Abstract
1. Daily i.p. administration, for eight days, of the cholinesterase inhibitor disulfoton to rats produced mild to moderate signs of intoxication (tremors, incontinence and diarrhoea) but no deaths.
2. Segments of ileum taken from the treated rats were subsensitive to carbachol but the vas deferens and the uterus did not exhibit any change in sensitivity to carbachol.
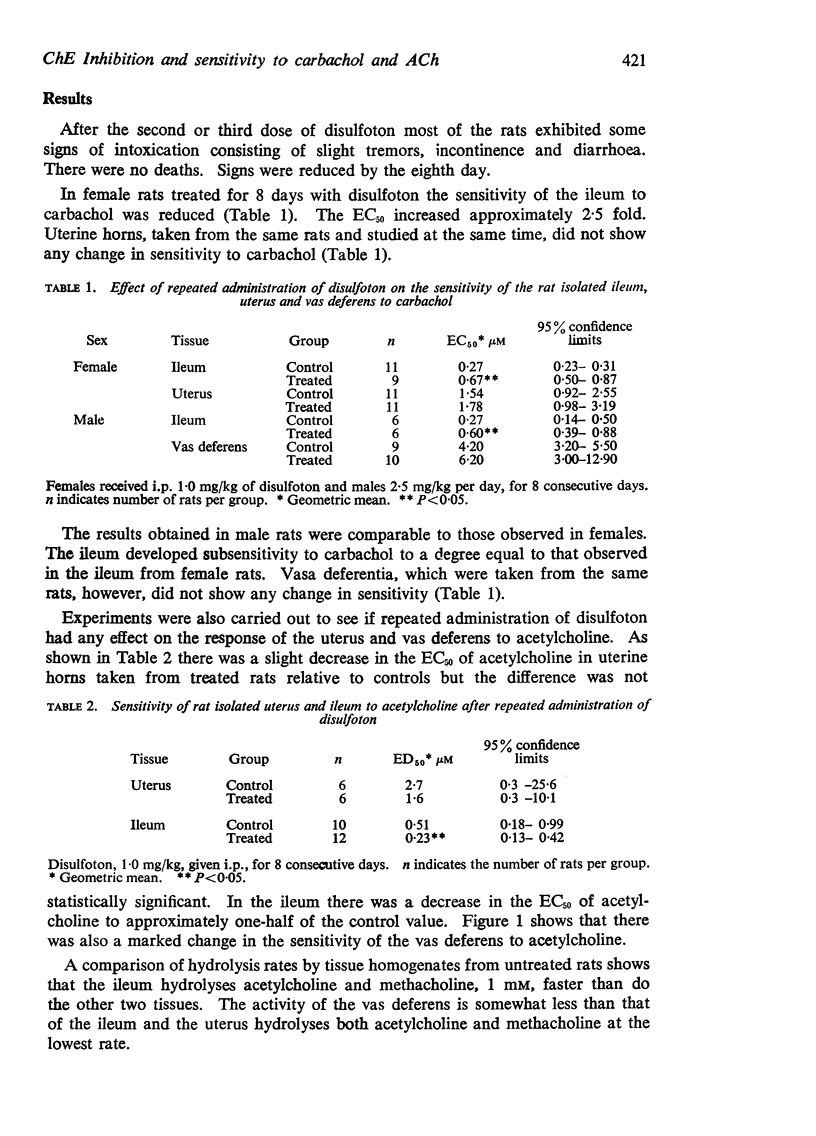
3. The sensitivity to acetylcholine was increased in the ileum and vas deferens but not in the uterus.
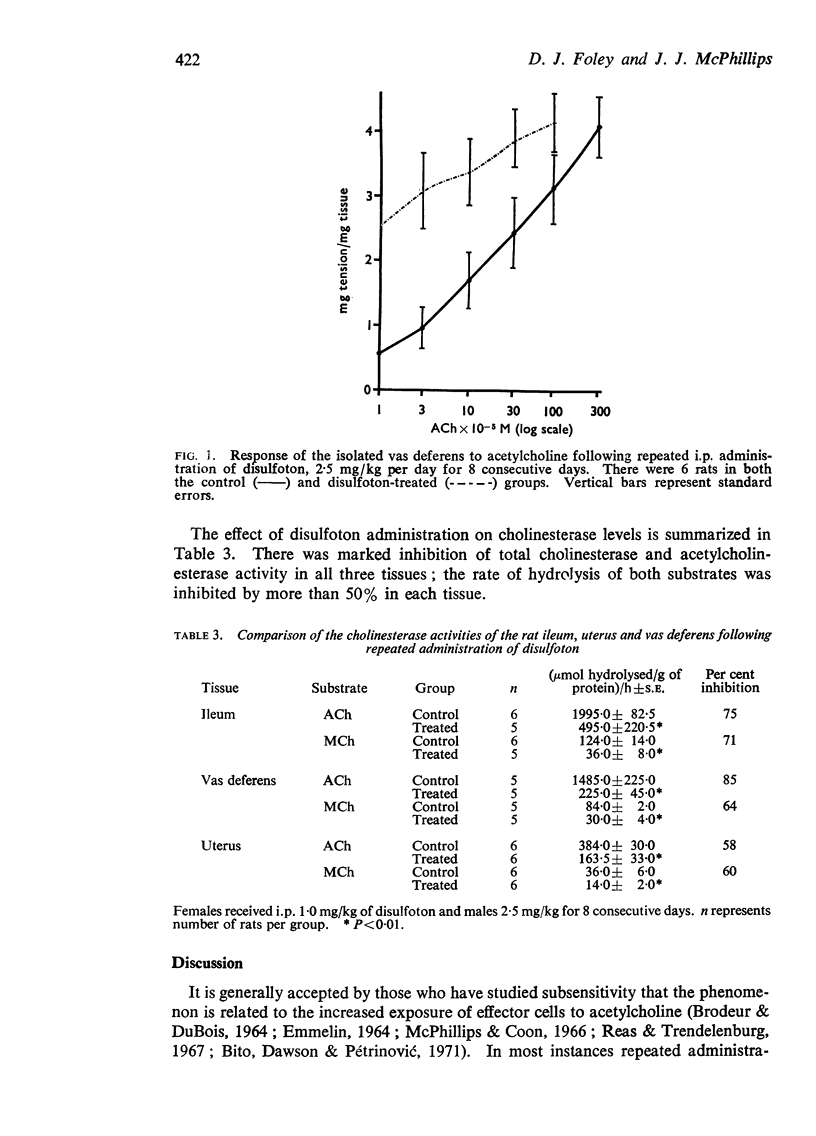
4. Acetylcholinesterase activity was 60-70% inhibited in all three tissues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N. The use and limitations of atropine for pharmacological studies on autonomic effectors. Pharmacol Rev. 1955 Dec;7(4):467–494. [PubMed] [Google Scholar]
- Adham N., Schenk E. A. Autonomic innervation of the rat vagina, cervix, and uterus and its cyclic cariation. Am J Obstet Gynecol. 1969 Jun 15;104(4):508–516. doi: 10.1016/s0002-9378(16)34239-9. [DOI] [PubMed] [Google Scholar]
- BOMBINSKI T. J., DUBOIS K. P. Toxicity and mechanism of action of di-syston. AMA Arch Ind Health. 1958 Mar;17(3):192–199. [PubMed] [Google Scholar]
- BRODEUR J., DUBOIS K. P. STUDIES ON THE MECHANISM OF ACQUIRED TOLERANCE BY RATS OF 0,0-DIETHYL S-2-(ETHYLTHIO)ETHYL PHOSPHORODITHIOATE (DI-SYSTON). Arch Int Pharmacodyn Ther. 1964 Jun 1;149:560–570. [PubMed] [Google Scholar]
- Bito L. Z., Dawson M. J., Petrinovic L. Cholinergic sensitivity: normal variability as a function of stimulus background. Science. 1971 May 7;172(3983):583–585. doi: 10.1126/science.172.3983.583. [DOI] [PubMed] [Google Scholar]
- Bito L. Z., Hyslop K., Hyndman J. Antiparasympathomimetic effects of cholinesterase inhibitor treatment. J Pharmacol Exp Ther. 1967 Jul;157(1):159–169. [PubMed] [Google Scholar]
- Carter G. W., Van Dyke K. A superior counting solution for water-soluble tritiated compounds. Clin Chem. 1971 Jul;17(7):576–580. [PubMed] [Google Scholar]
- DAWSON R. M., ROWLANDS I. W. Glycerylphosphorylcholine in the male reproductive organs of rats and guinea-pigs. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):26–34. doi: 10.1113/expphysiol.1959.sp001373. [DOI] [PubMed] [Google Scholar]
- EMMELIN N. Supersensitivity following "pharmacological denervation". Pharmacol Rev. 1961 Mar;13:17–37. [PubMed] [Google Scholar]
- Emmelin N. Action of acetylcholine on the responsiveness of effector cells. Experientia. 1964 May 15;20(5):275–275. doi: 10.1007/BF02151804. [DOI] [PubMed] [Google Scholar]
- Fleming W. W., Westfall D. P., De la Lande I. S., Jellett L. B. Log-normal distribution of equiefective doses of norepinephrine and acetylcholine in several tissues. J Pharmacol Exp Ther. 1972 May;181(2):339–345. [PubMed] [Google Scholar]
- HOLMSTEDT B. Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol Rev. 1959 Sep;11:567–688. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McPhillips J. J., Coon J. M. Adaptation to octamethyl pyrophosphoramide in rats. Toxicol Appl Pharmacol. 1966 Jan;8(1):66–76. doi: 10.1016/0041-008x(66)90102-5. [DOI] [PubMed] [Google Scholar]
- McPhillips J. J., Dar M. S. Resistance to the effect of carbachol on the cardiovascular system and on the isolated ileum of rats after subacute administration of an organophosphorus cholinesterase inhibitor. J Pharmacol Exp Ther. 1967 Jun;156(3):507–513. [PubMed] [Google Scholar]
- McPhillips J. J. Subsensitivity of the rat ileum to cholinergic drugs. J Pharmacol Exp Ther. 1969 Apr;166(2):249–254. [PubMed] [Google Scholar]
- Miller M. D., Marshall J. M. Uterine response to nerve stimulation; relation to hormonal status and catecholamines. Am J Physiol. 1965 Nov;209(5):859–865. doi: 10.1152/ajplegacy.1965.209.5.859. [DOI] [PubMed] [Google Scholar]
- PALLIE W., CORNER G. W., WEDDELL G. Nerve terminations in the myometrium of the rabbit. Anat Rec. 1954 Apr;118(4):789–811. doi: 10.1002/ar.1091180407. [DOI] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. J. Paralysis of autonomic ganglia by methonium salts. Br J Pharmacol Chemother. 1951 Mar;6(1):155–168. doi: 10.1111/j.1476-5381.1951.tb00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine S. E., McPhillips J. J. Specific subsensitivity of the rat atrium to cholinergic drugs. J Pharmacol Exp Ther. 1970 Nov;175(2):496–502. [PubMed] [Google Scholar]
- RICHARDSON K. C. The fine structure of autonomic nerve endings in smooth muscle of the rat vas deferens. J Anat. 1962 Oct;96:427–442. [PMC free article] [PubMed] [Google Scholar]
- RISLEY P. L., SKREPETOS C. N. HISTOCHEMICAL DISTRIBUTION OF CHOLINESTERASES IN THE TESTIS, EPIDIDYMIS AND VAS DEFERENS OF THE RAT. Anat Rec. 1964 Feb;148:231–249. doi: 10.1002/ar.1091480213. [DOI] [PubMed] [Google Scholar]
- Reas H. W., Trendelenburg U. Changes in the sensitivity of the sweat glands of the cat after denervation. J Pharmacol Exp Ther. 1967 Apr;156(1):126–136. [PubMed] [Google Scholar]
- Silva D. G. The ultrastructure of the myometrium of the rat with special reference to the innervation. Anat Rec. 1967 May;158(1):21–33. doi: 10.1002/ar.1091580104. [DOI] [PubMed] [Google Scholar]
- Westfall D. P., McClure D. C., Fleming W. W. The effects of denervation, decentralization and cocaine on the response of the smooth muscle of the guinea-pig vas deferens to various drugs. J Pharmacol Exp Ther. 1972 May;181(2):328–338. [PubMed] [Google Scholar]


