Abstract
1. A series of analogues of angiotensin II (ATII) has been used in the present experiments to characterize receptors for ATII in intestinal (rat stomach strip, rat colon) and vascular (rabbit aorta) smooth muscles. Two types of compounds have been chosen: (a) agonists with reduced potency, in which 4-Tyr, 6-His or 7-Pro had been substituted with L-Ala or with Gly and 1-amino-cyclopentane carboxylic acid (Acpc) and (b) competitive antagonists (8-Gly-ATII, 8-Leu-ATII).
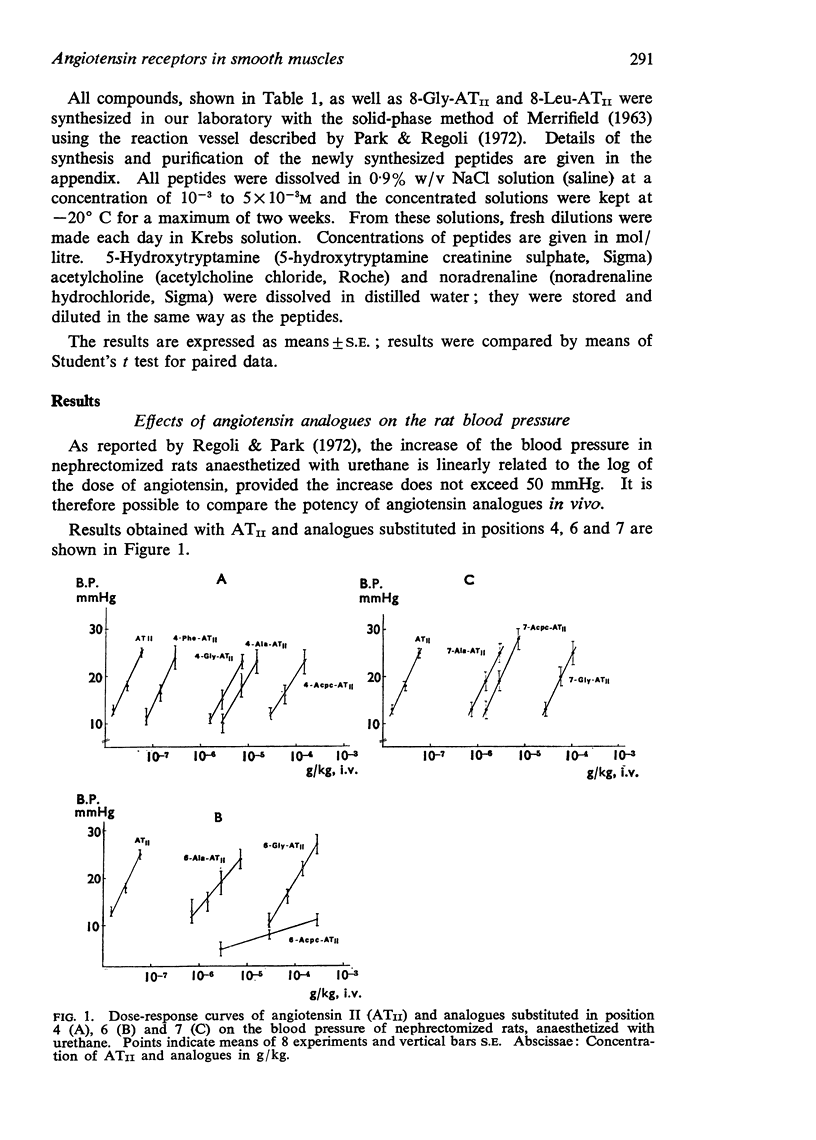
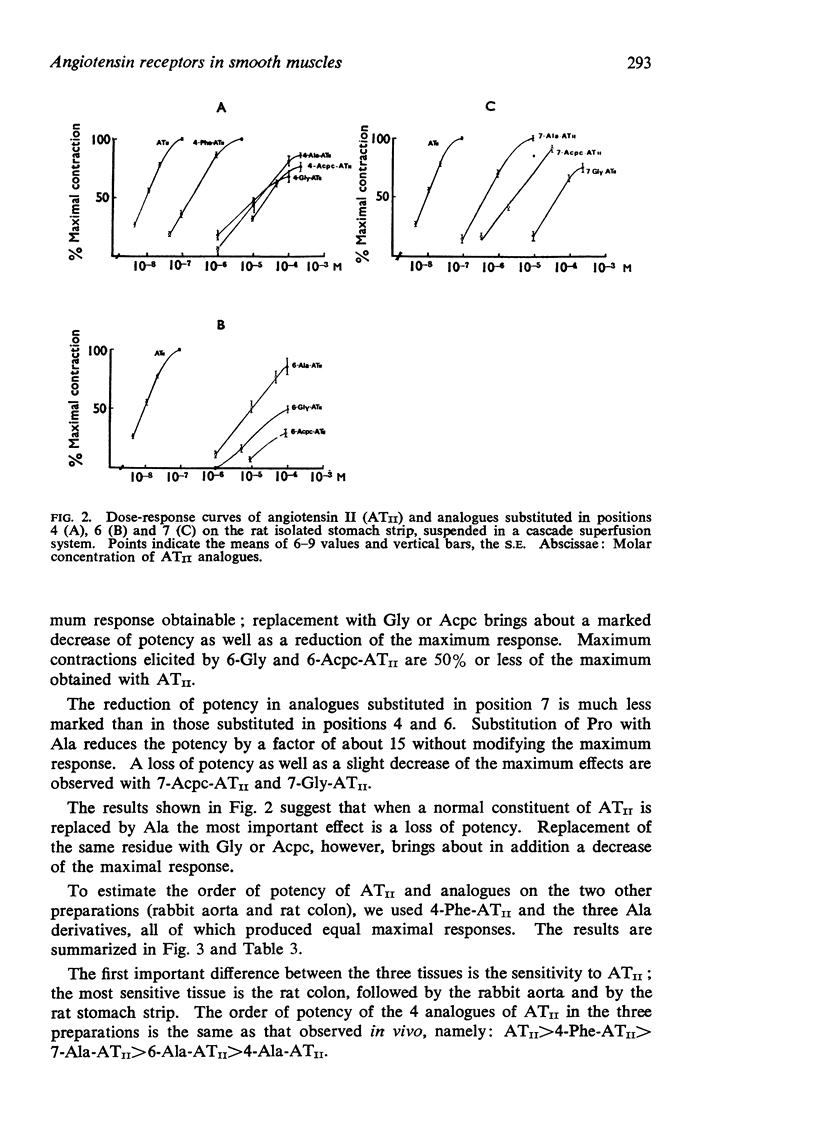
2. Replacement of 4-Tyr, 6-His and 7-Pro with L-Ala decreases the potency, but does not influence the maximum effect of the analogue, while substitution of the same residue with Gly and Acpc reduces both potency and maximum effect.
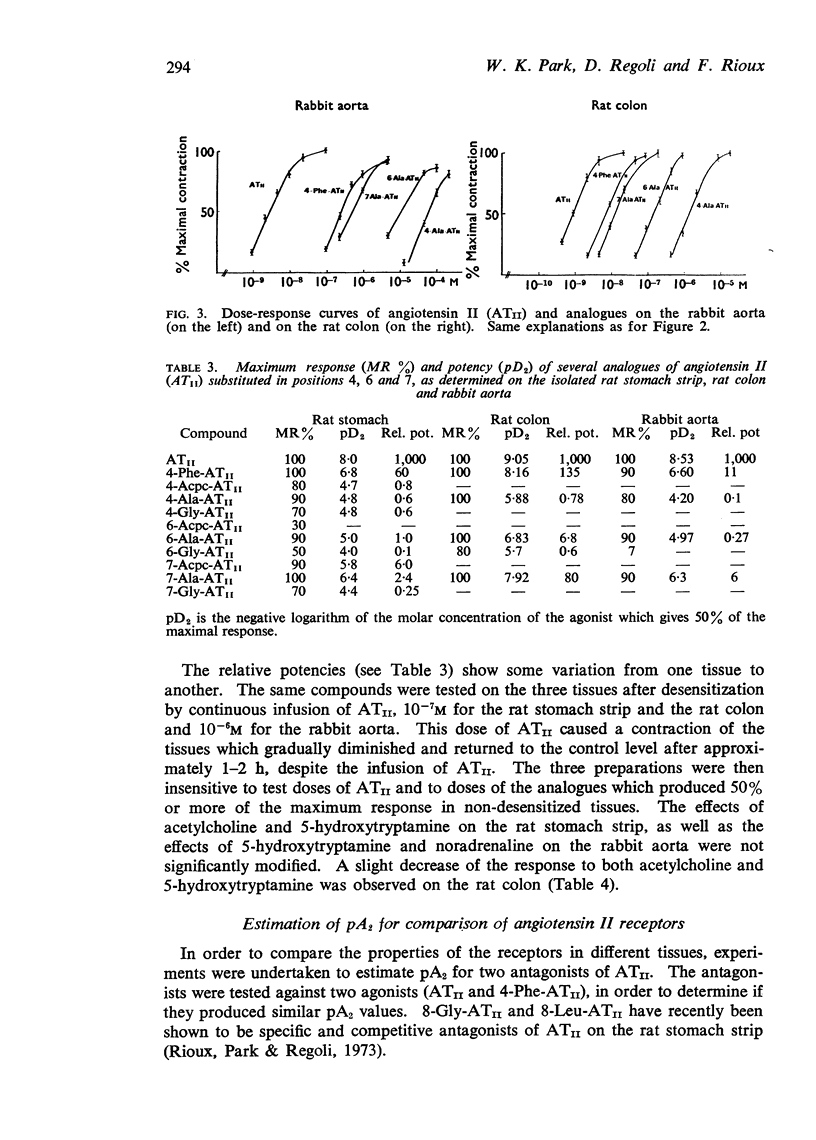
3. Compounds showing full size maximum responses were chosen to establish the following order of potency on the three preparations: ATII>4-Phe-ATII>7-Ala-ATII>6-Ala-ATII>4-Ala-ATII.
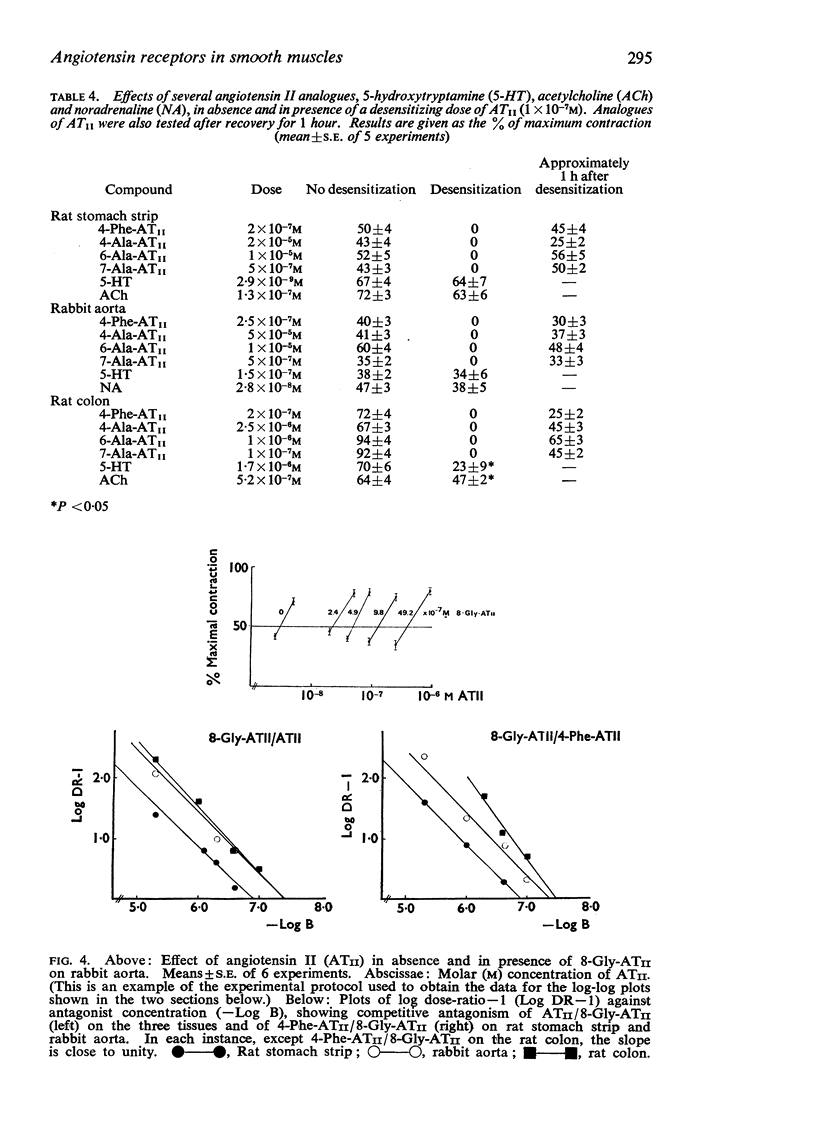
4. The four derivatives of ATII were completely inactive on tissues desensitized with ATII. The responses to 5-hydroxytryptamine, acetylcholine and noradrenaline were not significantly modified, except for the rat colon.
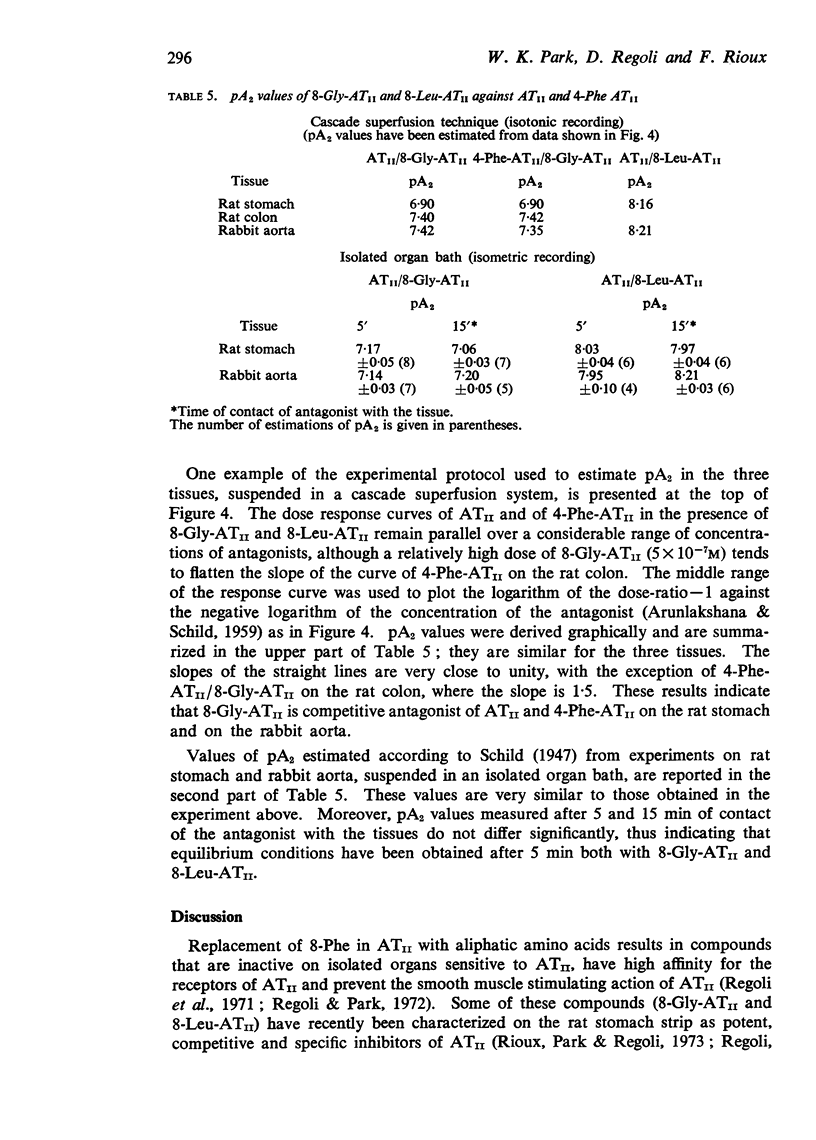
5. pA2 values for the two competitive antagonists against ATII and 4-Phe-ATII were estimated in the three preparations by the use of the cascade superfusion technique. For comparison, pA2 values were also estimated in rat stomach strips and rabbit aortas suspended in a normal organ bath, according to the method of Schild (1947). The similarities of the pA2 values obtained in two series of experiments indicate that (a) the cascade superfusion technique is suitable for this type of study and (b) the receptor for ATII in the three tissues may be the same.
6. It is suggested that receptors for ATII in intestinal and vascular smooth muscles may be the same, because (a) the order of potency of various agonists follows the same pattern, (b) the agonists are inactive on tissues desensitized with ATII, (c) pA2 values for competitive antagonists are similar in the three preparations.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F., BHADRAKOM S. Reactions of strips of rabbit aorta to epinephrine, isopropylarterenol, sodium nitrite and other drugs. J Pharmacol Exp Ther. 1953 Jun;108(2):129–143. [PubMed] [Google Scholar]
- Khairallah P. A., Toth A., Bumpus F. M. Analogs of angiotensin II. II. Mechanism of receptor interaction. J Med Chem. 1970 Mar;13(2):181–184. doi: 10.1021/jm00296a003. [DOI] [PubMed] [Google Scholar]
- PAGE I. H., BUMPUS F. M. Angiotensin. Physiol Rev. 1961 Apr;41:331–390. doi: 10.1152/physrev.1961.41.2.331. [DOI] [PubMed] [Google Scholar]
- Pals D. T., Masucci F. D., Sipos F., Denning G. S., Jr A specific competitive antagonist of the vascular action of angiotensin. II. Circ Res. 1971 Dec;29(6):664–672. doi: 10.1161/01.res.29.6.664. [DOI] [PubMed] [Google Scholar]
- Park W. K., Regoli D. New reaction vessel for solid-phase peptide synthesis. Can J Biochem. 1972 Jul;50(7):755–757. doi: 10.1139/o72-105. [DOI] [PubMed] [Google Scholar]
- Park W. K., Smeby R. R., Bumpus F. M. Synthesis of [5-isoleucine, 8-alanine]-angiotensin II by the solution method synthesis and the solid-phase method synthesis. Biochemistry. 1967 Nov;6(11):3458–3464. doi: 10.1021/bi00863a016. [DOI] [PubMed] [Google Scholar]
- REGOLI D., VANE J. R. A SENSITIVE METHOD FOR THE ASSAY OF ANGIOTENSIN. Br J Pharmacol Chemother. 1964 Oct;23:351–359. doi: 10.1111/j.1476-5381.1964.tb01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D., Park W. K., Rioux F., Chan C. S. Antagonists of angiotensin. Substitution of an aliphatic chain to phenyl ring in position 8. Rev Can Biol. 1971 Dec;30(4):319–329. [PubMed] [Google Scholar]
- Regoli D., Park W. K., Rioux F. II. Pharmacology of angiotensin antagonists. Can J Physiol Pharmacol. 1973 Feb;51(2):114–121. doi: 10.1139/y73-015. [DOI] [PubMed] [Google Scholar]
- Regoli D., Park W. K. The pressor and myotropic effects and the antagonistic properties of several analogues of angiotensin II. Can J Physiol Pharmacol. 1972 Feb;50(2):99–112. doi: 10.1139/y72-016. [DOI] [PubMed] [Google Scholar]
- Rioux F., Park W. K., Regoli D. I. Pharmacology of angiotensin antagonists. Can J Physiol Pharmacol. 1973 Feb;51(2):108–113. doi: 10.1139/y73-014. [DOI] [PubMed] [Google Scholar]
- Turker R. K., Yamamoto M. Y., Khairallah P. A., Bumpus F. M. Competative antagonism of 8-ala-angiotensin II to angiotensins I and II on isolated rabbit aorta and rat colon. Eur J Pharmacol. 1971;15(3):285–291. doi: 10.1016/0014-2999(71)90094-x. [DOI] [PubMed] [Google Scholar]
- VANE J. R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957 Sep;12(3):344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANE J. R. THE USE OF ISOLATED ORGANS FOR DETECTING ACTIVE SUBSTANCES IN THE CIRCULATING BLOOD. Br J Pharmacol Chemother. 1964 Oct;23:360–373. doi: 10.1111/j.1476-5381.1964.tb01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


