Abstract
In the moth Utetheisa ornatrix (Lepidoptera: Arctiidae), females mate preferentially with larger males. Large body mass is advantageous to both sexes: large males sire more young than small males, and large females have higher fecundity than small females. Here we report that body mass is heritable in both sexes, indicating that by choosing larger males females obtain genetic benefits for their offspring. Choosy females also receive extra nutrient and defensive alkaloid by way of their partner’s spermatophores, but these gifts do not affect the heritability of body mass. These results indicate that by exercising mate choice female Utetheisa receive both direct phenotypic and indirect genetic benefits.
Keywords: Fisherian selection, good genes, nuptial gift, pyrrolizidine alkaloid
In the arctiid moth Utetheisa ornatrix (henceforth called Utetheisa), the strategies of defense and reproduction are inexorably entwined (1). Both sexes, as larvae, sequester pyrrolizidine alkaloids (henceforth called alkaloids) from their foodplants, legumes of the genus Crotalaria (family Fabaceae). They retain the chemicals through metamorphosis, and the adult female bestows them upon the eggs (2). All developmental stages are protected as a result: the larvae and adults against spiders (3, 4); the eggs against coccinellid beetles (2) and ants (5).
At mating, the male transmits alkaloid to the female with the sperm package (spermatophore). The gift is substantial and provides the female with the option of supplementing her own endogenous supply of the chemicals. Indeed, it has been shown that in endowing the eggs, the female resorts both to her own alkaloid and to that received from the male (2). The male’s gift may also enhance the female’s own defense, which may be of particular importance when her alkaloid content is low (6). The spermatophore, which, on average, amounts to more than 10% of male body mass (7), also provides the female with added nutrient. She invests this nutrient in egg production. With each mating, she is able to increase her egg output by 15% (8). On average, females mate with four to five males over their lifespan (9), which we judge from laboratory longevities to be in the order of 3–4 weeks.
Three parameters correlate positively in virgin Utetheisa males: body mass, spermatophore mass, and body alkaloid content (7, 10, 11). Moreover, body alkaloid content, not surprisingly, correlates with the magnitude of the alkaloidal gift that the male is able to bestow upon the female (11). Larger males, in other words, contain more alkaloid and are able to donate spermatophores that are larger and richer in alkaloid (as well as, presumably, nutrient). By mating with larger males, therefore, female Utetheisa would have a means of accruing direct phenotypic benefits in substantial measure.
Earlier work showed that female Utetheisa do mate selectively with larger males. They do not gauge male body mass directly, but do so indirectly, by assessing the titer of a pheromone, hydroxydanaidal, that the male derives chemically from the alkaloid and produces in proportion to his alkaloid content (11, 12). The male airs this pheromone during close-range precopulatory interaction with the female by everting two brush-like devices (coremata) that are impregnated with the compound (12). Males devoid of alkaloid are unable to produce hydroxydanaidal and fare relatively poorly in courtship (12).
The critical question was whether, by choosing larger males, the female might benefit genetically in addition to phenotypically. Could body mass be heritable in Utetheisa? If so, females selecting for large males could receive genes that code for larger body mass. By receiving such genes, females could obtain genetic benefits for their progeny, because larger females are more fecund (8) and larger males sire more offspring (13).
Here we demonstrate that body mass is heritable in Utetheisa: larger parents of this moth beget larger offspring. Moreover, we show that neither spermatophore mass nor alkaloid possession affects the heritability of body mass: causing large males to produce small spermatophores had no effect on heritability, nor did dietary access to alkaloid.†
MATERIALS AND METHODS
Utetheisa.
All experimental Utetheisa were from laboratory colonies, which were established from wild stock collected in Highlands County, FL, and in Moore County, NC.
Larval Diets.
These were of two types (12): one based on pinto beans and lacking alkaloid [(−) diet], the other [(+) diet] also based on pinto beans, but containing a supplement of seeds of Crotalaria spectabilis, a major foodplant of Utetheisa. Utetheisa reared on (+) diet [herein called (+) Utetheisa] contain the principal alkaloid in C. spectabilis, monocrotaline, at a level (0.6 mg per adult) (15) commensurate with that of alkaloid in Utetheisa reared on C. spectabilis plants (0.7 mg per adult) (10). Utetheisa reared on (−) diet [herein called (−) Utetheisa] contain no detectable amount of alkaloid (12).
Adult Body Mass.
This parameter is subject to unpredictable variation, because adult Utetheisa differ in the time course over which they expel their meconial wastes after emergence. We know that pupal mass on day 7 after pupation (pupal duration is 9–11 days in Utetheisa) is a reliable correlate of adult body mass (r = 0.932, P < 0.001, n = 186, unpublished data, based on comparison of 7-day-old pupae and 3-day-old virgin adults), and we use this measure herein to express adult body mass. Adults that we claim to be size-matched differed by less than 5 mg in pupal mass.
Experimental Matings.
Males and females of known body mass were paired individually for 24 h in small, humidified, cylindrical containers (0.35 liter). Pairs were monitored at 6-h intervals to check on mating success [copulation lasts 10–12 h in Utetheisa (7)]. After mating, an ascertainment of weight loss of the male and weight gain of the female provided a basis for determining that spermatophore transfer had occurred.
Larval Rearing.
A standard procedure was adopted for rearing the progeny from experimental matings. Each mated female first was isolated in another cylindrical container, lined with wax paper, wherein she oviposited. Seven days later, after the eggs had hatched, four subsets of 8–10 larvae each were confined individually in small, cylindrical containers (0.1 liter) for separate parallel raising (this provided a measure of control for random environmental factors exerting a determinant effect on the larvae). Food supply, in the four separate chambers, was renovated every 4 days until pupation, after which, at pupal age of 7 days, the pupae were weighed for determination of adult mass.
Heritability Analysis.
For each set of progeny, we first calculated the mean body mass for each of the subsets of sons and daughters and then, on the basis of these means, calculated the overall body mass means for sons and daughters of the entire sample. The two overall means then were regressed against the fathers’ and mothers’ body mass means.
The method of heritability analysis used was a parent–offspring regression, where heritability (h2) is equal to the slope of offspring mean on the midparent mean (mean mass of the parents) or twice the slope of offspring mean on a single-parent mean (16). It was not necessary to adjust regression coefficients and SEs because, as became apparent from the data in experiment 1, the variance in size was the same for both sexes (two-tailed variance ratio test, P = 0.98).
Experiment 1: Heritability of Body Mass.
Sixty matings were effected between 3-day-old, randomly selected, virgin, (+) adults. Heritability and correlation coefficients were determined for all parent–offspring combinations, and heritabilities (regression slopes) were compared by using analyses of covariance (ANCOVAs) (16).
Experiment 2: Effect of Spermatophore Mass on Heritability of Body Mass.
Ordinarily, after mating, a male Utetheisa requires a span of 6–7 days before it is able again to produce a full-size spermatophore. If induced to mate a second time after a shorter span, it produces a subsized spermatophore (7). We were able to induce 51 of the 60 males used in experiment 1 to mate a second time, 2 days after the first mating. Each male was paired with a female that was also (+) and was a sister and size match of the first mate.
Heritabilities between first and second matings were compared with ANCOVAs, and paired t tests were performed to compare the body mass of offspring from the first and second matings.
Experiment 3: Effect of Alkaloid Possession on Heritability of Body Mass.
The intent of this experiment was to determine whether dietary alkaloid intake affects the heritability of body mass. The basic protocol consisted of mating two closely related males of different dietary background [brothers, size-matched, one (+) and the other (−)] to their counterparts among a pair of females [sisters, size-matched, one (+) and the other (−)]. Offspring were reared on the diet of the parents. Thirty-four such sets of double matings were performed. Heritabilities were expressed as regressions of offspring mass on midparent mass.
RESULTS
Experiment 1: Heritability of Body Mass.
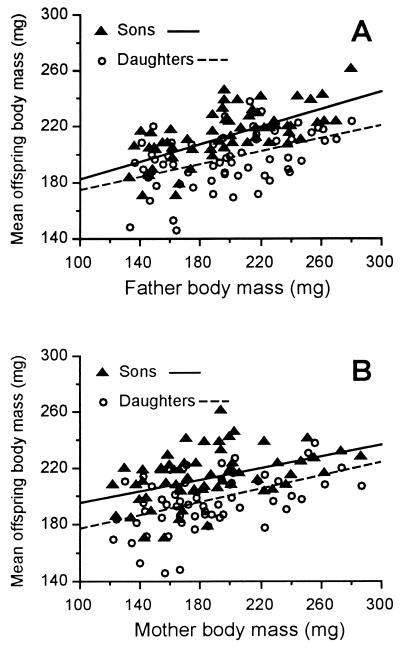
Parental body mass was significantly positively correlated with offspring body mass for all parent–offspring combinations, indicating that larger parents produced larger offspring (Table 1, r values). Heritability estimates were significantly greater than zero for all parent–offspring combinations (Table 1, h2 values).
Table 1.
Heritability estimates (h2 ± SEM) and correlation coefficients (r) for parent–offspring body mass data from experiment 1 (n = 60)
| Fathers | r | Mothers | r | Midparent | r | |
|---|---|---|---|---|---|---|
| Sons | 0.618 ± 0.094 | 0.653 | 0.404 ± 0.112 | 0.426 | 0.497 ± 0.057 | 0.753 |
| Daughters | 0.460 ± 0.116 | 0.458 | 0.474 ± 0.116 | 0.471 | 0.454 ± 0.070 | 0.648 |
| All offspring | 0.538 ± 0.092 | 0.610 | 0.440 ± 0.102 | 0.496 | 0.476 ± 0.052 | 0.771 |
All heritabilities and correlations are significantly different from zero (P < 0.001 for h2; P < 0.01 for r).
There were no differences between father–son and father–daughter heritabilities (ANCOVA, P = 0.30; Fig. 1A) or between mother–son and mother–daughter heritabilities (ANCOVA, P = 0.67; Fig. 1B). Moreover, the father–son regression slope was not significantly different from the mother–son regression slope (ANCOVA, P = 0.15), and the father–daughter regression slope was not significantly different from the mother–daughter slope (ANCOVA, P = 0.93).
Figure 1.
Experiment 1. (A) Mean body mass of sons and daughters plotted as a function of body mass of their father (n = 60; see Table 1). (B) Mean body mass of sons and daughters plotted as a function of body mass of their mother (n = 60; see Table 1).
Experiment 2: Effect of Spermatophore Mass on Heritability of Body Mass.
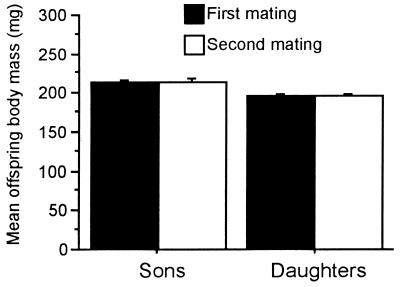
None of the parent–offspring heritabilities pertinent to the second mating of the 51 males differed significantly from their counterparts pertinent to the first mating of these males (ANCOVA: father–son, P = 0.54; father–daughter, P = 0.45; mother–son, P = 0.70; mother–daughter, P = 0.76), indicating that spermatophore size was of no consequence. Moreover, the offspring did not differ significantly in body mass between the two matings (Fig. 2).
Figure 2.
Experiment 2. Mean body mass of sons and daughters from first and second mating of a set of males (n = 51). Offspring mass did not differ significantly between matings (paired t test: sons, P = 0.72; daughters, P = 0.75). (Bars = SEM.)
Experiment 3: Effect of Alkaloid Possession on Heritability of Body Mass.
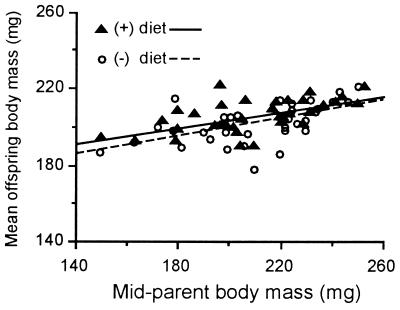
Midparent body mass and offspring body mass were positively correlated for both diets (r(+ diet) = 0.581, r(− diet) = 0.569; P < 0.01 for each), and the heritability of body mass (h2 ± SEM) was significantly greater than zero for both diets (h2(+ diet) = 0.201 ± 0.100, h2 (− diet) = 0.235 ± 0.120; P < 0.001 for each; Fig. 3). The heritabilities did not differ significantly between individuals reared on the two diets (ANCOVA, P = 0.66).
Figure 3.
Experiment 3. Mean body mass of offspring plotted as a function of midparent body mass for individuals reared on (+) and (−) diets (n = 34 per diet).
DISCUSSION
It seems established that body mass in Utetheisa, the trait upon which the female bases her choice in courtship, is heritable. The trait is evidently under genetic control, and its heritability appears to be unaffected by such phenotypic variables as spermatophore size or dietary access to alkaloid.
As regards receipt of benefits from sexual selection, Utetheisa appears to be a moth that “has it all.” By accessing large males, the female is guaranteed to receive sizable outright gifts (direct phenotypic benefits) in the form of alkaloid and nutrient (1, 8). But, as implied by our findings, she is apt to receive indirect genetic benefits as well. By pairing with large males, she is able to impart on her sons the very quality, largeness, that led to the courtship success of their father (10, 12, 13) and on her daughters the largeness that correlates with increased fecundity in the female (8). Within the strictures of current behavioral terminology (17–20), Utetheisa thus can be said to reap both “Fisherian” and “good genes” benefits from sexual selection.
Also of interest, and not surprising given that body mass is heritable and a sexually selected trait in Utetheisa, is that male size is an exaggerated feature in this species. Contrary to what is generally true for Lepidoptera (21), the male in Utetheisa is larger than the female (ref. 13; Figs. 1 and 2).
We predict that as regards sexual strategy, Utetheisa will turn out not to be unique. Other insects are bound to be found in which both direct (phenotypic) and indirect (genetic) benefits from sexual selection are similarly combined.
Acknowledgments
We are indebted to H. K. Reeve for his insight, advice, and critical comments on the manuscript. M. C. Andrade, P. Buston, H. Farris, P. W. Sherman, and P. Starks provided helpful comments on earlier versions of the paper. We also thank K. Amerman for technical assistance and K. Keyser for providing field-collected Utetheisa. Research support was provided by a Grant-in-Aid of Research from Sigma Xi, The Scientific Research Society (to V.K.I.), and National Institutes of Health Grant AI02908 (to T.E.).
ABBREVIATION
- ANCOVA
analysis of covariance
Footnotes
This paper is no. 161 in the series “Defense Mechanisms of Arthropods.” Paper no. 160 is ref. 14.
References
- 1.Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1995;92:50–55. doi: 10.1073/pnas.92.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dussourd D E, Ubik K, Harvis C, Resch J, Meinwald J, Eisner T. Proc Natl Acad Sci USA. 1988;85:5992–5996. doi: 10.1073/pnas.85.16.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisner T, Meinwald J. In: Pheromone Biochemistry. Prestwich G D, Blumquist G J, editors. Orlando, FL: Academic; 1987. pp. 251–269. [Google Scholar]
- 4.Eisner T, Eisner M. Psyche. 1991;98:111–118. [Google Scholar]
- 5.Hare J F, Eisner T. Oecologia. 1993;96:9–18. doi: 10.1007/BF00318024. [DOI] [PubMed] [Google Scholar]
- 6.González A, Rossini C, Eisner M, Eisner T. Proc Natl Acad Sci USA. 1999;96:5570–5574. doi: 10.1073/pnas.96.10.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaMunyon C W, Eisner T. Proc Natl Acad Sci USA. 1994;91:7081–7084. doi: 10.1073/pnas.91.15.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaMunyon C W. Ecol Entomol. 1997;22:69–73. [Google Scholar]
- 9.Pease R W., Jr J Lepid Soc. 1968;22:197–209. [Google Scholar]
- 10.Conner W E, Roach B, Benedict E, Meinwald J, Eisner T. J Chem Ecol. 1990;16:543–552. doi: 10.1007/BF01021785. [DOI] [PubMed] [Google Scholar]
- 11.Dussourd D E, Harvis C, Resch J, Meinwald J, Eisner T. Proc Natl Acad Sci USA. 1991;88:9224–9227. doi: 10.1073/pnas.88.20.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conner W E, Eisner T, Vander Meer R K, Guerrero A, Meinwald J. Behav Ecol Sociobiol. 1981;9:227–235. [Google Scholar]
- 13.LaMunyon C W, Eisner T. Proc Natl Acad Sci USA. 1993;90:4689–4692. doi: 10.1073/pnas.90.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González, A., Schroeder, F. C., Attygalle, A. B., Svatos, A., Meinwald, J. & Eisner, T. (1999) Chemoecology, in press.
- 15.Bogner F, Eisner T. Experientia. 1992;48:97–102. doi: 10.1007/BF01923618. [DOI] [PubMed] [Google Scholar]
- 16.Falconer D S. Introduction to Quantitative Genetics. 4th Ed. New York: Longman; 1996. [Google Scholar]
- 17.Andersson M. Sexual Selection. Princeton, NJ: Princeton Univ. Press; 1994. [Google Scholar]
- 18.Kirkpatrick M, Ryan M J. Nature (London) 1991;350:33–38. [Google Scholar]
- 19.Promislow D E, Smith E A, Pearse L. Proc Natl Acad Sci USA. 1998;95:10687–10692. doi: 10.1073/pnas.95.18.10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welch A M, Semlitsch R D, Gerhardt H C. Science. 1998;280:1928–1930. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- 21.Opler P A, Krizek G O. Butterflies East of the Great Plains. Baltimore: John Hopkins Univ. Press; 1984. pp. 12–13. [Google Scholar]