Abstract
1. Crude venom (TSV) from the Australian tiger snake (Notechis scutatus scutatus) has both presynaptic and postsynaptic effects at the neuromuscular junctions of toads.
2. TSV (50 μg/ml) rapidly blocked indirectly elicited muscle twitches without affecting the compound action potential in the sciatic nerve or twitches elicited by direct stimulation.
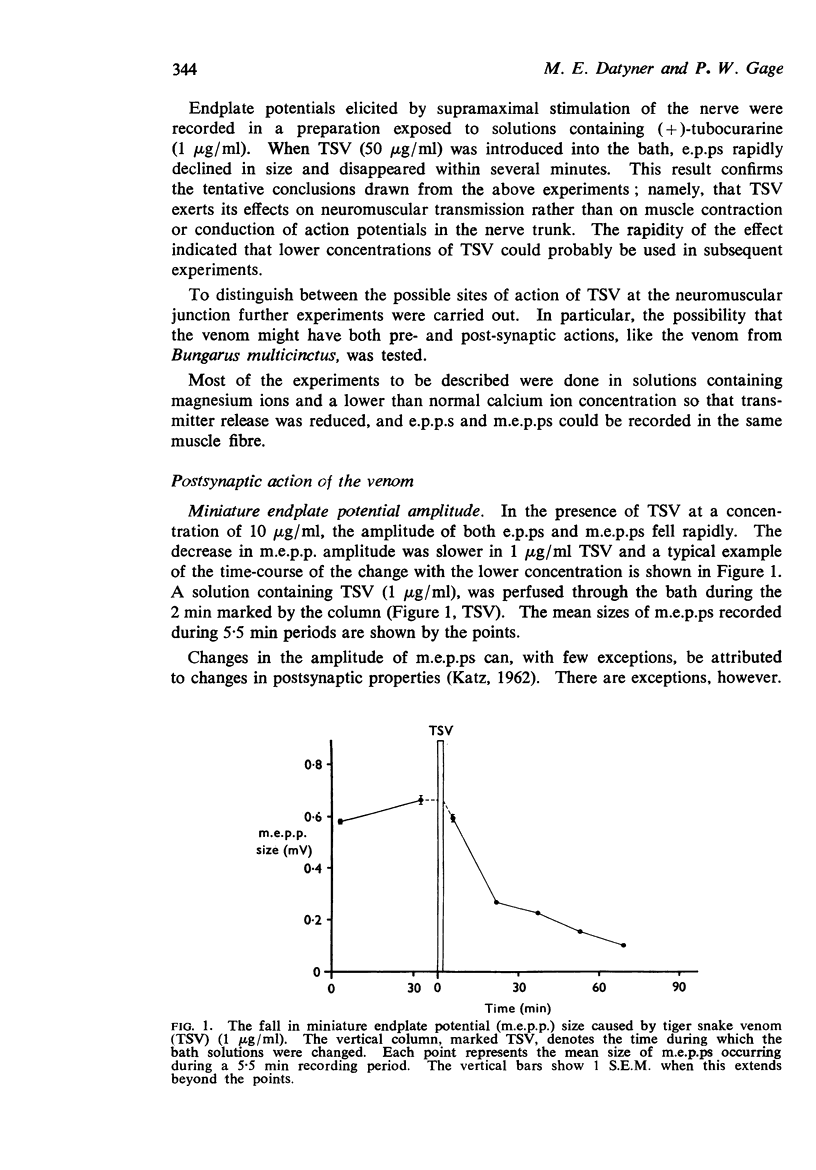
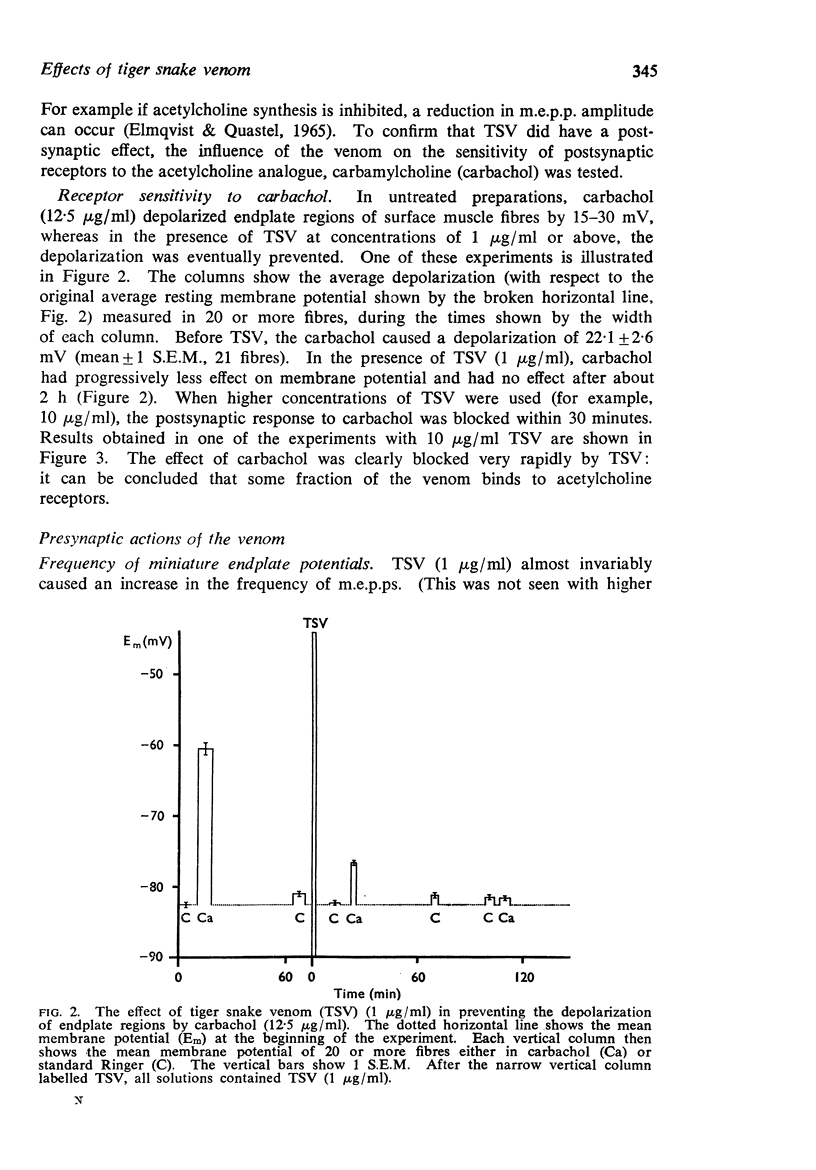
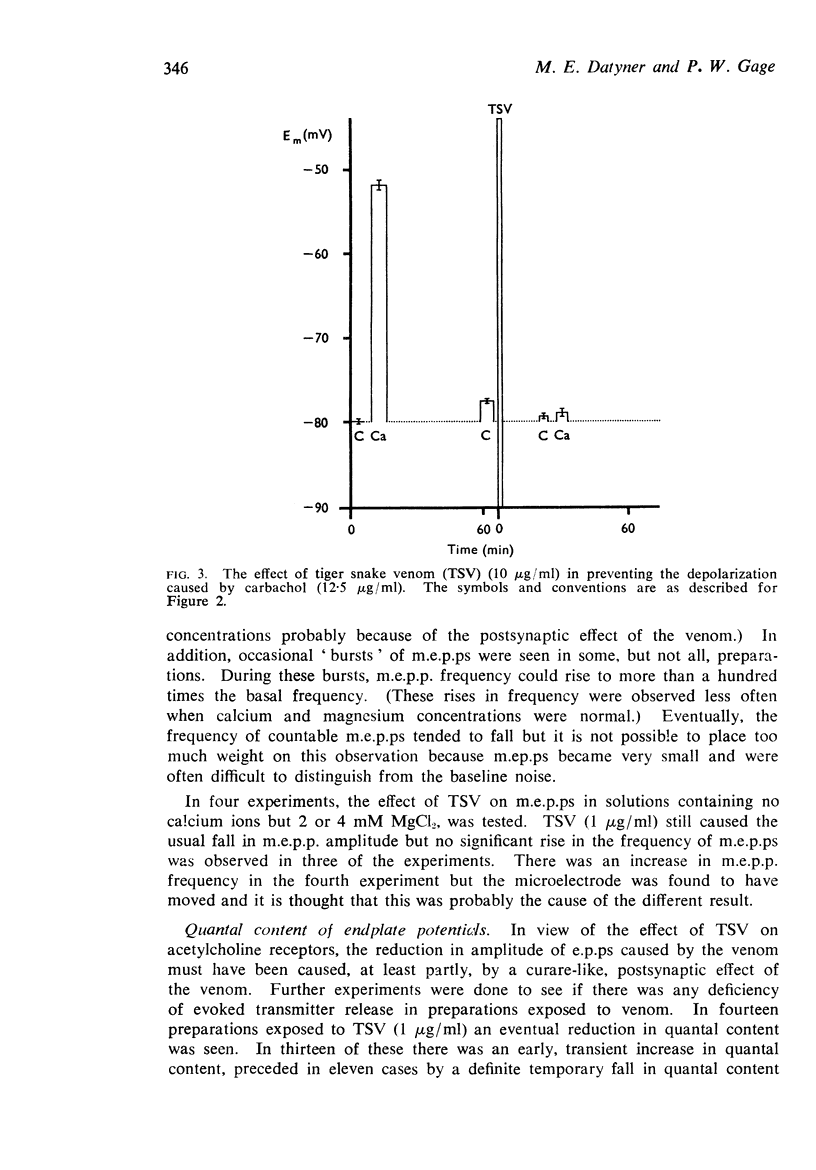
3. Low concentrations of the venom (1-10 μg/ml) reduced the amplitude of miniature endplate potentials (m.e.p.ps) and inhibited the depolarization of muscle fibres normally caused by carbachol. It was concluded that a fraction of the venom binds to acetylcholine receptors.
4. The frequency of m.e.p.ps was at first increased by TSV at a concentration of 1 μg/ml. Occasional, high frequency `bursts' of m.e.p.ps were recorded in some preparations. The mean frequency of m.e.p.ps appeared to fall after several hours in the venom.
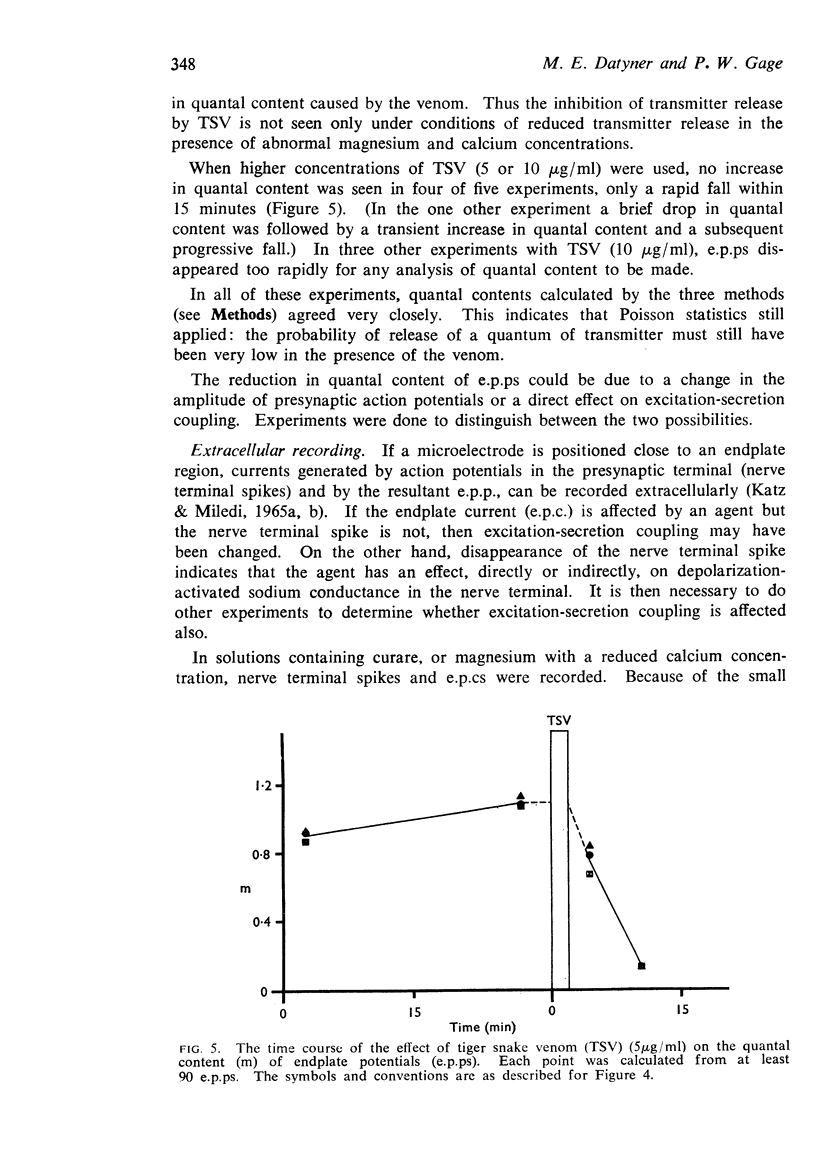
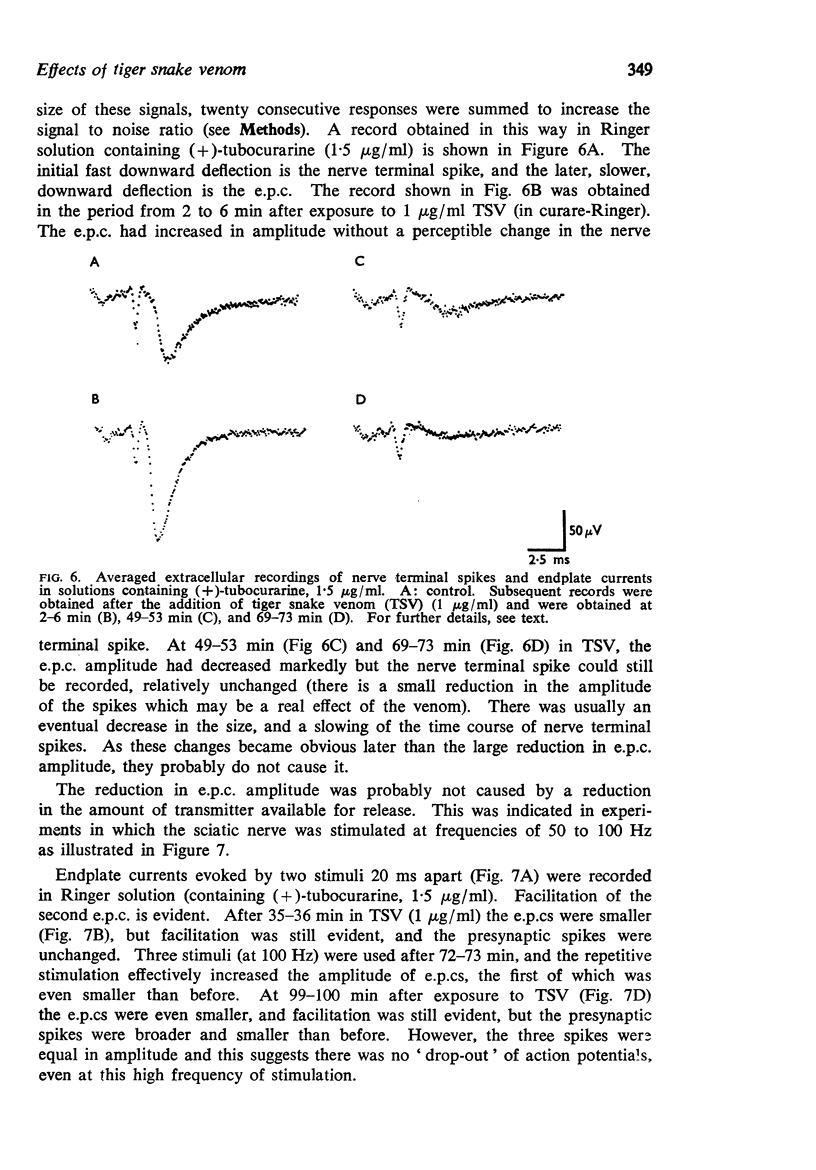
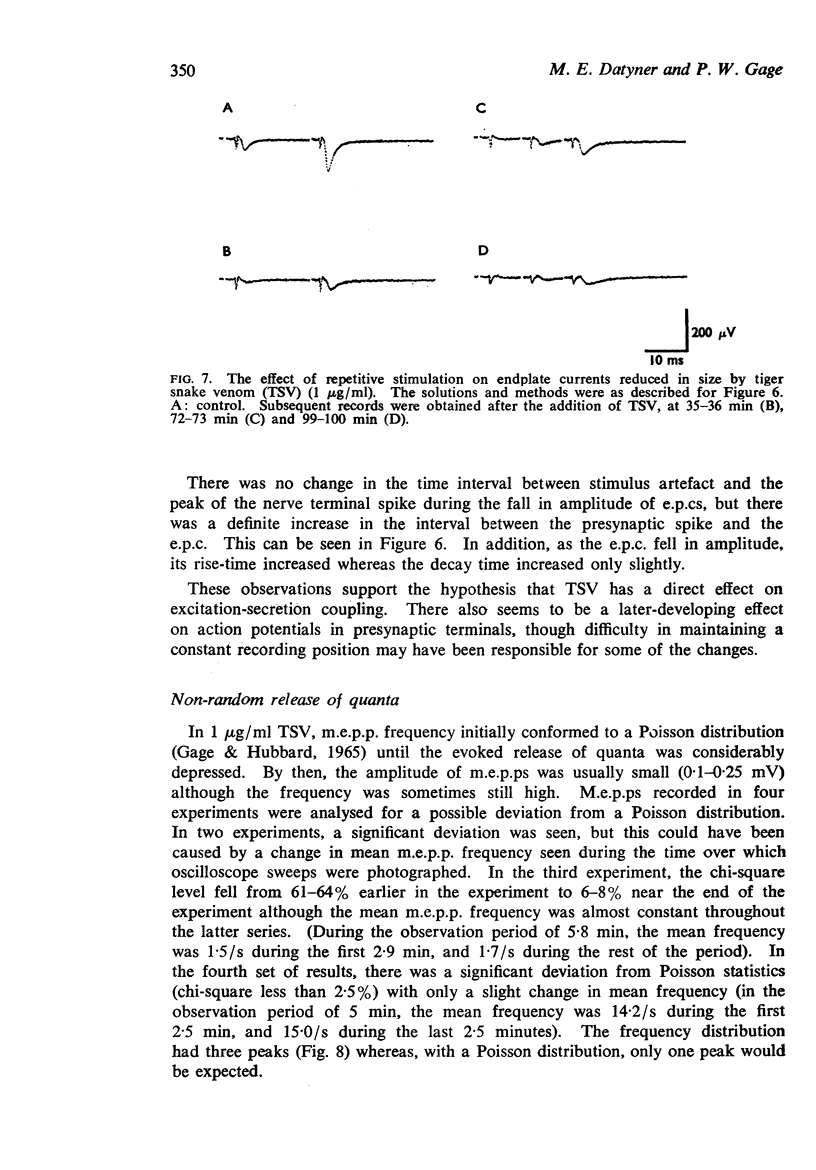
5. The quantal content of endplate potentials (e.p.ps) was reduced by the venom. With low concentrations (1 μg/ml), an initial increase in quantal content was often seen. When the quantal content was markedly depressed there was no parallel reduction in the amplitude of nerve terminal spikes recorded extracellularly, though a later fall in size and slowing of time course was often seen.
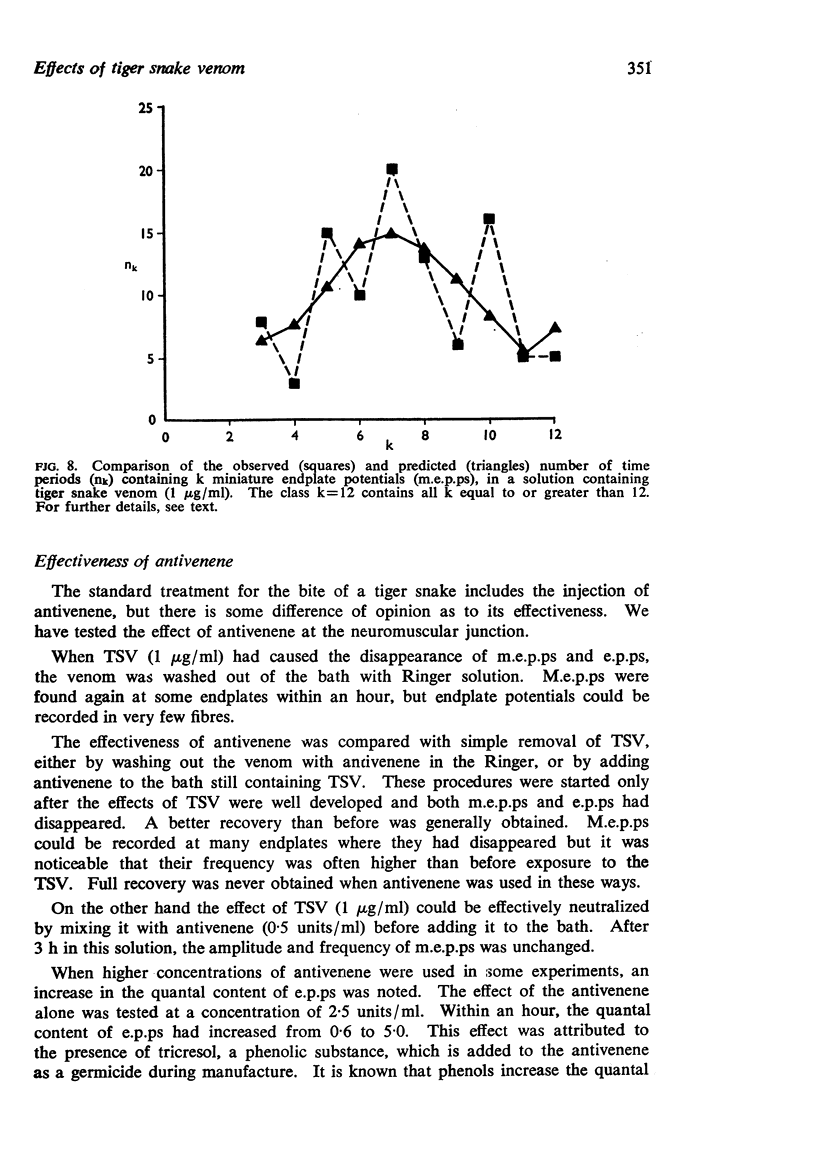
6. There was evidence that TSV eventually changed the normal Poisson characteristics of the spontaneous release of quanta and this may be correlated with electronmicroscopic changes in nerve terminals.
7. Tiger snake antivenene counteracted the postsynaptic, but not the presynaptic effects of TSV when they had developed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Kelly R. B., Sargent P. B., Williamson P., Hall Z. W. Binding of -bungarotoxin to acetylcholine receptors in mammalian muscle (snake venom-denervated muscle-neonatal muscle-rat diaphragm-SDS-polyacrylamide gel electrophoresis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):147–151. doi: 10.1073/pnas.69.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B. Acetylcholine receptor. I. Identification and biochemical characteristics of a cholinergic receptor of guinea pig cerebral cortex. J Biol Chem. 1972 Jan 10;247(1):130–145. [PubMed] [Google Scholar]
- Chen I. L., Lee C. Y. Ultrastructural changes in the motor nerve terminals caused by beta-bungarotoxin. Virchows Arch B Cell Pathol. 1970;6(4):318–325. doi: 10.1007/BF02899133. [DOI] [PubMed] [Google Scholar]
- Datyner M. E., Gage P. W. Australian tiger snake venom--an inhibitor of transmitter release. Nat New Biol. 1973 Feb 21;241(112):246–247. doi: 10.1038/newbio241246a0. [DOI] [PubMed] [Google Scholar]
- Dulhunty A., Gage P. W. Selective effects of an octopus toxin on action potentials. J Physiol. 1971 Oct;218(2):433–445. doi: 10.1113/jphysiol.1971.sp009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker D., Harris J. B., Thesleff S. Action of a cobra neurotoxin on denervated rat skeletal muscle. Eur J Pharmacol. 1971 Jul;15(2):254–256. doi: 10.1016/0014-2999(71)90182-8. [DOI] [PubMed] [Google Scholar]
- Earl J. E., Excell B. J. The effects of toxic components of Naja nivea (Cape cobra) venom on neuromuscular transmission and muscle membrane permeability. Comp Biochem Physiol A Comp Physiol. 1972 Mar;41(3):597–615. doi: 10.1016/0300-9629(72)90015-1. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Hartzell H. C. Acetylcholine receptors: number and distribution at neuromuscular junctions in rat diaphragm. Science. 1972 Apr 14;176(4031):189–191. doi: 10.1126/science.176.4031.189. [DOI] [PubMed] [Google Scholar]
- Franklin G. I., Potter L. T. Studies of the binding of -bungarotoxin to membrane-bound and detergent-dispersed acetylcholine receptors from Torpedo electric tissue. FEBS Lett. 1972 Nov 15;28(1):101–106. doi: 10.1016/0014-5793(72)80687-2. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. Evidence for a Poisson distribution of miniature end-plate potentials and some implications. Nature. 1965 Oct 23;208(5008):395–396. doi: 10.1038/208395a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fambrough D. M. Acetylcholine receptors. Distribution and extrajunctional density in rat diaphragm after denervation correlated with acetylcholine sensitivity. J Gen Physiol. 1972 Sep;60(3):248–262. doi: 10.1085/jgp.60.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Karlsson E., Eaker D., Rydén L. Purification of a presynaptic neurotoxin from the venom of the australian tiger snake Notechis scutatus scutatus. Toxicon. 1972 Jun;10(4):405–413. doi: 10.1016/0041-0101(72)90066-9. [DOI] [PubMed] [Google Scholar]
- Karlsson E., Heilbronn E., Widlund L. Isolation of the nicotinic acetylcholine receptor by biospecific chromatography on insolubilized Naja naja neurotoxin. FEBS Lett. 1972 Nov 15;28(1):107–111. doi: 10.1016/0014-5793(72)80688-4. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Chang C. C. Modes of actions of purified toxins from elapid venoms on neuromuscular transmission. Mem Inst Butantan. 1966;33(2):555–572. [PubMed] [Google Scholar]
- Lester H. A. Postsynaptic action of cobra toxin at the myoneural junction. Nature. 1970 Aug 15;227(5259):727–728. doi: 10.1038/227727a0. [DOI] [PubMed] [Google Scholar]
- Meldrum B. S. Actions of whole and fractionated indian cobra (naja naja) venom on skeletal muscle. Br J Pharmacol Chemother. 1965 Aug;25(1):197–205. doi: 10.1111/j.1476-5381.1965.tb01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J. C., Olsen R. W., Menez A., Fromageot P., Boquet P., Changeux J. P. Some physical properties of the cholinergic receptor protein from Electrophorus electricus revealed by a tritiated alpha-toxin from Naja nigricollis venom. Biochemistry. 1972 Mar 28;11(7):1200–1210. doi: 10.1021/bi00757a014. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molinoff P., Potter L. T. Isolation of the cholinergic receptor protein of Torpedo electric tissue. Nature. 1971 Feb 19;229(5286):554–557. doi: 10.1038/229554a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Mechanism of action of tetrodotoxin and saxitoxin on excitable membranes. Fed Proc. 1972 May-Jun;31(3):1124–1132. [PubMed] [Google Scholar]
- OTSUKA M., NONOMURA Y. The action of phenolic substances on motor nerve endings. J Pharmacol Exp Ther. 1963 Apr;140:41–45. [PubMed] [Google Scholar]
- Raftery M. A., Schmidt J., Clark D. G. Specificity of -bungarotoxin binding to Torpedo californica electroplax. Arch Biochem Biophys. 1972 Oct;152(2):882–886. doi: 10.1016/0003-9861(72)90285-8. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Schmidt J., Clark D. G., Wolcott R. G. Demonstration of a specific -bungarotoxin binding component in electrophorus electricus electroplax membranes. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1622–1629. doi: 10.1016/0006-291x(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. Use of affinity chromatography for acetylcholine receptor purification. Biochem Biophys Res Commun. 1972 Oct 17;49(2):572–578. doi: 10.1016/0006-291x(72)90449-4. [DOI] [PubMed] [Google Scholar]


