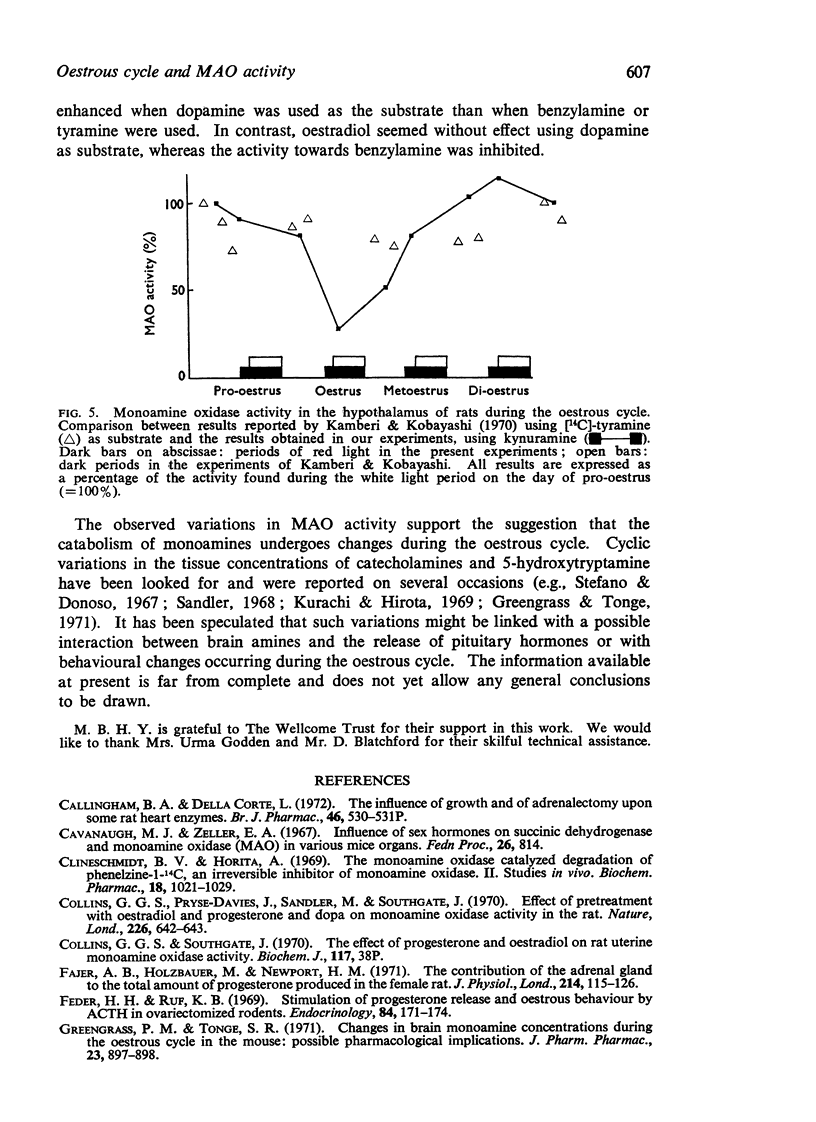
Abstract
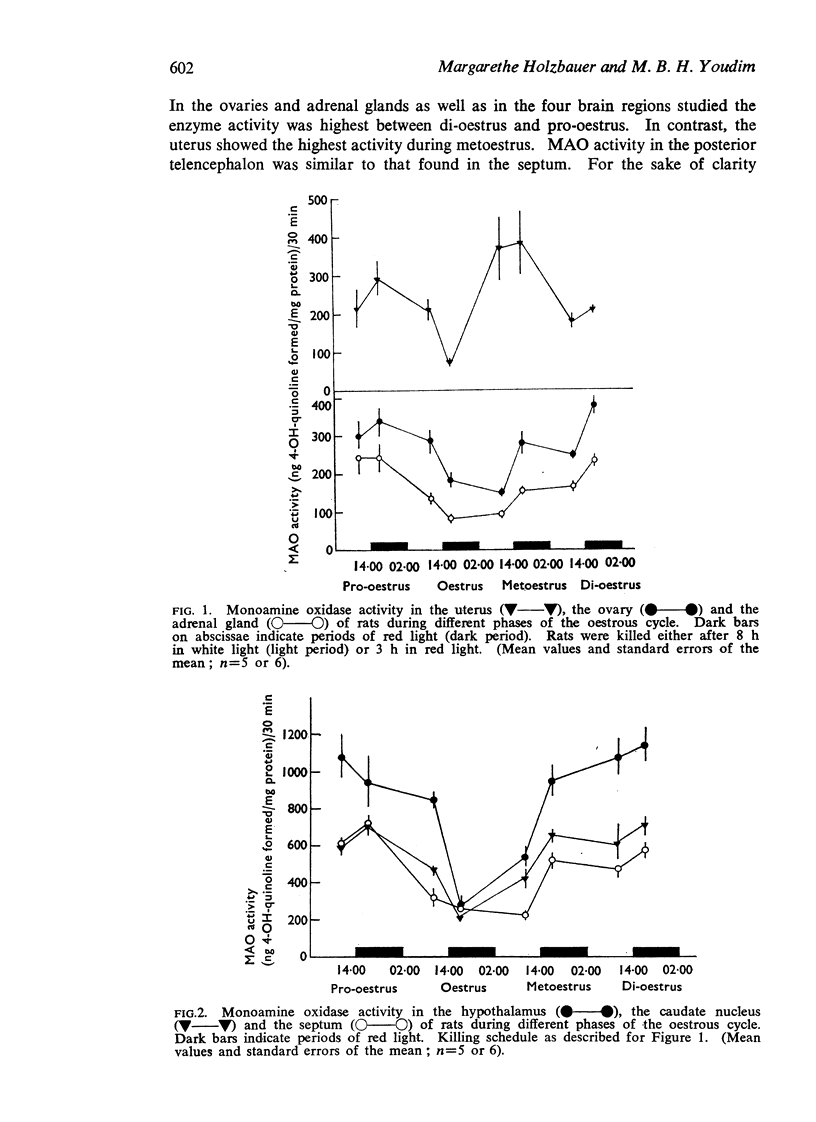
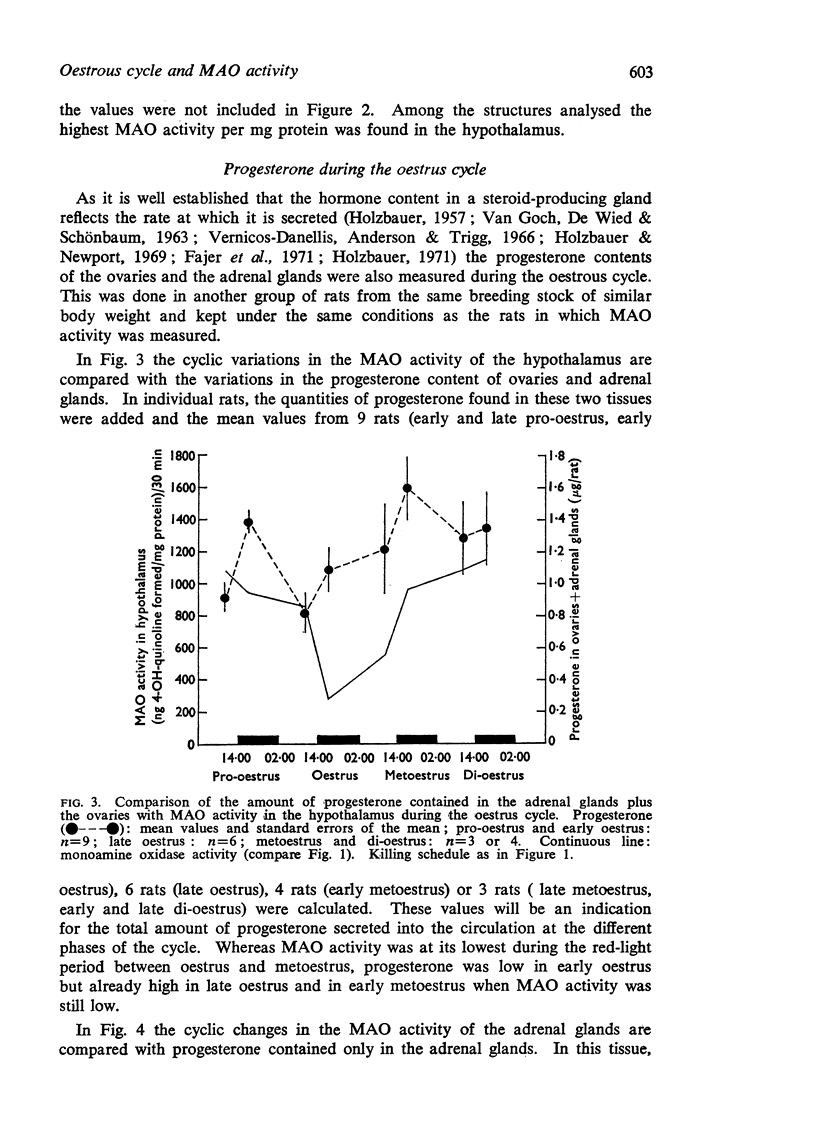
1. During the oestrous cycle of the rat, monoamine oxidase (MAO) activity was studied in the uterus, the ovaries and the adrenal glands and also in 4 brain regions which are rich in monoamines. In all these structures the activity was lowest during the night between oestrus and metoestrus. High values were found in di-oestrus, with the exception of the uterus in which the MAO activity reached its peak during metoestrus.
2. A stimulating effect of progesterone on uterine, ovarian and adrenal MAO activity was observed.
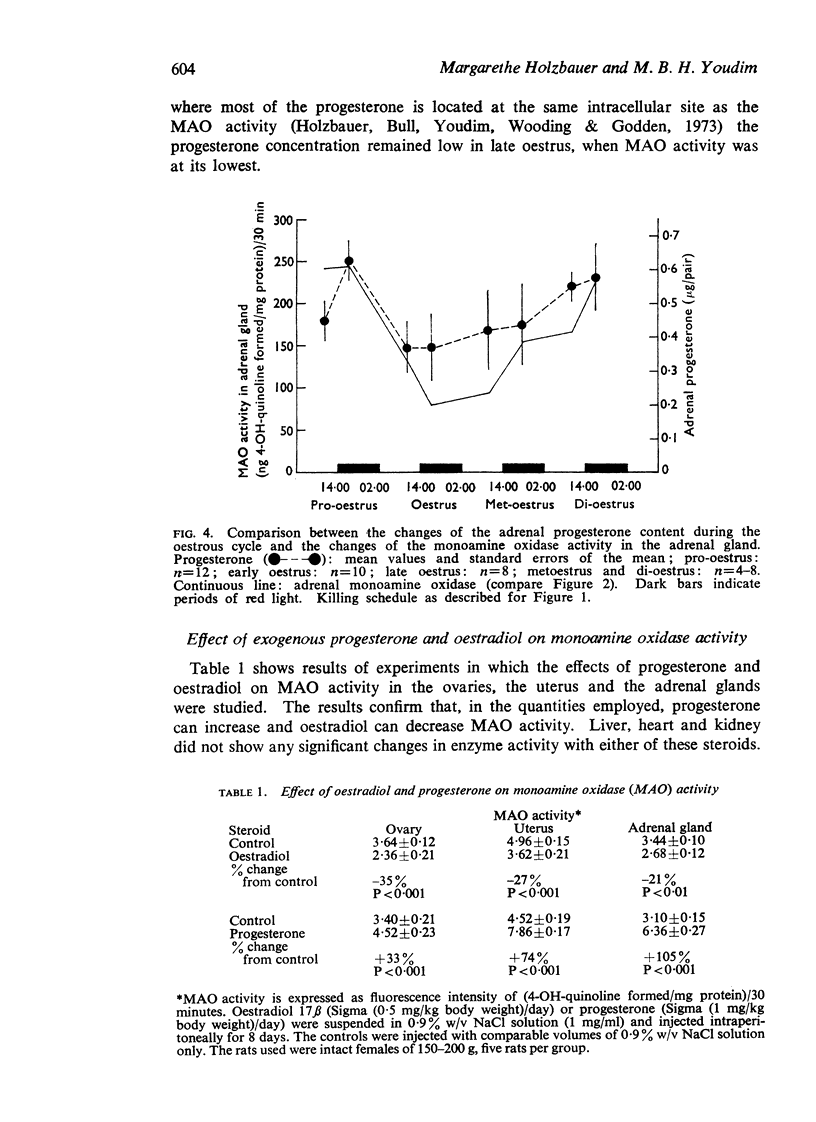
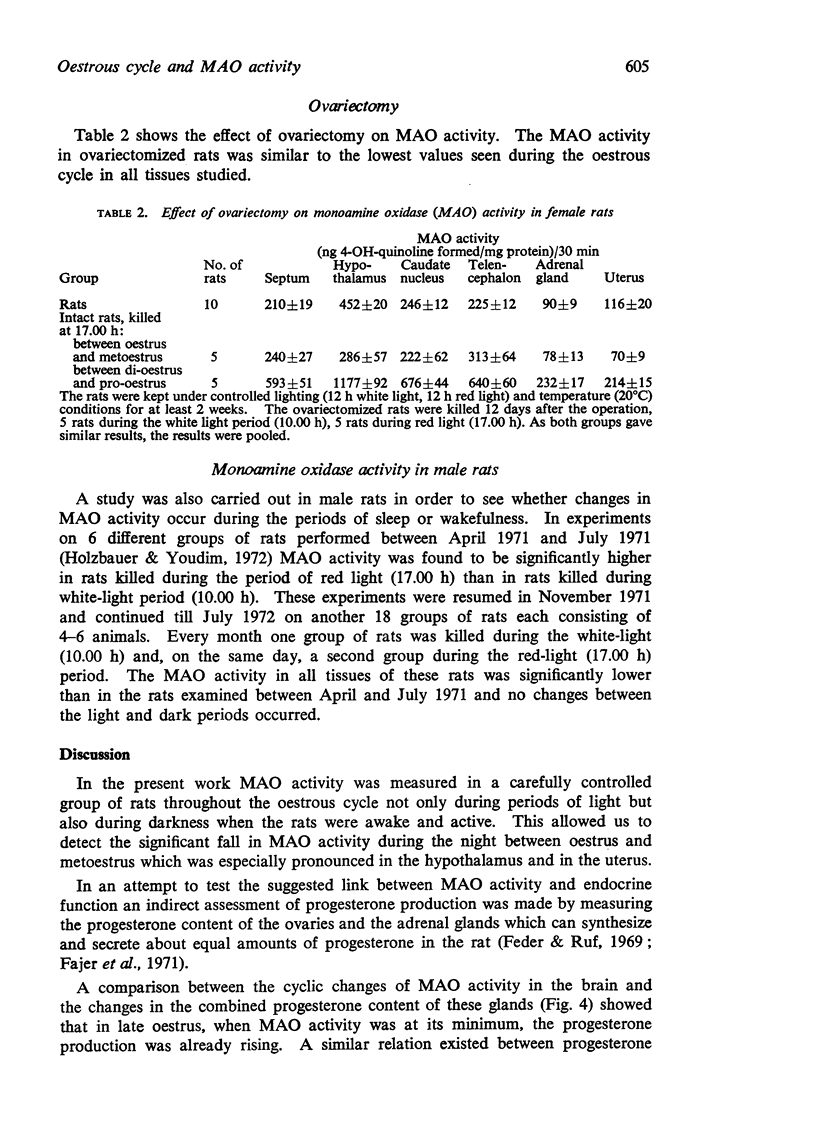
3. An index for the cyclic variations in progesterone production was obtained by measuring the adrenal and ovarian progesterone content at the same stages of the cycle as MAO activity.
4. The sum of the amounts of progesterone contained in the ovaries and adrenal glands was low in early oestrus but increased during late oestrus when MAO activity was at its minimum. Progesterone contents were high in met- and di-oestrus when MAO activity was also high.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callingham B. A., Della Corte L. The influence of growth and of adrenalectomy upon some rat heart enzymes. Br J Pharmacol. 1972 Nov;46(3):530P–531P. [PMC free article] [PubMed] [Google Scholar]
- Clineschmidt B. V., Horita A. The monoamine oxidase catalyzed degradation of phenelzine-l-14C, an irreversible inhibitor of monoamine oxidase--II. Studies in vivo. Biochem Pharmacol. 1969 May;18(5):1021–1028. doi: 10.1016/0006-2952(69)90105-1. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Pryse-Davies J., Sandler M., Southgate J. Effect of pretreatment with oestradiol, progesterone and DOPA on monoamine oxidase activity in the rat. Nature. 1970 May 16;226(5246):642–643. doi: 10.1038/226642a0. [DOI] [PubMed] [Google Scholar]
- Collins G. G., Southgate J. The effect of progesterone and oestradiol on rat uterine monoamine oxidase activity. Biochem J. 1970 Apr;117(2):38P–38P. doi: 10.1042/bj1170038pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajer A. B., Holzbauer M., Newport H. M. The contribution of the adrenal gland to the total amount of progesterone produced in the female rat. J Physiol. 1971 Apr;214(1):115–126. doi: 10.1113/jphysiol.1971.sp009422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder H. H., Ruf K. B. Stimulation of progesterone release and estrous behavior by ACTH in ovariectomized rodents. Endocrinology. 1969 Jan;84(1):171–174. doi: 10.1210/endo-84-1-171. [DOI] [PubMed] [Google Scholar]
- Greengrass P. M., Tonge S. R. Changes in brain monoamine concentrations during the oestrous cycle in the mouse: possible pharmacological implications. J Pharm Pharmacol. 1971 Nov;23(11):897–898. doi: 10.1111/j.2042-7158.1971.tb10216.x. [DOI] [PubMed] [Google Scholar]
- HOLZBAUER M. The corticosterone content of rat adrenals under different experimental conditions. J Physiol. 1957 Dec 3;139(2):294–305. doi: 10.1113/jphysiol.1957.sp005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbauer M., Bull G., Youdim M. B., Wooding F. B., Godden U. Subcellular distribution of steroids in the adrenal gland. Nat New Biol. 1973 Mar 28;242(117):117–119. doi: 10.1038/newbio242117a0. [DOI] [PubMed] [Google Scholar]
- Holzbauer M. In vivo production of steroids with central depressant actions by the ovary of the rat. Br J Pharmacol. 1971 Nov;43(3):560–569. doi: 10.1111/j.1476-5381.1971.tb07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbauer M., Newport H. M. Adrenal secretion rates and adrenal tissue concentrations of pregnenolone, progesterone, 11 beta OH-androstenedione and some other steroids in young pigs and dogs. J Physiol. 1969 Feb;200(3):821–848. doi: 10.1113/jphysiol.1969.sp008724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbauer M., Newport H. M. The effect of stress on the concentration of 3beta-hydroxypregn-5-en-20-one (pregnenolone) and pregn-4-ene-3,20-dione (progesterone) in the adrenal gland of the rat. J Physiol. 1967 Nov;193(1):131–140. doi: 10.1113/jphysiol.1967.sp008347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbauer M., Youdim M. B. Proceedings: Influence of endocrine glands on central and peripheral monoamine oxidase activity. Br J Pharmacol. 1972 Feb;44(2):355P–356P. [PMC free article] [PubMed] [Google Scholar]
- Kamberi I. A., Kobayashi Y. Monoamine oxidase activity in the hypothalamus and various other brain areas and in some endocrine glands of the rat during the estrus cycle. J Neurochem. 1970 Feb;17(2):261–268. doi: 10.1111/j.1471-4159.1970.tb02209.x. [DOI] [PubMed] [Google Scholar]
- Krajl M. A rapid microfluorimetric determination of monoamine oxidase. Biochem Pharmacol. 1965 Nov;14(11):1684–1686. doi: 10.1016/0006-2952(65)90025-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Salseduc M. M., Jofre I. J., Izquierdo J. A. Monoamineoxidase (E.C.1.4.3.4) and catechol-O-methyltransferase (E.C.2.1.1.a) activity in cerebral structures and sexual organs of rats during their sexual cycle. Med Pharmacol Exp Int J Exp Med. 1966;14(2):113–119. doi: 10.1159/000135774. [DOI] [PubMed] [Google Scholar]
- Sandler M., Youdim M. B. Multiple forms of monoamine oxidase: functional significance. Pharmacol Rev. 1972 Jun;24(2):331–348. [PubMed] [Google Scholar]
- Sandler R. Concentration of norepinephrine in the hypothalamus of the rat in relation to the estrous cycle. Endocrinology. 1968 Dec;83(6):1383–1386. doi: 10.1210/endo-83-6-1383. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate J. Endometrial monoamine oxidase: the effect of sex steroids. Adv Biochem Psychopharmacol. 1972;5:263–269. [PubMed] [Google Scholar]
- Southgate J., Grant E. C., Pollard W., Pryse-Davies J., Sandler M. Cyclical variations in endometrial monoamine oxidase: correlation of histochemical and quantitative biochemical assays. Biochem Pharmacol. 1968 May;17(5):721–726. doi: 10.1016/0006-2952(68)90008-7. [DOI] [PubMed] [Google Scholar]
- Stefano F. J., Donoso A. O. Norepinephrine levels in the rat hypothalamus during the estrous cycle. Endocrinology. 1967 Dec;81(6):1405–1406. doi: 10.1210/endo-81-6-1405. [DOI] [PubMed] [Google Scholar]
- Vernikos-Danellis J., Anderson E., Trigg L. Changes in adrenal corticosterone concentration in rats: method of bio-assay for ACTH. Endocrinology. 1966 Sep;79(3):624–630. doi: 10.1210/endo-79-3-624. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K., Hawkins R. A., Stocker J. F. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969 Jul;85(1):103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]
- Youdim M. B., Collins G. G., Sandler M. Multiple forms of rat brain monoamine oxidase. Nature. 1969 Aug 9;223(5206):626–628. doi: 10.1038/223626a0. [DOI] [PubMed] [Google Scholar]
- Youdim M. B. Multiple forms of mitochondrial monoamine oxidase. Br Med Bull. 1973 May;29(2):120–122. doi: 10.1093/oxfordjournals.bmb.a070980. [DOI] [PubMed] [Google Scholar]
- Zolovick A. J., Pearse R., Boehlke K. W., Eleftheriou B. E. Monoamine oxidase activity in various parts of the rat brain during the estrous cycle. Science. 1966 Nov 4;154(3749):649–649. doi: 10.1126/science.154.3749.649. [DOI] [PubMed] [Google Scholar]


