Abstract
We examined the biogeographic patterns implied by early hominid phylogenies and compared them to the known dispersal patterns of Plio-Pleistocene African mammals. All recent published phylogenies require between four and seven hominid dispersal events between southern Africa, eastern Africa, and the Malawi Rift, a greater number of dispersals than has previously been supposed. Most hominid species dispersed at the same time and in the same direction as other African mammals. However, depending on the ages of critical hominid specimens, many phylogenies identify at least one hominid species that dispersed in the direction opposite that of contemporaneous mammals. This suggests that those hominids may have possessed adaptations that allowed them to depart from continental patterns of mammalian dispersal.
Keywords: phylogeny, human evolution
A critical biogeographic question in early human evolution concerns the relationships between the eastern and southern African hominids. Given that the mammalian fossil record preserves evidence of faunal interchange between these two regions (1, 2), it is very unlikely that hominids in one region evolved entirely independently from those in the other. Yet, despite the strong probability that early hominids dispersed between eastern and southern Africa, few studies (1–4) have examined the paleobiogeography of early hominid species. These studies agree that either two, or three, dispersal events must have taken place and that, in general, the early hominids followed the continental dispersal patterns of other large-bodied mammals (Fig. 1a). Some of these studies are predicated by the hypothesis that hominid speciation and dispersal events are driven by environmental change, and in particular by the hypothesis that mammals shift their ranges to match changes in the distributions of vegetational zones (2, 5, 6).
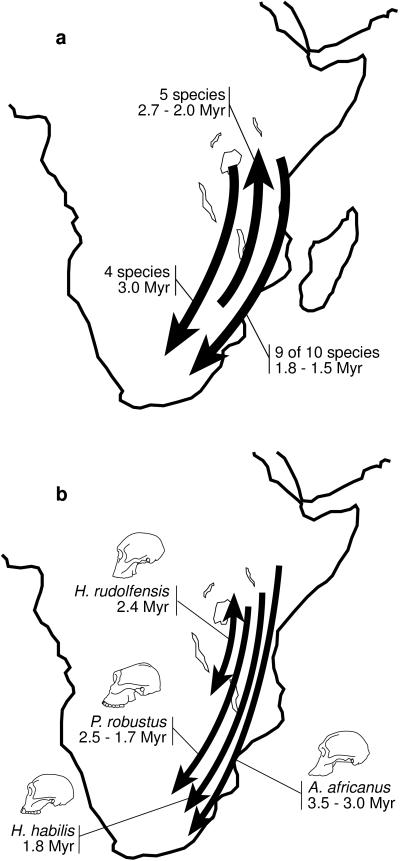
Figure 1.
Plio-Pleistocene dispersals of African mammals. (a) Three waves of mammalian dispersal (1), including a southward dispersal at 3.0 Myr of Canis, Diceros, Australopithecus, and Metridiochoerus, a northward dispersal between 2.7 and 2.0 Myr of Cercopithecoides, Connochaetes, Parmularius, Tragelaphus, and Antidorcas, and a southward dispersal between 1.8 and 1.5 Myr of Theropithecus, Nyctereutes, Equus, Metridiochoerus, Homo, Kobus, Hippotragus, and two species of Tragelaphus. Hipparion disperses northward at 1.7 Myr when other mammals are dispersing southward. Each wave of dispersals is either significantly or nearly significantly different from random. Other studies have placed H. habilis in the northward event (2) and P. robustus in the late southward event (3), and H. rudolfensis may have dispersed with other eastern African mammals into the Corridor at ≈2.4 Myr (2). (b) Early hominid dispersals implied by Fig. 2 b, c, and e (29, 30), under the assumption that H. habilis originated in eastern Africa (see Table 1). The direction of the H. rudolfensis dispersal is unclear. Different patterns are implied if H. habilis originated in southern Africa. Fig. 2b also includes a northward migration at ≈2.7 Myr of the common ancestor of Homo and Paranthropus (not shown).
Prior studies have addressed the impact of ecology on hominid biogeography, but the biogeographic implications of phylogeny have yet to be fully explored. Obtaining a reliable phylogeny is a crucial, initial step in the formulation of any biogeographic hypothesis because, if a species in one region is descended from an ancestor in another region, then a vicariance (splitting of ranges) or dispersal event must have taken place (7, 8). The present study examines the biogeographic implications of various early hominid phylogenies (Fig. 2) and compares the inferred dispersal patterns of the early hominids to those of other large mammals.
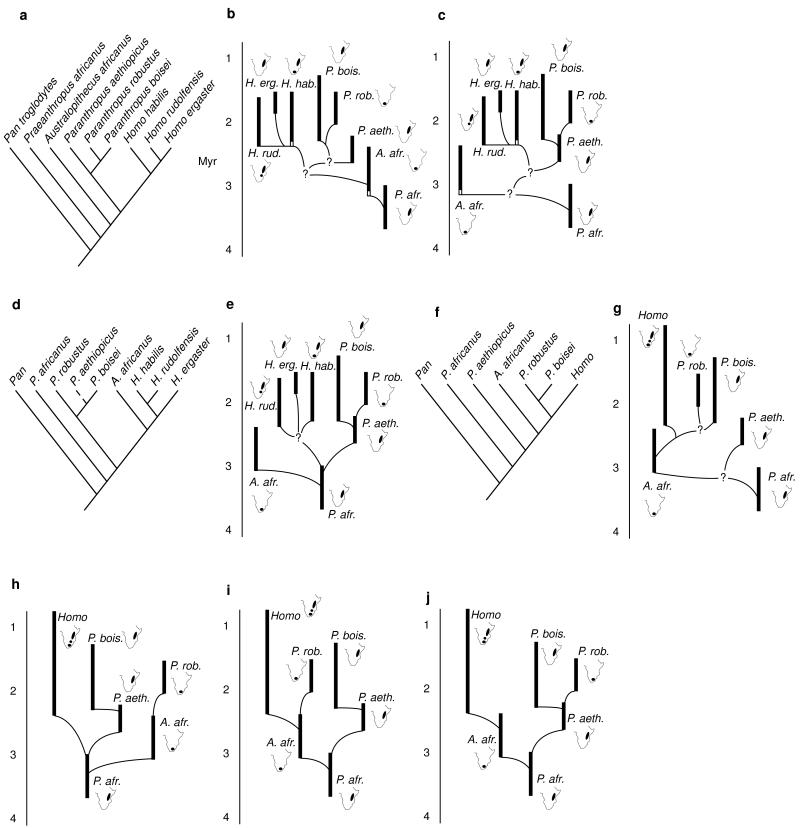
Figure 2.
Early hominid phylogenies. (a–c) Cladogram and phyletic trees of Strait et al. (30). (d and e) Cladogram and phyletic tree of Wood (29). (f and g) Cladogram and phyletic tree of Skelton and McHenry (24). (h and i) Phyletic trees consistent with Walker et al. (14). (j) Phyletic tree of Delson (25) and Grine (26). For each taxon in the phyletic trees, a map of Africa is shown indicating the regions in which its fossils are found. Dashed line in d represents an implied relationship. Black vertical bars indicate the known time ranges of hominid species. White vertical bars in b and c indicate inferred time ranges. Time scale in millions of years (Myr) is given to the left of each phyletic tree. All phylogenies are depicted by using the alpha taxonomy of Strait et al. (30). Note that the Pliocene hominids traditionally attributed to Australopithecus afarensis are here referred to Praeanthropus africanus because the inclusion of this hypodigm within Australopithecus has the effect of making that genus paraphyletic [see Strait et al. (30) for a more complete review].
Note that dispersal is used here in a generic sense to refer to the movement of taxa across the landscape and thus comprises range expansion, range shift, and dispersal across a faunal barrier (7). The present study does not test whether hominid species arose as a result of vicariance (8). Although vicariance may have played a role in early hominid evolution, only one early hominid species (Homo habilis sensu stricto, hereafter referred to as H. habilis) is known from both eastern and southern Africa. Thus, because vicariance implies that an ancestral species will have a larger range than its descendants, it is likely that most hominid vicariance events were preceded by range expansion (a type of dispersal).
Biogeographic patterns were obtained by plotting information about the geographic distribution of species onto early hominid cladograms and phyletic trees. This procedure assumes that the chronological and geographic ranges of hominid species are closely approximated by the times (6, 9–19) and places at which their fossils are found. The validity of this assumption undoubtedly varies on a locality-by-locality basis, and the discovery of hominid fossils in Chad (20) has reminded researchers that the absence of evidence is not evidence of absence, but it is necessary to make the assumption to generate working biogeographic hypotheses. Obviously, the possibility that the fossil record has been affected by taphonomic bias, such that fossil preservation and the stratigraphic record are better in eastern than in southern Africa, means that biogeographic conclusions must be drawn cautiously.
Nonetheless, the hominid fossil record is not so poor as to make biogeographical inferences untenable. Obviously, new fossil discoveries have the potential to overturn certain of the results of the present study (a truism of paleontological research). However, unless a new hominid species is discovered that largely invalidates the basic patterns that are common to current phylogenetic hypotheses, new hominid fossils will not substantially alter the biogeographic patterns reported here unless hominid species endemic to eastern Africa are found in southern Africa (and vice versa), or if any of six hominid species (Paranthropus robustus, Paranthropus boisei, Paranthropus aethiopicus, H. habilis, Homo rudolfensis, Homo ergaster) are found to be older than 2.8 million years (Myr). These conditions provide reasonable “confidence limits” for the present study because the geographic distributions of Plio-Pleistocene hominids have been established over the course of decades of research and because, of the six species listed above, only one (P. aethiopicus) is currently known to be older than 2.4 Myr (6, 10, 12–19). Regardless, even if new fossil discoveries necessitate a dramatic revision of early hominid phylogeny, the methods described here will allow researchers to determine the biogeographic patterns implied by any new tree.
MATERIALS AND METHODS
Patterns implied by cladograms and phyletic trees (Fig. 2) were documented by treating geography as an ordered cladistic character in which regions correspond to character states. Two main regions were recognized: eastern and southern Africa. A third region, “the Corridor,” represented by the Malawi section of the Rift Valley, also was defined because at least one hominid specimen has been reported from there (16) and because the Corridor is likely to have served as a faunal conduit between the other two regions (2). Although mammal dispersals could have bypassed the Corridor by passing instead through south-central Africa, the fossil record is not adequate to test this possibility. Biogeographic patterns were reconstructed by using parsimony to identify the regions occupied by the hypothetical ancestors located at the internal nodes of the cladograms and phyletic trees. In other words, each character state change in the cladograms and trees corresponded to a dispersal event. The geographic distribution of hominid speciation events also was recorded to determine whether those speciations tended to be focused in any particular region.
Note that the formal methods of cladistic biogeography, in which biogeographic patterns are tested by examining area cladograms (8), cannot be applied here because data are available from too few regions. Cladistic biogeography requires faunal information from at least three areas, and although three regions are defined here, only eastern and southern Africa have been well sampled. Faunal representation from the Corridor is limited (21).
Taxa examined here comprise the Plio-Pleistocene hominid species known from between 3.5 and 1.5 Myr that have been included in phylogenetic reconstructions (Fig. 2). Ardipithecus ramidus (22) and Australopithecus anamensis (23) are likely to be near the ancestry of later hominids and were used as an outgroup to determine the polarity of the “geography” cladistic character. In other words, it was assumed that eastern Africa was the region occupied by the last common ancestor of the hominids considered here. Note that this does not preclude the possibility that the last common ancestor of chimpanzees and humans arose outside of eastern Africa. Certainly, the hominid fossils from Chad (20) indicate that there must have been at least one hominid dispersal between eastern and central Africa.
Some of the phylogenetic analyses (14, 24–26) did not investigate phylogenetic relationships within the genus Homo. For those trees, all possible relationships within the genus were examined, with the exception that H. ergaster was never assumed to be directly ancestral to H. rudolfensis or H. habilis. Moreover, in the case of biogeographic patterns that are equally parsimonious, it was assumed for the sake of simplicity that species originated in either eastern or southern Africa. This is consistent with previous investigations of early hominid biogeography, and, although there are no strong grounds on which one can justify the assumption, its utilitarian value is that it greatly reduces the number of possible biogeographic patterns implied by some of the phylogenies.
Biogeographic patterns implied by phyletic trees were complicated by the fact that two hominid species are known from more than one region [i.e., H. habilis (eastern and southern Africa) and H. rudolfensis (eastern Africa and the Corridor)]. The regions in which these species first occur are unclear because of ambiguities concerning the age and taxonomic affinities of critical specimens (16, 17, 19, 27, 28). Accordingly, each phyletic tree was examined four times, taking into account all possible combinations of the centers of origin of those species.
RESULTS
Biogeographic patterns implied by early hominid phylogenies are summarized in Table 1. It is evident that published phylogenies do not all agree on a single biogeographic pattern. However, as researchers make decisions concerning fundamental questions about phylogeny, taxonomy, and chronology, then biogeographic patterns become clearer. For example, consider the phylogenetic hypotheses based on our own work (Fig. 2 b, c, and e). Of these, Wood (29) does not consider the earliest putative Homo specimens from southern Africa as belonging to H. habilis. Consequently, only one biogeographic pattern is consistent with a strict reading of his phylogenetic hypothesis (Fig. 1b). In contrast, Strait et al. (30) included the southern African specimens within H. habilis, meaning that the biogeographic implications of those phylogenies must accommodate uncertainty as to the center of origin of that species. If H. habilis did not arrive in southern Africa until 1.8 Myr, then the resulting biogeographic patterns are very similar or identical to those implied by Wood’s (29) phylogeny (Fig. 1b). If, on the other hand, H. habilis originated in southern Africa, then more complicated biogeographic patterns are implied (Table 1).
Table 1.
Dispersal and speciation events implied by early hominid phylogenies
| Tree type | Tree | Dispersal‡ with speciation | Dispersal‡ without speciation | Speciation without dispersal§
|
|
|---|---|---|---|---|---|
| East | South | ||||
| Cladogram | Fig. 2a | 1, 4 | 12 or 14, 13 | A, C, E, F, G, H | |
| Fig. 2d | 1, 4 | 12 or 14, 13 | A, C, E, F, G, H | ||
| Fig. 2f | 1, 4 | 12 or 14, 13 | A, C, E, F, G, H | ||
| Phyletic tree* | Fig. 2b | 1, 3, 4, 6 | 14, 16 or 13 | A, C, E, F, G?, H | |
| Fig. 2c | 1, 3, 4 | 14, 16 or 13 | A, C, E, F, G?, H | ||
| Fig. 2e | 1, 3, 4 | 14, 16, or 13 | A, C, E, F, G?, H | ||
| Fig. 2g | 1, at least two others | 14, 16 or 13 | at least A, C | ||
| Fig. 2h | 1, 3, at least one other | 14, 16 or 13 | at least A, C, E | D | |
| Fig. 2i | 1, at least one other | 14, 16 or 13 | at least A, C, E | D | |
| Fig. 2j | 1, 4, at least one other | 14, 16 or 13 | at least A, C, E | ||
| Phyletic tree† | Fig. 2b | 1, 4 & 7 or 8 & 10, 9 & 11 or 17 | 15, 16 or 13 | A, C?, E?, G?, H? | D?, F |
| Fig. 2c | 1, 2 or 8, 4, 9 & 11 or 17 | 15, 16 or 13 | A, C?, E, G?, H? | F? | |
| Fig. 2e | 1, 2, 3 or 17,4 | 15, 16 or 13 | A, C, E, G?, H? | ||
| Fig. 2g | 1, 10, at least one other | 15, 16 or 13 | at least A, C | at least D | |
| Fig. 2h | at least 1, 2, or 5 | 15, 16 or 13 | at least A, C, E | at least D | |
| Fig. 2i | 1, at least one other | 15, 16 or 13 | at least A, C, E | at least D | |
| Fig. 2j | 1, 4, at least one other | 15, 16 or 13 | A, C, E | at least D | |
Italicized numbers represent southward dispersals. Bold-faced numbers represent northward dispersals. Underlined numbers represent dispersals that occur only if H. rudolfensis appeared first in the Malawi Rift. Letters represent speciation events that are not associated with dispersals. “East” and “South” refer to regions in Africa.
Assumes that H. habilis appears first in eastern Africa.
Assumes that H. habilis appears first in southern Africa.
Dispersals: 1, A. africanus or its ancestor disperses from East to South between 3.5 and 3.0 Myr; 2, H. habilis or its ancestor, East to South, ≈2.5 Myr; 3, H. rudolfensis or its ancestor, East to the Corridor, ≈ 2.4 Myr; 4, P. robustus or its ancestor, East to South between 2.5 and 1.7 Myr; 5, H. habilis or its ancestor, the Corridor to South, ≈2.5 Myr; 6, the common ancestor of Homo and Paranthropus, South to East, ≈2.7 Myr; 7, the common ancestor of Paranthropus, South to East, ≈2.6 Myr; 8, P. aethiopicus or its ancestor, South to East, ≈2.5 Myr; 9, H. rudolfensis or its ancestor, South to the Corridor, ≈2.4 Myr, 10, P. boisei or its ancestor, South to East, ≈2.2 Myr; 11, H. ergaster or its ancestor, the Corridor to East, ≈1.9 Myr; 12, H. habilis East to Souh, ≈2.5 Myr; 13, H. rudolfensis, East to the Corridor, ≈2.4 Myr; 14, H. habilis, East to South, ≈1.8 Myr; 15, H. habilis, South to East, ≈2.3 Myr; 16, H. rudolfensis, the Corridor to East, ≈2.4 Myr; 17, the common ancestor of H. rudolfensis and H. ergaster, South to East, ≈2.5 Myr.
Speciation events without dispersals: A, P. africanus; B, A. africanus; C, P. aethiopicus; D, P. robustus; E, P. boisei; F, H. habilis; G, H. rudolfensis; H, H. ergaster.
Ultimately, the choice of biogeographic pattern depends on the cladogram or phyletic tree that is preferred. However, despite the fact that early hominid phylogenies differ markedly in the details of their branching patterns, certain generalizations about hominid biogeography emerge from this study (Table 1). All of the cladograms and phyletic trees agree that Australopithecus africanus, or its ancestor [a category that might include recently discovered fossils from Sterkfontein’s Member 2 (31)], dispersed from eastern to southern Africa between 3.5 and 3.0 Myr. All of the cladograms imply four episodes of southward dispersal. The phyletic trees all indicate that H. rudolfensis dispersed between eastern Africa and the Malawi Rift around 2.4 Myr, but the direction of the dispersal is unclear. Moreover, H. habilis dispersed either northward at ≈2.3 Myr or southward at ≈1.8 Myr. In addition, all of the cladograms and many of the phyletic trees indicate that P. robustus, or its ancestor, dispersed from eastern to southern Africa between 2.5 and 1.7 Myr [depending on the ages of the fossils at Drimolen and Kromdraai (6, 13, 15, 18)].
DISCUSSION
In contrast to the two, or three, dispersals identified by previous investigations, the trees examined in the present study require between four and seven. This suggests that patterns of early hominid biogeography were more complicated than those indicated by earlier studies. Moreover, these patterns allow one to test whether hominid dispersals conformed to the mammalian patterns identified by Turner and Wood (1). Such a test depends critically on the taxonomy and chronology of particular hominid specimens from southern Africa. Hominids conform entirely to the mammalian patterns (Fig. 1a) if H. habilis originated in eastern Africa [implying that putative early Homo fossils from southern Africa are either younger than Sterkfontein’s Member 4 or do not belong to H. habilis (28)] and if recently discovered P. robustus fossils from Drimolen (18) are comparable in age to Swartkrans Member 1 and Kromdraai Member B, both currently thought to be ≈1.7 Myr (6, 15). Such agreement between hominid and mammalian dispersal patterns supports the hypothesis that hominids and other mammals responded similarly to environmental perturbations.
However, if H. habilis originated in southern Africa (implying that H. habilis is present in Sterkfontein 4), then all cladograms and several phyletic trees indicate that this species, or its ancestor, dispersed from eastern to southern Africa at a time when contemporaneous mammals were moving northward. The same could be said for P. robustus, or its ancestor, if Drimolen is older than Swartkrans 1 or if Kromdraai B dates to ≈2.0 Myr (13). If these species departed from continental patterns of mammalian dispersal, then it is probable that one or both of them possessed behavioral or anatomical adaptations that allowed them to do so. This possibility needs to be tested against the paleontological and archaeological records. A departure from mammalian trends would further suggest that biogeographic models based on ecological hypotheses, such as Vrba’s (5) Habitat Theory, have important and interesting exceptions.
All cladograms and phyletic trees identify eastern Africa as the region responsible for producing most (if not all) hominid species, either through speciation events within the region or because some eastern African populations dispersed to southern Africa and differentiated into regional species. Although taphonomic bias may contribute to this pattern, it nonetheless begs the question of what were the evolutionary mechanisms responsible for the frequent speciation of hominids in eastern Africa. Although several reconstructions of early hominid habitats have attempted to explain hominid speciation patterns (2–4, 32, 33), these studies either have not considered fully the implications of early hominid phylogeny or have made assumptions about biogeography that are inconsistent with the patterns found here.
CONCLUSION
Phylogeny has significant implications for interpretations of early hominid biogeography. In particular, hominid dispersals between eastern and southern Africa appear to have been more frequent that has previously been thought, and some of those dispersals may have opposed prevailing mammalian trends. If the latter is true, then attention should be paid to determining whether such dispersals were facilitated by the evolution of anatomical or behavioral adaptations in those hominid species. Most phylogenies also indicate that eastern Africa hosted a majority of hominid speciation events. An integration of ecological, phylogenetic, and archaeological data, as well as any relevant evidence about local habitats within regions, should lead to a more complete understanding of hominid dispersal and speciation patterns.
Acknowledgments
We thank W. Howells, D. Pilbeam, W. Kimbel, P. Herendeen, K. Behrensmeyer, D. Lieberman, J. Clark, R. Potts, B. Richmond, O. Pearson, and R. McCarthy for their valuable comments. We also thank T. Bromage, R. Foley, E. O’Brien, C. Peters, and M. Rosenzweig for providing access to manuscripts in press. This research was supported by The Henry Luce Foundation.
ABBREVIATION
- Myr
million years
References
- 1.Turner A, Wood B A. J Hum Evol. 1993;24:147–168. [Google Scholar]
- 2.Bromage T G, Schrenk F. J Hum Evol. 1995;28:109–114. [Google Scholar]
- 3.Foley R A. Another Unique Species. New York: Longman; 1987. [Google Scholar]
- 4.Foley, R. A., in African Biogeography, Climate Change, and Human Evolution, eds. Bromage, T. G. & Schrenk, F. (Oxford Univ. Press, New York), in press.
- 5.Vrba E S. J Mammal. 1992;73:1–28. [Google Scholar]
- 6.Vrba E S. In: Paleoclimate and Evolution with Emphasis on Human Origins. Vrba E S, Denton G H, Partridge T C, Burke L H, editors. New Haven, CT: Yale Univ. Press; 1995. pp. 24–45. [Google Scholar]
- 7.Myers A A, Giller P S. In: Analytical Biogeography. Myers A A, Giller P S, editors. London: Chapman & Hall; 1988. pp. 1–12. [Google Scholar]
- 8.Nelson G, Platnick N. Systematics and Biogeography: Cladistics and Vicariance. New York: Columbia Univ. Press; 1981. [Google Scholar]
- 9.Partridge T C. S Afr J Sci. 1982;78:300–301. [Google Scholar]
- 10.Feibel C S, Brown F H, McDougall I. Am J Phys Anthropol. 1989;78:595–622. doi: 10.1002/ajpa.1330780412. [DOI] [PubMed] [Google Scholar]
- 11.Kimbel W H, Johanson D C, Rak Y. Nature (London) 1994;368:449–451. doi: 10.1038/368449a0. [DOI] [PubMed] [Google Scholar]
- 12.W. H. Kimbel (1995) in Paleoclimate and Evolution with Emphasis on Human Origins, eds. Vrba, E. S., Denton, G. H., Partridge, T. C. & Burke, L. H. (Yale Univ. Press, New Haven, CT), pp. 425–437.
- 13.Vrba E S. L’Homo Erectus et Alpalce de l’Homme de Tautavel Parmi les Hominides Fossils. Paris: Centre Nationale de Recherche Scientifique; 1982. pp. 707–752. [Google Scholar]
- 14.Walker A C, Leakey R E F, Harris J M, Brown F H. Nature (London) 1986;322:517–522. [Google Scholar]
- 15.Delson E. In: Evolutionary History of the “Robust” Australopithecines. Grine F E, editor. New York: Aldine de Gruyter; 1988. pp. 317–324. [Google Scholar]
- 16.Schrenk F, Bromage T G, Betzler C G, Ring U, Juwayeyi Y M. Nature (London) 1993;365:833–835. doi: 10.1038/365833a0. [DOI] [PubMed] [Google Scholar]
- 17.Kimbel W H, Johanson D C, Rak Y. Am J Phys Anthropol. 1997;103:235–262. doi: 10.1002/(SICI)1096-8644(199706)103:2<235::AID-AJPA8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Keyser A. In: Abstracts of Contributions to the Dual Congress 1998. Raath M A, Soodyall H, Barkhan D, Kuykendall K L, Tobias P V, editors. Johannesburg: Univ. of the Witwatersrand Press; 1998. [Google Scholar]
- 19.Suwa G, White T D, Howell F C. Am J Phys Anthropol. 1996;101:247–282. doi: 10.1002/(SICI)1096-8644(199610)101:2<247::AID-AJPA9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 20.Brunet M, Beauvilain A, Coppens Y, Heintz E, Moutaye A H E, Pilbeam D R. C R Acad Sci. 1996;322:907–913. [Google Scholar]
- 21.T. G. Bromage, F. Schrenk & Juwayeyi, Y. M. (1995) J. Hum. Evol.28, 37–57. [DOI] [PubMed]
- 22.White T D, Suwa G, Asfaw B. Nature (London) 1994;371:306–312. doi: 10.1038/371306a0. [DOI] [PubMed] [Google Scholar]
- 23.Leakey M G, Feibel C S, McDougall I, Walker A. Nature (London) 1995;376:565–571. doi: 10.1038/376565a0. [DOI] [PubMed] [Google Scholar]
- 24.Skelton R R, McHenry H M. J Hum Evol. 1992;23:309–349. [Google Scholar]
- 25.Delson E. Nature (London) 1986;322:496–497. [Google Scholar]
- 26.Grine F E. In: The Evolutionary History of the “Robust” Australopithecines. Grine F E, editor. New York: Aldine de Gruyter; 1988. pp. 223–246. [Google Scholar]
- 27.Kimbel W H, Rak Y. In: Species, Species Concepts, and Primate Evolution. Kimbel W H, Martin L B, editors. New York: Plenum; 1993. pp. 461–485. [Google Scholar]
- 28.Ahern J C M. Am J Phys Anthropol. 1998;105:461–480. doi: 10.1002/(SICI)1096-8644(199804)105:4<461::AID-AJPA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Wood B A. J Hum Evol. 1992;22:361–365. [Google Scholar]
- 30.Strait D S, Grine F E, Moniz M A. J Hum Evol. 1997;32:17–82. doi: 10.1006/jhev.1996.0097. [DOI] [PubMed] [Google Scholar]
- 31.Clarke R J, Tobias P V. Science. 1995;269:521–524. doi: 10.1126/science.7624772. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien, E. M. & Peters, C. R., in African Biogeography, Climate Change, and Human Evolution, eds. Bromage, T. G. & Schrenk, F. (Oxford Univ. Press, New York), in press.
- 33.Rosenzweig, M. L., in African Biogeography, Climate Change, and Human Evolution, eds. Bromage, T. G. & Schrenk, F. (Oxford Univ. Press, New York), in press.