal saline according to protocol. The animals were randomized for delayed DPR, which was initiated 2 or 4 hours after conventional resuscitation. To simulate DPR, solution A was drained from the tissue bath, and solution B (Delflex) replaced it in the bath either 2 or 4 hours after conventional resuscitation, for the 2-hour and 4-hour delayed-DPR groups, respectively. Solution B (Delflex) was maintained in the tissue bath to suffuse the ileum continuously for the remaining 100 minutes of the experiment in both groups. Microvascular and hemodynamic measurements as well as core body temperature and tissue bath pH and temperature were performed immediately after initiation of simulated DPR and at 20-minute intervals for the remainder of the experiment. During the simulated DPR protocol, solution B (Delflex) was in direct contact only with the small segment of ileum under observation in the tissue bath. At the end of the experiment, a single dose of the endothelium-independent, receptor-independent sodium nitruprusside (10−4M) was added to the tissue bath to assess the maximum dilation capacity of each arteriole. Data on microvascular diameters were normalized as a percentage of the baseline and also as a percentage of the maximum dilation capacity.
Statistical Analysis
All data are presented as mean ± standard error of the mean unless stated otherwise. The percentage change of vessel diameter from baseline was assessed by repeated measures two-way analysis of variance (ANOVA), followed by Dunnett’s multiple-range test to evaluate changes from baseline within the same animal. A result was considered significant if the probability of a type 1 error was a p value less than 0.05.
RESULTS
There was no significant difference in baseline hemodynamics and intestinal microvascular diameters between the two experimental groups. As expected, hemorrhagic shock caused a significant reduction in MAP from baseline in both experimental groups. Conventional resuscitation from hemorrhagic shock restored and maintained MAP during the entire period of the experiment in both groups without subsequent additional fluid infusion (Figs. 2 and 3, upper panel).
Fig. 2.
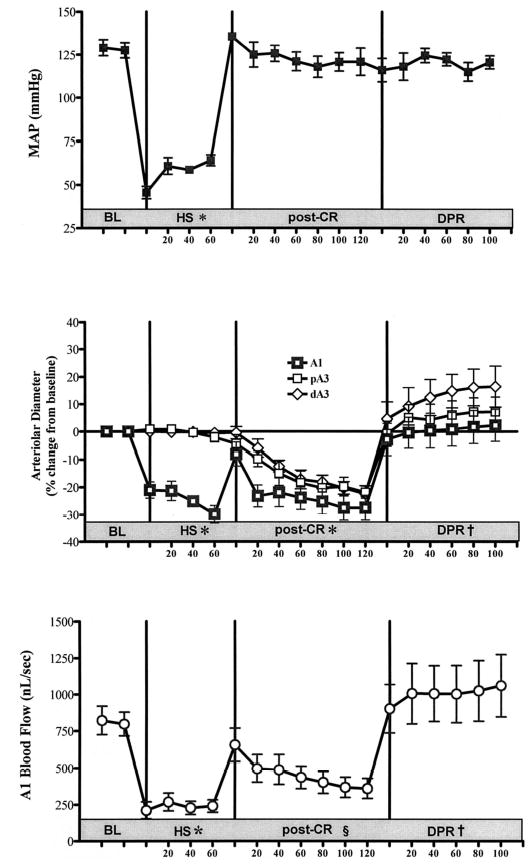
Effects of a 2-hour delay in the initiation of direct peritoneal resuscitation (DPR) on mean arterial pressure (MAP) (upper panel), microvascular diameter (middle panel), and intestinal A1 blood flow (lower panel). BL, baseline; HS, hemorrhagic shock; post-CR, postconventional resuscitation; A1, intestinal inflow arteriole; pA3, proximal A3 premucosal arteriole; dA3, distal A3 premucosal arteriole. * p < 0.001 versus BL by two-way analysis of variance (ANOVA) and Bonferroni posttest. § p < 0.05 versus BL by two-way ANOVA and Bonferroni posttest. † p < 0.01 versus post-conventional resuscitation by two-way ANOVA and Bonerroni posttest.
Fig. 3.
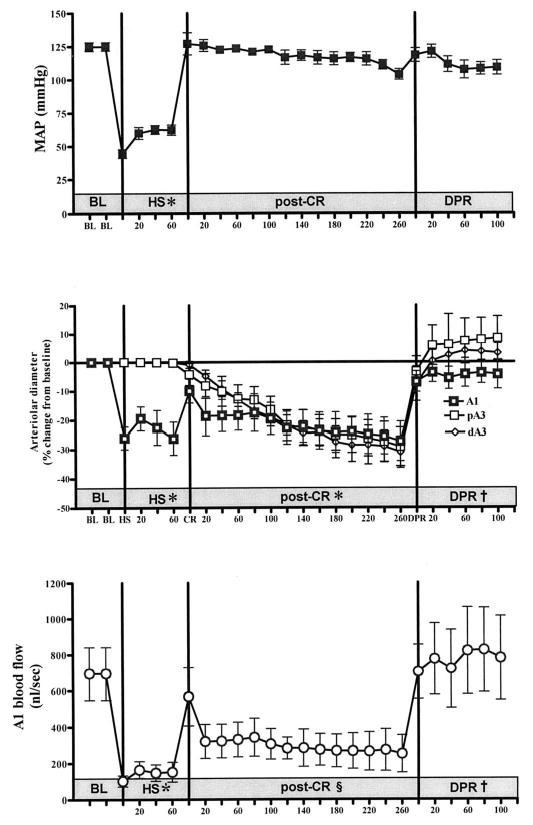
Effects of a 4-hour delay in initiation of direct peritoneal resuscitation (DPR) on mean arterial pressure (MAP) (upper panel), microvascular diameter (middle panel), and intestinal A1 blood flow (lower panel). BL, baseline; HS, hemorrhagic shock; post-CR, postconventional resuscitation; A1, intestinal inflow arteriole; pA3, proximal A3 premucosal arteriole; dA3, distal A3 premucosal arteriole. *p < 0.001 versus BL by two-way analysis of variance (ANOVA) and Bonferroni posttest. §p < 0.05 versus BL by two-way ANOVA and Bonferroni posttest. † p < 0.01 versus post-conventional resuscitation by two-way ANOVA and Bonerroni posttest.
There was a differential response of the intestinal microvasculature to hemorrhagic shock (Figs. 2 and 3, middle panel). There was a marked constriction of the inflow A1 arterioles (~100 μm), which was not seen in the smaller premucosal A3 vessels (8–15 μm). Conventional resuscitation initially restored A1 diameters to prehemorrhagic shock baseline levels. This was followed by a progressive vasoconstriction of all intestinal arterioles. Delayed initiation of DPR to 2 or 4 hours, respectively, produced an instantaneous and sustained vasodilation from postconventional resuscitation diameters in all intestinal arterioles (39% ± 7.1% and 40% ± 10.8%). There was no significant difference in the magnitude of the DPR-mediated dilation in the intestinal arterioles between the 2-hour and 4-hour delay animal groups.
Hemorrhagic shock caused a significant reduction in intestinal A1 blood flow (Figs. 2 and 3, lower panel). Conventional resuscitation initially restored A1 blood flow to prehemorrhagic shock baseline values. This was followed by a progressive decrease in blood flow (average, −48% and −64%) of baseline flows 2 and 4 hours after conventional resuscitation, respectively. Delayed initiation of DPR to 2 and 4 hours, r espectively, increased and sustained A1 blood flow from postconventional resuscitation blood flow (+195% and +325%).
Delayed DPR-mediated intestinal microvascular reactivity expressed as a percentage change from postconventional resuscitation diameter is depicted in Figure 4. Delayed DPR to 2 hours after conventional resuscitation (upper panel) produced a generalized vasodilation at all intestinal arteriolar levels. This vascular reactivity was similar in magnitude to the dilation produced by a 4-hour delay in initiation of DPR therapy (lower panel).
Fig. 4.
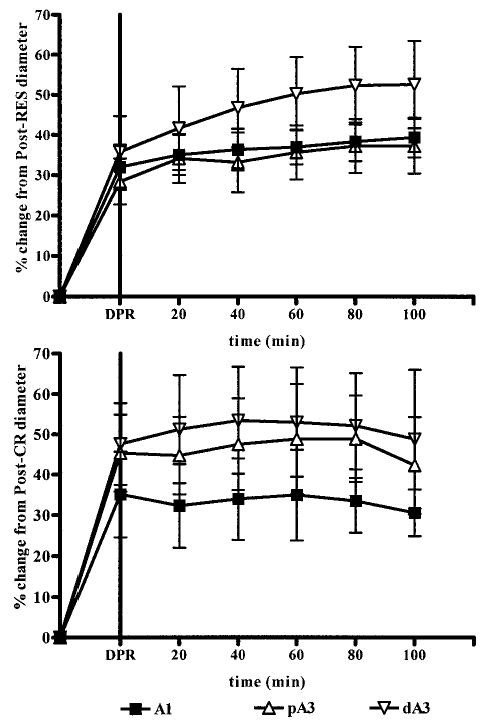
Magnitude of direct peritoneal resuscitation (DPR)-mediated dilation in intestinal microvasculature after 2-hour and 4-hours delays in DPR therapy initiation (upper and lower panels, respectively). A1, intestinal inflow arteriole; pA3, proximal A3 premucosal arteriole; dA3, distal A3 premucosal arteriole.
DISCUSSION
Conventional resuscitation from trauma and hemorrhagic shock sometimes culminates in multisystem organ failure and death. This has been attributed to three major pathophysiologic events: (1) a progressive splanchnic vasoconstriction and hypoperfusion, (2) a gut-derived exaggerated systemic inflammatory response, and (3) an obligatory tissue fluid sequestration.19,20 The mode by which these events translate into distal tissue injury and organ failure remains to be elucidated fully. There is overwhelming evidence to suggest that the persistent splanchnic hypoperfusion noted after resuscitation from hemorrhagic shock causes activation of the systemic inflammatory response, which is launched through lymphatic drainage of the gut.3,6,21–24 The current study confirmed previous observations that in the intestine and other splanchnic organs, adequate conventional resuscitation that restores baseline hemodynamics results in a progressive intestinal microvascular vasoconstriction and hypoperfusion. In addition, the results show that this established postresuscitation microvascular derangement can be reversed by a clinical glucose-based peritoneal dialysis solution (Delflex) applied topically on a short segment of the terminal ileum.
The mechanism by which the splanchnic microcirculation relapses to vasoconstriction after restoration and maintenance of central hemodynamics is not fully understood. However, a number of studies have shown that this vasoconstriction is associated with impairment of the endothelium-dependent dilation response, a postperfusion burst of superoxide and oxygen free radical formation, and decreased production of basal levels of constitutive nitric oxide.25–29 Furthermore, it is important to note that resuscitation from hemorrhagic shock also is known to alter membrane potential, disturb transmembrane ion equilibrium and membrane fluidity, alter cell volume, deplete energy stores, and accelerate the production of certain vasoactive substances while suppressing others. These pathophysiologic events reflect major alterations in organ microcirculation and tissue perfusion that cannot be reversed by vigorous intravenous fluid therapy.1,28 Unfortunately, measurements of blood pressure, heart rate, urine output, and central venous pressure used commonly as clinical end points of adequate intravenous resuscitation are inadequate indicators of tissue perfusion.30,31 Clearly, adjunct resuscitation techniques that can restore tissue perfusion without superfluous effects on hemodynamics are necessary.
In the current study, intravital microscopy was used to monitor microvascular reactivity and blood flow changes in a small segment of the terminal ileum (2–4 cm) that had intact nerve and vascular supplies. This segment was positioned in a relatively large bath (60 mL) in which temperature, pH, partial pressure of oxygen (pO2), partial pressure of carbon dioxide (pCO2), and osmolality were monitored and controlled while direct observations of the intestinal microcirculation were made simultaneously. The authors’ previous experience with this technique for resuscitated hemorrhagic shock and sepsis1,2,32 demonstrated that vessel diameters of the intestinal microcirculation are highly stable over 4 to 6 hours of observation when a nonvasoactive Krebs–Ringer solution is used to suffuse the intestine continuously at a controlled temperature of 37°C and a pH of 7.35 to 7.40.1,2 Thus, the stability of the baseline measurements suggests that the vessel reactivity changes in the current study can be attributed only to a specific experimental intervention rather than to a shift in microvascular baselines.
In the current study, the instantaneous reversal of the postresuscitation vasoconstriction and hypoperfusion that occurred without a change in systemic MAP can be attributed to the topical exposure of the intestinal segment to the Delflex solution. The experimental protocol with a short segment of intestine presumably simulates the microvascular events that would occur if DPR were applied to the whole peritoneal cavity. Although the mechanisms of Delflex-mediated dilation have not been clearly defined, it is unlikely that the systemic effects of glucose absorption are active in the current protocol because only a 2- to 4-cm segment of the intestine was exposed to the Delflex solution. In other experiments, in which the entire peritoneal cavity was exposed to the Delflex solution, a moderate increase in blood glucose was observed.12 Although blood glucose was not measured in the current study, the authors doubt that systemic glucose absorption was a factor in the instantaneous dilation effects noted in the experimental preparation. Delflex, like other glucose-based clinical peritoneal dialysis solutions, produces vasodilation in both visceral and parietal microvascular beds by mechanisms related to the solution’s hyperosmolality and its lactate buffer anion system.33,34 The authors have shown that there is a vasodilation response after mucosal glucose absorption that is mediated by an adenosine receptor–mediated mechanism.35 Although local metabolic glucose absorption was active in the small intestinal segment under observation in the current experimental preparation, it probably accounted for only one fourth of the dilation effect observed in this study.
Fluid shifts in response to a hypertonic solution in the peritoneal cavity are complex. Instillation of such a fluid in the peritoneal cavity generates an osmotic force proportional to its glucose concentration and changes the intraperitoneal hydrostatic pressure in a nonlinear fashion depending on the instilled volume.36–38 These intraperitoneal forces cause simultaneous osmotic-driven water flux into the peritoneal cavity, and in the opposite direction, a hydrostatic-driven fluid convection into tissue bordering the peritoneal cavity, especially the abdominal muscle.36–38 Whereas the osmotic water flow is limited by dissipation of the osmotic gradient attributable to glucose absorption, the hydrostatic-driven water flow promotes tissue hydration, restores lymph flow, and forms a slow resuscitation compartment, which expands the vascular volume overtime. In a different study, the authors have shown that DPR placed in the entire cavity as an adjunct to resuscitation causes antifluid sequestration and immuno-modulation effects in a rat model of severe hemorrhagic shock and conventional resuscitation.39 In this model, initiation of DPR simultaneously with conventional resuscitation normalized body weight and tissue water contents and caused a marked production of interleukin-10 (IL-10) in the liver and intestine, as compared with conventional resuscitation alone. This is associated with a significant downregulation of liver IL-6 and intestinal tumor necrosis factor-α (TNF-α) and a 100% survival after 72 hours, as compared with 40% mortality over 24 hours among the conventionally treated animals.39 In other studies the authors have shown that exposure of the whole peritoneal cavity to 30 mL of Delflex solution at the time of resuscitation from hemorrhagic shock in rats causes a 30% increase in whole organ blood flow (measured with colored microspheres) in the gut, more than a 50% increase in spleen and pancreas flow, and more than a 100% increase in lung, psoas muscle, and diaphragm flow.12 Such hyperperfusion was not observed when 30 mL of saline was instilled into the peritoneal cavity. Therefore, it appears from the data that DPR, when used as an adjunct to conventional resuscitation, augments splanchnic and lung blood flow in the postresuscitation period by a local change in vascular resistance.
The rationale for using the authors’ technique of DPR as an adjunct to conventional resuscitation is to prevent or reverse the splanchnic vasoconstriction and hypoperfusion. This new resuscitation technique uses a clinical peritoneal dialysis solution known for its vasoactive properties and for its ability to enhance local blood flow.33,34 The magnitude of the dilation and hyperperfusion observed in the current study is similar to those reported in the authors’ previous study, in which DPR was initiated simultaneously with conventional resuscitation.11 Therefore, DPR appears to produce the same vasodilation effect regardless of the time of initiation. Direct peritoneal resuscitation is conceptually different from conventional intravascular resuscitation or low-volume intravascular hypertonic saline resuscitation. The solution is administered intraperitoneally as a topical adjunct to intravascular volume resuscitation. In addition to its favorable effects on vascular reactivity and blood flow, DPR has other potential therapeutic benefits, which include the reversal of the capillary no-flow phenomenon seen at reperfusion after hemorrhagic shock. This occurs because DPR enhances both arterial inflow and venous outflow, and appears to prevent the swelling of the capillary endothelial lining. Also, DPR alters the systemic inflammatory response presumably by suppression of neutrophil activation and modulation of neutrophil function.40–42 In addition, DPR can potentially reverse the shock/resuscitation-mediated fluid shifts and electrolyte imbalance noted with conventional resuscitation, presumably by an osmotic effect. Other beneficial effects of DPR include correction of the systemic acidosis by the buffer system of the peritoneal solution, and by enhancement of tissue perfusion.
With hemorrhagic shock, neurohumoral reflexes mediated by catecholamines, vasopressin, and angiotensin II promote vasoconstriction in certain vascular beds to ensure that an adequate fraction of the cardiac output supplies oxygen and nutrients to vital organs.43,44 This occurs at the expense of other vascular beds such as the gut.45 At the microvascular level, shock results in impairment of local mechanisms that control local vascular tone, organ blood flow, and the downstream capillary surface area.46 At the capillary level, shock reduces the capillary luminal diameter by 20% to 25% because of endothelial cell swelling.47 This endothelial swelling is prompted by lactic acidosis in a pH-dependent fashion and can be blocked by specific Na+ channel inhibitors. Therefore, this observation is thought to be the result of intracellular regulatory mechanisms, namely, Na+/H+ exchange and bicarbonate-transporting carriers.48,49 Another adaptive response to hemorrhagic shock is the compulsory translocation of body water, electrolytes, and proteins to restore an effective blood volume and suppress baroreceptor activity. With resuscitation, however, there is further cellular swelling associated with an increase in cellular H2O, Na+, K+, Ca++, and adenosine triphosphate (ATP).50,51 Although these remote and local compensatory adjustment mechanisms are intended to maintain adequate tissue perfusion in accord with tissue demand, the occurrence of a time-dependent circulatory insufficiency at the tissue level becomes self-destructive when perfusion falls below the metabolic needs of the parenchymal cells. Therefore, enhancement of tissue perfusion, especially in splanchnic organs, is crucial in reversing the pathophysiology of shock. In animal models, a number of pharmacologic interventions such as pentoxifylline, magnesium chloride, and adenosine or complement inhibition have been shown to preserve microvascular blood flow and improve resuscitation outcome.7,52–55 Thus, any technique that improves local tissue perfusion without negative effects on central hemodynamic function would have clinical value. Direct peritoneal resuscitation appears to have these qualities.
In conclusion, addition of DPR to conventional resuscitation protocols, either as a simultaneous therapy or at a delayed time after conventional resuscitation, prevents progressive splanchnic ischemia and produces a sustained generalized vasodilation and hyperperfusion without adverse effects on hemodynamics. The molecular mechanism of these favorable effects awaits further investigation.
DISCUSSION
Dr. Carl J. Hauser (Newark, New Jersey): Thank you. The resuscitation of hemorrhagic shock to standardized hemodynamic end points such as blood pressure and urine output often leaves patients with subtle ongoing tissue perfusion defects. Because the gut is highly susceptible to such defects and splanchnic vasoconstriction can help convert enterocytes to an inflammatory phenotype, it is of concern that these may contribute to SIRS.
In prior work, these authors have shown that peritoneal dialysis using hypertonic saline glucose solutions in addition to conventional resuscitation seems to modify body water shifts that can impair gut perfusion and activate leukocytes systemically. They call this therapy “direct peritoneal resuscitation,” although they state that it does not act through blood volume expansion. So, in that sense, the term “resuscitation” seems a little out of place to me. I would prefer to call it “hypertonic peritoneal dialysis.”
That being the case, the authors now extend their prior work and have evaluated DPR as a posttreatment for shock. Using vital microscopy, they show that the addition of direct peritoneal resuscitation to shocked and resuscitated rats appears to alleviate splanchnic vasoconstriction and to improve gut perfusion.
The manuscript is well written. The results are clear and convincing. There are some questions that I still think should be addressed.
The authors are convinced that the effects of DPR are independent of plasma volume changes. I think they probably are right, but I think that it’s probably best to measure plasma volume directly to prove that this is correct and that the sparing of gut perfusion is not simply a volume expansion effect. Furthermore, the composition of the dialysate really is not well spelled out in the paper, and the individual components of such fluids can be crucial. I think, in that respect, the study probably lacks some controls. For instance, would peritoneal dialysis with hypertonic saline produce similar results? One has to wonder whether by doing this dialysis, we’re simply washing out vasoconstrictor substances from the area, or whether this is, in fact, a reversal of osmotic swelling, for instance, of endothelial cells, and subsequent inflammation.
The authors also speculate that DPR may act by diminishing acidosis. If so, I’d like to know if they measured systemic base deficit or lactate. Also, in that respect, if dialysates are always buffered, could the provision of the systemic buffer also have contributed?
I really enjoyed this presentation. There’s been very little really new under the sun in the realm of resuscitation for a long time, and we need innovative thinking such as this.
Dr. Rao R. Ivatury (Richmond, Virginia): I thank Dr. Garrison for allowing me the privilege of reading their manuscript in advance, and also for an outstanding presentation. This is really a very intriguing study, not only because we are resuscitating by DPR. I have some questions about whether velocity in the vessels really equates with mucosal blood flow and tissue oxygenation. Can we equal the two? A lot of recent studies begin to talk about the problem of shock as not necessarily from a delay or a low oxygen delivery, but as a result of interference with tissue energetics. In that context, would it be advisable for you to measure the mucosal phoxy-hemoglobin levels, cytochrome A8:3 ratios, or NADH:NAD ratios to tell us something about what exactly a cell is doing?
Furthermore, I’m also intrigued by your intravital microscopy. We began to do this, but we are grappling with the problem of standardizing the definitions of the results from microconstriction versus dilation. So, what exactly is the interrater variability in the computation of these vessel diameters by observation of the computerized images, especially those you showed with severe constriction? Is this a systemic effect? Do you have any data on the base deficit or lactate? Can we do these microcirculatory studies elsewhere from the gut and also show the same beneficial effects? And would you speculate on the mechanism?
I enjoyed the presentation. Thank you.
Dr. H. Gill Cryer (Los Angeles, California): Thank you. Neal. I really enjoyed this paper. It’s good to see the work is still going on in that laboratory at the rate that you are doing it.
I have a couple of questions. John Goshee, quite a few years ago, did some studies when he was using glucose as a perfusate. Again, I’m not clear as to what the peritoneal dialysate has in it, but when glucose was absorbed by the mucosal side of the bowel, it was a potent vasodilator that virtually could not be stopped. No matter what vasoconstrictor you give, glucose absorption in the intestine will overcome it and cause vasodilation. The bowel that you’re observing is the mucosal side in contact with the resuscitation solution, so that this could be more of a reaction to mucosal absorption of glucose. Or is it possible that there is absorption through the serosal side?
It wasn’t clear to me exactly how you were doing the resuscitation. I enjoyed the paper.
Dr. Slate Wilson (Portland, Oregon): I, too, think this is an elegant study. Those pictures are just great. What this does to me as a trauma surgeon is make me fantasize about possible clinical trials. I’m wondering whether anybody is dreaming of getting institutional review board permission to start doing something in shock from a particularly nonabdominal source.
Dr. R. Neal Garrison (Louisville, Kentucky, closing): Thank you for those kind comments. Dr. Hauser. I think the issue of whether it’s resuscitation versus dialysis and what we call it is purely semantics. We could say basically “peritoneal exposure” I guess. It isn’t dialysis, and we purposely did not use that term because “dialysis” implies that you’re washing out something, whereas we’re simply putting it in. We do not have plasma volume changes or haven’t measured them, but with this model, in which we’re using it, I doubt that this is the effect because we have only a very small segment of intestine exposed to the fluid. The fluid shifts simply would not occur in a small, 2-cm segment of intestine exposed to the tissue bath. Therefore, I doubt that there are any significant plasma volume exchanges.
You mentioned the hypertonic saline issue, and we think the mechanism is the tonicity and the osmolarity of the solution. This is a glucose-based solution. The dialysis fluid is basically saline with a lot of glucose in it, and the osmolarity is about 390. We think it does prevent the swelling of the endothelial cell and maintains its integrity so that the membrane function can occur. You can prevent this vasoconstriction by inhibiting complement with the administration of heparin, which has no anticoagulant effect but some membrane effect, or you can prevent it by delivery of magnesium chloride ATP. The animal does fine, the vessels do fine, and the blood pressure still is 40 with the ATP solution. We did not measure lactate, so we do not know what those levels are, but we assuming that they were elevated at least during the hemorrhage period.
Dr. Ivatury, I appreciate your comments. We have done experiments in which we have looked at laser Doppler oxygen flow probes on the intestine on the mucosal side, and it does correlate with the flow characteristics of the vessel. Reliability of the views is really very tight. We select the vessels, so you know investigator credibility is really very good and the numbers are very tight. When you perform this technique of videomicroscopy, as you had mentioned to me beforehand, the problem is the baseline of a vessel. This is one animal observation, so you start with a baseline, and then you can measure the changes from that baseline state. If you just put it on in the middle of shock you don’t know what your baseline blood vessel is, but they are reliable anatomy-wise in terms of their diameter when the baseline diameter can be established. Again, I’m not sure of the mechanisms, but I suspect that they involve osmotic control of the endothelial cell membrane.
Dr. Cryer, we appreciate your comments. These experiments were performed on your microscope. The setups are the same, and the equipment is the same as when you were there. You are correct. Dr. Goshe presented some data that showed similar findings. When glucose was given, we thought it was the glucose absorption. It turns out that it isn’t the glucose absorption, but the osmolarity of the solution. The jejunum is responsive to glucose under an adenosine mechanism of action, but you can block adenosine receptors and still get the dilation effect in the terminal ileum where these experiments were done. The mucosa is in contact with the solution. It is in the tissue bath.
Dr. Wilson, I appreciate your comments. I am trying to get institutional review board approval to do this clinically. We need to proceed because it is a solution that is used every day clinically, and I think it will be an adjuvant to resuscitation for trauma patients.
Thank you for the privilege of the floor.
Footnotes
This project was supported by NIH research Grant # R01 HL76163–01, funded by the National Heart, Lung, and Blood Institute and the United States Army Medical Resources and Material Command, and by a VA Merit Review grant. Part of this study was presented and published in an Abstract form at the 62nd AAST annual meeting, September 11–13, 2003, Minneapolis, Minnesota.
References
- 1.Zakaria ER, Spain DA, Harris PD, Garrison RN. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002;18:567–573. doi: 10.1097/00024382-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. doi: 10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–145. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Upperman JS, Deitch EA, Guo W, Lu Q, Xu D. Posthemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock. 1998;10:407–414. doi: 10.1097/00024382-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez RJ, Moore EE, Ciesla DJ, Biffl WL, Johnson JL, Silliman CC. Mesenteric lymph is responsible for posthemorrhagic shock systemic neutrophil priming. J Trauma. 2001;51:1069–1072. doi: 10.1097/00005373-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Complement inhibition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782–791. doi: 10.1067/msy.1998.91489. [DOI] [PubMed] [Google Scholar]
- 8.Spain DA, Fruchterman TM, Matheson PJ, Wilson MA, Martin AW, Garrison RN. Complement activation mediates intestinal injury after resuscitation from hemorrhagic shock. J Trauma. 1999;46:224–233. doi: 10.1097/00005373-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Watkins JM, Spain DA, Krysztopik RJ, Downard PJ, Wilson MA, Garrison RN. Heparin preserves intestinal perfusion after hemorrhage and resuscitation. J Surg Res. 1996;66:154–158. doi: 10.1006/jsre.1996.0388. [DOI] [PubMed] [Google Scholar]
- 10.Flynn WJ, Jr, Gosche JR, Garrison RN. Intestinal blood flow is restored with glutamine or glucose suffusion after hemorrhage. J Surg Res. 1992;52:499–504. doi: 10.1016/0022-4804(92)90318-t. [DOI] [PubMed] [Google Scholar]
- 11.Zakaria ER, Garrison RN, Spain DA, Matheson PJ, Harris PD, Richardson JD. Intraperitoneal resuscitation improves intestinal blood flow following hemorrhagic shock. Ann Surg. 2003;237:704–713. doi: 10.1097/01.SLA.0000064660.10461.9D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zakaria ER, Hurt RT, Matheson PJ, Garrison RN. A novel method of peritoneal resuscitation improves organ perfusion after hemorrhagic shock. Am J Surg. 2003;186:443–448. doi: 10.1016/j.amjsurg.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Holcomb JB. Fluid resuscitation in modern combat casualty care: lessons learned from Somalia. J Trauma. 2003;54:S46–S51. doi: 10.1097/01.TA.0000051936.91915.A2. [DOI] [PubMed] [Google Scholar]
- 14.Dubick MA, Atkins JL. Small-volume fluid resuscitation for the far-forward combat environment: current concepts. J Trauma. 2003;54:S43–S45. doi: 10.1097/01.TA.0000064514.42470.3B. [DOI] [PubMed] [Google Scholar]
- 15.Holmes JF, Sakles JC, Lewis G, Wisner DH. Effects of delaying fluid resuscitation on an injury to the systemic arterial vasculature. Acad Emerg Med. 2002;9:267–274. doi: 10.1111/j.1553-2712.2002.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 16.Girolami A, Little RA, Foex BA, Dark PM. Hemodynamic responses to fluid resuscitation after blunt trauma. Crit Care Med. 2002;30:385–392. doi: 10.1097/00003246-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Rhee P, Koustova E, Alam HB. Searching for the optimal resuscitation method: recommendations for the initial fluid resuscitation of combat casualties. J Trauma. 2003;54:S52–S62. doi: 10.1097/01.TA.0000064507.80390.10. [DOI] [PubMed] [Google Scholar]
- 18.Bohlen HG, Gore RW. Preparation of rat intestinal muscle and mucosa for quantitative microcirculatory studies. Microvasc Res. 1976;11:103–110. doi: 10.1016/0026-2862(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 19.Lucas CE. The water of life: a century of confusion. J Am Coll Surg. 2001;192:86–93. doi: 10.1016/s1072-7515(00)00761-4. [DOI] [PubMed] [Google Scholar]
- 20.Moon PF, Hollyfield-Gilbert MA, Myers TL, Kramer GC. Effects of isotonic crystalloid resuscitation on fluid compartments in hemorrhaged rats. Shock. 1994;2:355–361. doi: 10.1097/00024382-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Moore E, Moore FA, Franciose RJ, Kim FJ, Biffl WI, Banerjee A. Postischemic gut serves as a priming bed for circulation neutrophils that provoke multiple organ failure. J Trauma. 1994;37:881–887. doi: 10.1097/00005373-199412000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Zallen G, Moore EE, Johnson JL, Tamura DY, Ciesla DJ, Silliman CC. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83:83–88. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 23.Zhu XL, Ayala A, Zellweger R, Morrison MH, Chaudry IH. Peritoneal macrophages show increased cytokine gene expression following haemorrhagic shock. Immunology. 1994;83:378–383. [PMC free article] [PubMed] [Google Scholar]
- 24.Zinner MJ, Gurll NJ, Reynolds DG. The effect of hemorrhagic shock and resuscitation on regional blood flow in cynomulgus monkeys. Circ Shock. 1977;4:291–296. [PubMed] [Google Scholar]
- 25.Fruchterman TM, Spain DA, Matheson PJ, et al. Small intestinal production of nitric oxide is decreased following resuscitated hemorrhage. J Surg Res. 1998;80:102–109. doi: 10.1006/jsre.1998.5421. [DOI] [PubMed] [Google Scholar]
- 26.Liaudet L, Szabo A, Soriano FG, Zingarelli B, Szabo C, Salzman AL. Poly (ADP-ribose) synthetase mediates intestinal mucosal barrier dysfunction after mesenteric ischemia. Shock. 2000;14:134–141. doi: 10.1097/00024382-200014020-00010. [DOI] [PubMed] [Google Scholar]
- 27.Osband AJ, Deitch EA, Lu Q, et al. The role of oxidant-mediated pathways in the cytotoxicity of endothelial cells exposed to mesenteric lymph from rats subjected to trauma-hemorrhagic shock. Shock. 2003;20:269–273. doi: 10.1097/01.shk.0000079422.72656.66. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Hauptman JG, Chaudry IH. Hepatocellular dysfunction occurs early after hemorrhage and persists despite fluid resuscitation. J Surg Res. 1990;48:464–470. doi: 10.1016/0022-4804(90)90014-s. [DOI] [PubMed] [Google Scholar]
- 29.Wang P, Ba ZF, Burkhardt J, Chaudry IH. Measurement of hepatic blood flow after severe hemorrhage: lack of restoration despite adequate resuscitation. Am J Physiol. 1992;262:G92–G98. doi: 10.1152/ajpgi.1992.262.1.G92. [DOI] [PubMed] [Google Scholar]
- 30.Ivatury RR, Simon RJ, Islam S, Fueg A, Rohman M, Stahl WM. A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg. 1996;183:145–154. [PubMed] [Google Scholar]
- 31.Scalea TM, Maltz S, Yelon J, Trooskin SZ, Duncan AO, Sclafani SJ. Resuscitation of multiple trauma and head injury: role of crystalloid fluids and inotropes. Crit Care Med. 1994;22:1610–1615. [PubMed] [Google Scholar]
- 32.Zhao H, Spain DA, Matheson PJ, Vaughn C, Harris PD, Garrison RN. Sustained infection induces two distinct microvascular mechanisms in the splanchnic circulation. Surgery. 2000;128:513–519. doi: 10.1067/msy.2000.108114. [DOI] [PubMed] [Google Scholar]
- 33.Miller FN, Nolph KD, Joshua IG, Wiegman DL, Harris PD, Andersen DB. Hyperosmolality, acetate, and lactate: dilatory factors during peritoneal dialysis. Kidney Int. 1981;20:397–402. doi: 10.1038/ki.1981.152. [DOI] [PubMed] [Google Scholar]
- 34.Zakaria ER, Spain DA, Harris PD, Garrison RN. Generalized dilation of the visceral microvasculature by peritoneal dialysis solutions. Perit Dial Int. 2002;22:593–601. [PubMed] [Google Scholar]
- 35.Matheson PJ, Spain DA, Harris PD, Garrison RN, Wilson MA. Glucose and glutamine gavage increase portal vein nitric oxide metabolite levels via adenosine A2b activation. J Surg Res. 1999;84:57–63. doi: 10.1006/jsre.1999.5604. [DOI] [PubMed] [Google Scholar]
- 36.Zakaria ER, Lofthouse J, Flessner MF. In vivo hydraulic conductivity of muscle: effects of hydrostatic pressure. Am J Physiol. 1997;273:2774–2782. doi: 10.1152/ajpheart.1997.273.6.H2774. [DOI] [PubMed] [Google Scholar]
- 37.Zakaria ER, Lofthouse J, Flessner MF. In vivo effects of hydrostatic pressure on interstitium of abdominal wall muscle. Am J Physiol. 1999;276:517–529. doi: 10.1152/ajpheart.1999.276.2.H517. [DOI] [PubMed] [Google Scholar]
- 38.Zakaria ER, Lofthouse J, Flessner MF. Hydrostatic and osmotic pressures modulate partitioning of tissue water in abdominal muscle during dialysis. Perit Dial Int. 1999;19:208–211. [PubMed] [Google Scholar]
- 39.Garrison RN, Conn AC, Harris PD, Zakaria ER. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2004;136:900–908. doi: 10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 40.Wieczorowska-Tobis K, Styszynski A, Polubinska A, Radkowski M, Breborowicz A, Oreopoulos DG. Hypertonicity of dialysis fluid suppresses intraperitoneal inflammation. Adv Perit Dial. 2000;16:262–266. [PubMed] [Google Scholar]
- 41.Styszynski A, Podkowka R, Wieczorowska-Tobis K, et al. Glucose suppresses peritoneal inflammatory reactions and mesothelial hyperplasia caused by intraperitoneal saline infusion. Adv Perit Dial. 2002;18:21–25. [PubMed] [Google Scholar]
- 42.White R, Ram S. Peritoneal dialysis solution attenuates microvascular leukocyte adhesion induced by nitric oxide synthesis inhibition. Adv Perit Dial. 1996;12:53–56. [PubMed] [Google Scholar]
- 43.Toung T, Reilly PM, Fuh KC, Ferris R, Bulkley GB. Mesenteric vasoconstriction in response to hemorrhagic shock. Shock. 2000;13:267–273. doi: 10.1097/00024382-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15:329–343. doi: 10.1097/00024382-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 45.Mackway-Jones K, Foex BA, Kirkman E, Little RA. Modification of the cardiovascular response to hemorrhage by somatic afferent nerve stimulation with special reference to gut and skeletal muscle blood flow. J Trauma. 1999;47:481–485. doi: 10.1097/00005373-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Zweifach BW. Mechanisms of blood flow and fluid exchange in microvessels: hemorrhagic hypotension model. Anesthesiology. 1974;41:157–168. doi: 10.1097/00000542-197408000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Mazzoni MC, Borgstrom P, Intaglietta M, Arfors KE. Lumenal narrowing and endothelial cell swelling in skeletal muscle capillaries during hemorrhagic shock. Circ Shock. 1989;29:27–39. [PubMed] [Google Scholar]
- 48.Behmanesh S, Kempski O. Mechanisms of endothelial cell swelling from lactacidosis studied in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H1512–H1517. doi: 10.1152/ajpheart.2000.279.4.H1512. [DOI] [PubMed] [Google Scholar]
- 49.Mazzoni MC, Intaglietta M, Cragoe EJ, Jr, Arfors KE. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol. 1992;73:1467–1473. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- 50.Day B, Friedman SM. Intracellular sodium and potassium changes in vascular smooth muscle during hemorrhagic shock. Surg Gynecol Obstet. 1978;147:25. [PubMed] [Google Scholar]
- 51.Illner H, Shires GT. The effect of hemorrhagic shock on potassium transport in skeletal muscle. Surg Gynecol Obstet. 1980;150:17–25. [PubMed] [Google Scholar]
- 52.Wang P, Ba ZF, Morrison MH, Ayala A, Dean RE, Chaudry IH. Mechanism of the beneficial effects of ATP-MgCl2 following trauma- hemorrhage and resuscitation: downregulation of inflammatory cytokine (TNF, IL-6) release. J Surg Res. 1992;52:364–371. doi: 10.1016/0022-4804(92)90117-i. [DOI] [PubMed] [Google Scholar]
- 53.Wang P, Ba ZF, Stepp KJ, Chaudry IH. Pentoxifylline attenuates the depressed endothelial cell function and vascular muscle contractility following trauma and hemorrhagic shock. J Trauma. 1995;39:121–126. doi: 10.1097/00005373-199507000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Waxman K, Eloi L, Dinh L, Scannell G, Tominaga GT. Pentoxifylline alone versus pentoxifylline combined with superoxide dismutase prolongs survival in a rat hemorrhagic shock model. Resuscitation. 1993;26:237–242. doi: 10.1016/0300-9572(93)90144-f. [DOI] [PubMed] [Google Scholar]
- 55.Wu X, Kentner R, Stezoski J, et al. Intraperitoneal, but not enteric, adenosine administration improves survival after volume-controlled hemorrhagic shock in rats. Crit Care Med. 2001;29:1767–1773. doi: 10.1097/00003246-200109000-00019. [DOI] [PubMed] [Google Scholar]


