Abstract
1. Electrophysiological techniques were utilized to study the actions of 5-hydroxytryptamine (5-HT) and picrotoxin on the superior cervical ganglion of the cat.
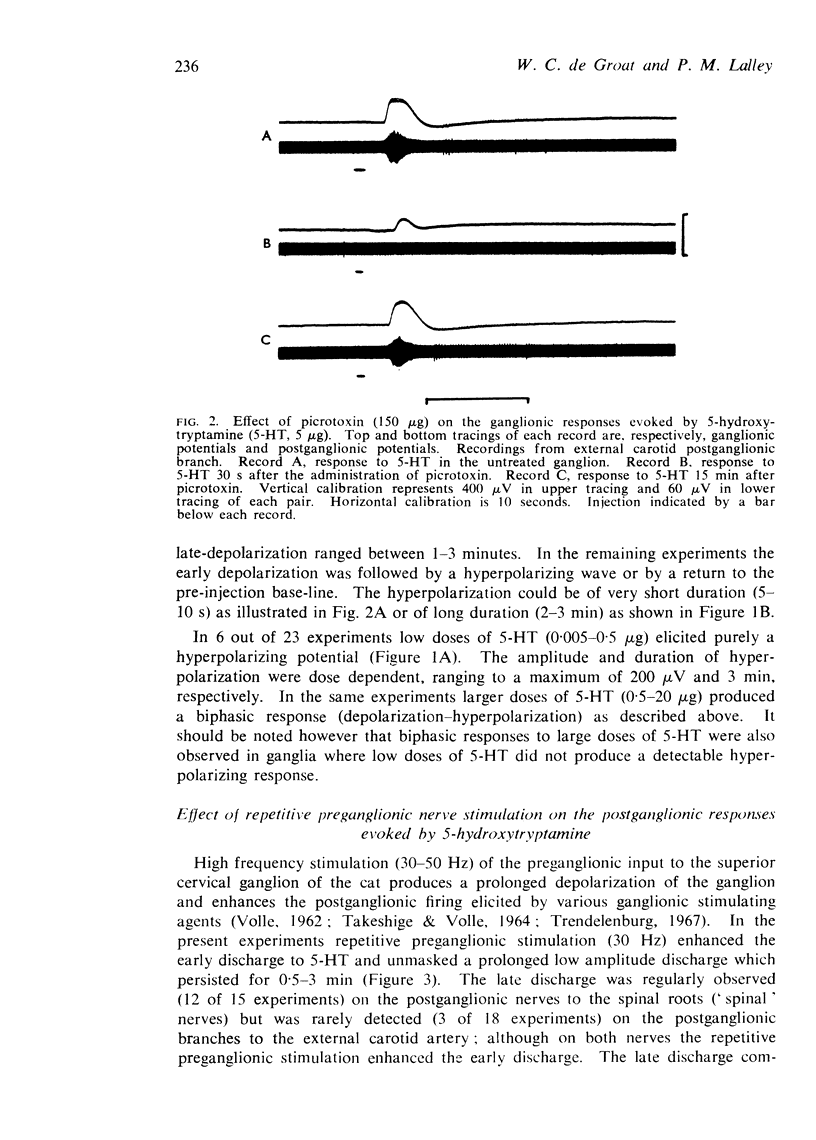
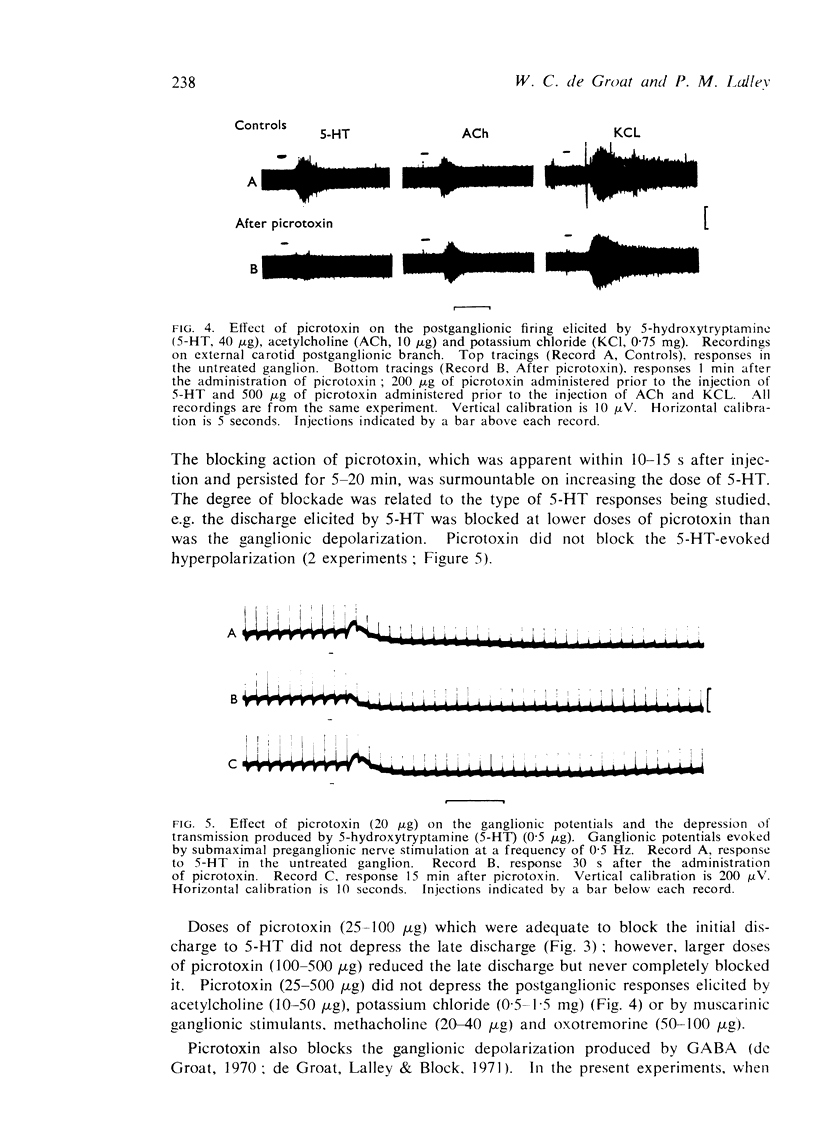
2. The intra-arterial administration of 5-HT to the ganglion elicited both depressant and excitatory actions. In low doses (0·01-0·5 μg) the amine produced a depression of ganglionic transmission. In larger doses (2-50 μg) it produced an excitation of ganglion cells (early discharge) and an initial enhancement of transmission, which was followed by depression. Picrotoxin (25-500 μg, i.a.) blocked the initial excitatory effects of 5-HT but did not block the depression. Picrotoxin did not antagonize the excitatory actions of injected cholinomimetic agents or potassium chloride.
3. In ganglia conditioned by repetitive stimulation of the preganglionic nerve (30 Hz for 30 s) 5-HT also elicited a late-occurring and very prolonged discharge on certain postganglionic nerves (`spinal') but not on others (external carotid). The late discharge was only partially depressed by picrotoxin.
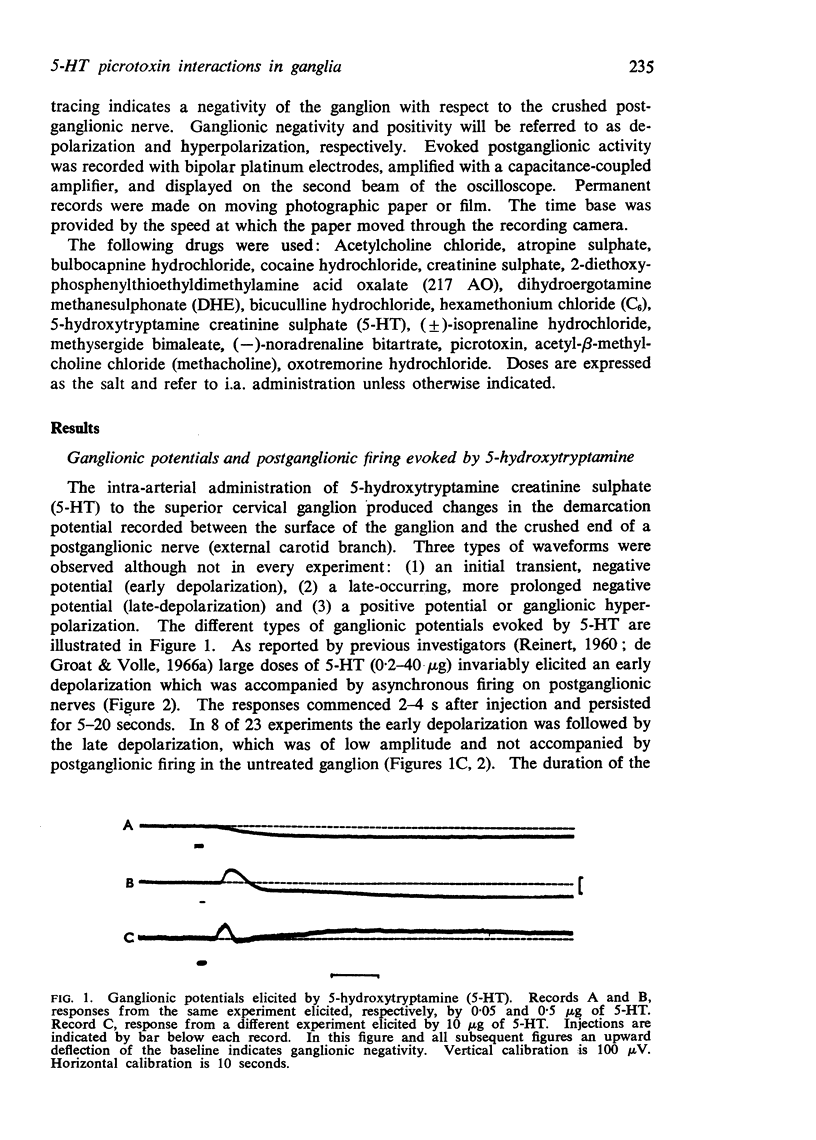
4. Recordings from the surface of the superior cervical ganglion revealed that 5-HT produced three types of ganglionic potentials: (1) an initial transient depolarization which coincided with the early discharge, (2) a late-occurring, prolonged depolarization which coincided with the late discharge, and (3) a hyperpolarization which in some experiments accompanied the depression of transmission. The late depolarization and hyperpolarization were not observed in every experiment. Picrotoxin (25-500 μg) blocked the initial depolarization, but did not block the late depolarization or the hyperpolarization.
5. It is concluded the 5-HT can produce three distinct responses in the superior cervical ganglion: a depressant effect and two types of excitation. It seems likely that depression and excitation occur via the activation of different receptors, since picrotoxin selectively blocks the latter. The finding that picrotoxin is a 5-HT antagonist in peripheral ganglia raises the possibility that picrotoxin might also influence tryptaminergic mechanisms in the central nervous system.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bülbring E., Gershon M. D. Serotonin participation in the vagal inhibitory pathway to the stomach. Adv Pharmacol. 1968;6(Pt A):323–333. doi: 10.1016/s1054-3589(08)61188-6. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., DAVIS R. Pharmacological studies upon neurones of the lateral geniculate nucleus of the cat. Br J Pharmacol Chemother. 1962 Apr;18:217–246. doi: 10.1111/j.1476-5381.1962.tb01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Crawford J. M. Central synaptic transmission--microelectrophoretic studies. Annu Rev Pharmacol. 1969;9:209–240. doi: 10.1146/annurev.pa.09.040169.001233. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- DEGROAT W. C., VOLLE R. L. GANGLIONIC ACTIONS OF OXOTREMORINE. Life Sci. 1963 Aug;8:618–623. doi: 10.1016/0024-3205(63)90116-4. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A. Gamma-aminobutyric acid antagonism and presynaptic inhibition in the frog spinal cord. Science. 1972 Jan 21;175(4019):331–333. doi: 10.1126/science.175.4019.331. [DOI] [PubMed] [Google Scholar]
- Davidson N., Southwick C. A. Amino acids and presynaptic inhibition in the rat cuneate nucleus. J Physiol. 1971 Dec;219(3):689–708. doi: 10.1113/jphysiol.1971.sp009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C. GABA-depolarization of a sensory ganglion: antagonism by picrotoxin and bicuculline. Brain Res. 1972 Mar 24;38(2):429–432. doi: 10.1016/0006-8993(72)90726-3. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Lalley P. M., Saum W. R. Depolarization of dorsal root ganglia in the cat by GABA and related amino acids: antagonism by picrotoxin and bicuculline. Brain Res. 1972 Sep 15;44(1):273–277. doi: 10.1016/0006-8993(72)90383-6. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Saum W. R. Adrenergic inhibition in mammalian parasympathetic ganglia. Nat New Biol. 1971 Jun 9;231(23):188–189. doi: 10.1038/newbio231188a0. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Saum W. R. Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol. 1972 Jan;220(2):297–314. doi: 10.1113/jphysiol.1972.sp009708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Volle R. L. Interactions between the catecholamines and ganglionic stimulating agents in sympathetic ganglia. J Pharmacol Exp Ther. 1966 Nov;154(2):200–215. [PubMed] [Google Scholar]
- De Groat W. C., Volle R. L. The actions of the catecholamines on transmission in the superior cervical ganglion of the cat. J Pharmacol Exp Ther. 1966 Oct;154(1):1–13. [PubMed] [Google Scholar]
- DeGroat W. C., Lalley P. M., Block M. The effects of bicuculline and GABA on the superior cervical ganglion of the cat. Brain Res. 1971 Feb 5;25(3):665–668. doi: 10.1016/0006-8993(71)90473-2. [DOI] [PubMed] [Google Scholar]
- GADDUM J. H., PICARELLI Z. P. Two kinds of tryptamine receptor. Br J Pharmacol Chemother. 1957 Sep;12(3):323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GYERMEK L., BINDLER E. Action of indole alkylamines and amidines on the inferior mesenteric ganglion of the cat. J Pharmacol Exp Ther. 1962 Nov;138:159–164. [PubMed] [Google Scholar]
- GYERMEK L., BINDLER E. Blockade of the ganglionic stimulant action of 5-hydroxytryptamin. J Pharmacol Exp Ther. 1962 Mar;135:344–348. [PubMed] [Google Scholar]
- HERTZLER E. C. 5-Hydroxytryptamine and transmission in sympathetic ganglia. Br J Pharmacol Chemother. 1961 Dec;17:406–413. doi: 10.1111/j.1476-5381.1961.tb01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L., Comroe J. H., Jr Reflex apnea, bradycardia, and hypotension produced by serotonin and phenyldiguanide acting on the nodose ganglia of the cat. Circ Res. 1971 Aug;29(2):145–155. doi: 10.1161/01.res.29.2.145. [DOI] [PubMed] [Google Scholar]
- Levy R. A., Repkin A. H., Anderson E. G. The effect of bicuculline on primary afferent terminal excitability. Brain Res. 1971 Sep 10;32(1):261–265. doi: 10.1016/0006-8993(71)90178-8. [DOI] [PubMed] [Google Scholar]
- Machová J., Boska D. The effect of 5-hydroxytryptamine, dimethylphenylpiperazinium and acetylcholine on transmission and surface potential in the cat sympathetic ganglion. Eur J Pharmacol. 1969 Aug;7(2):152–158. doi: 10.1016/0014-2999(69)90004-1. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. EFFECTS OF DRUGS ON VERTEBRATE MECHANORECEPTORS. Pharmacol Rev. 1964 Dec;16:341–380. [PubMed] [Google Scholar]
- ROBERTSON P. A. Potentiation of 5-hydroxytryptamine by the true-cholinesterase inhibitor 284C51. J Physiol. 1954 Jul 28;125(1):37–8P. [PubMed] [Google Scholar]
- Saum W. R., De Groat W. C. Parasympathetic ganglia: activation of an adrenergic inhibitory mechanism by cholinomimetic agents. Science. 1972 Feb 11;175(4022):659–661. doi: 10.1126/science.175.4022.659. [DOI] [PubMed] [Google Scholar]
- Saum W. R., Groat W. C. Antagonism by bulbocapnine of adrenergic inhibition in parasympathetic ganglia in the urinary bladder. Brain Res. 1972 Feb 25;37(2):340–344. doi: 10.1016/0006-8993(72)90682-8. [DOI] [PubMed] [Google Scholar]
- TAKESHIGE C., VOLLE R. L. MODIFICATION OF GANGLIONIC RESPONSES TO CHOLINOMIMETIC DRUGS FOLLOWING PREGANGLIONIC STIMULATION, ANTICHOLINESTERASE AGENTS AND PILOCARPINE. J Pharmacol Exp Ther. 1964 Dec;146:335–343. [PubMed] [Google Scholar]
- TRENDELENBURG U. The action of 5-hydroxytryptamine on the nictitating membrane and on the superior cervical ganglion of the cat. Br J Pharmacol Chemother. 1956 Mar;11(1):74–80. doi: 10.1111/j.1476-5381.1956.tb01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENDELENBURG U. The action of histamine, pilocarpine and 5-hydroxytryptamine on transmission through the superior cervical ganglion. J Physiol. 1957 Jan 23;135(1):66–72. doi: 10.1113/jphysiol.1957.sp005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebecis A. K., Phillis J. W. The effects of topically applied biogenic monoamines on the isolated toad spinal cord. Comp Biochem Physiol. 1967 Nov;23(2):553–563. doi: 10.1016/0010-406x(67)90407-0. [DOI] [PubMed] [Google Scholar]
- Tebécis A. K., Di Maria A. A re-evaluation of the mode of action of 5-hydroxytryptamine on lateral geniculate neurones: comparison with catecholamines and LSD. Exp Brain Res. 1972 Apr 27;14(5):480–493. doi: 10.1007/BF00236590. [DOI] [PubMed] [Google Scholar]
- VOLLE R. L. Enhancement of postganglionic responses to stimulating agents following repetitive preganglionic stimulation. J Pharmacol Exp Ther. 1962 Apr;136:68–74. [PubMed] [Google Scholar]
- de Groat W. C. The actions of gamma-aminobutyric acid and related amino acids on mammalian autonomic ganglia. J Pharmacol Exp Ther. 1970 Apr;172(2):384–396. [PubMed] [Google Scholar]


