Abstract
1. The effects of phenoxyalkyltrimethylammonium and triethylammonium bromides on the frog rectus preparation and on the isolated guinea-pig ileum in the presence of hexamethonium have been compared with those of analogous phenylalkyltrimethylammonium and triethylammonium bromides. Affinity constants have been measured when possible.
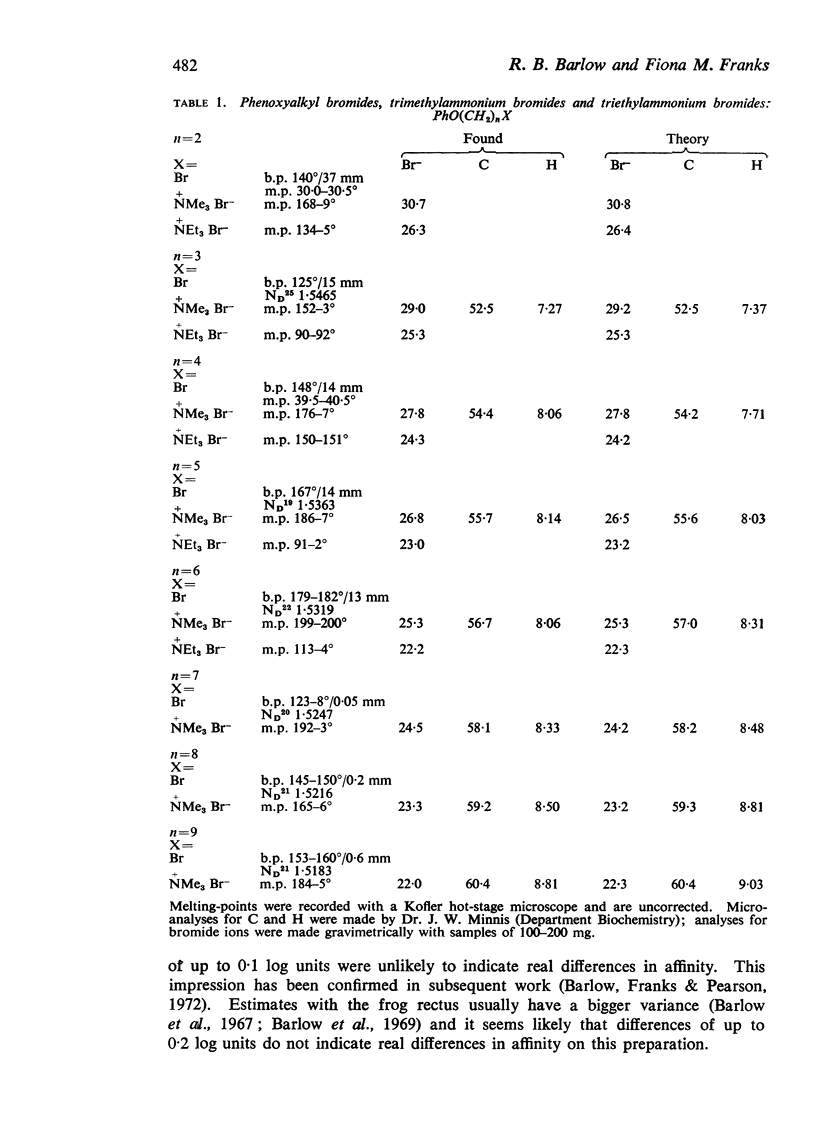
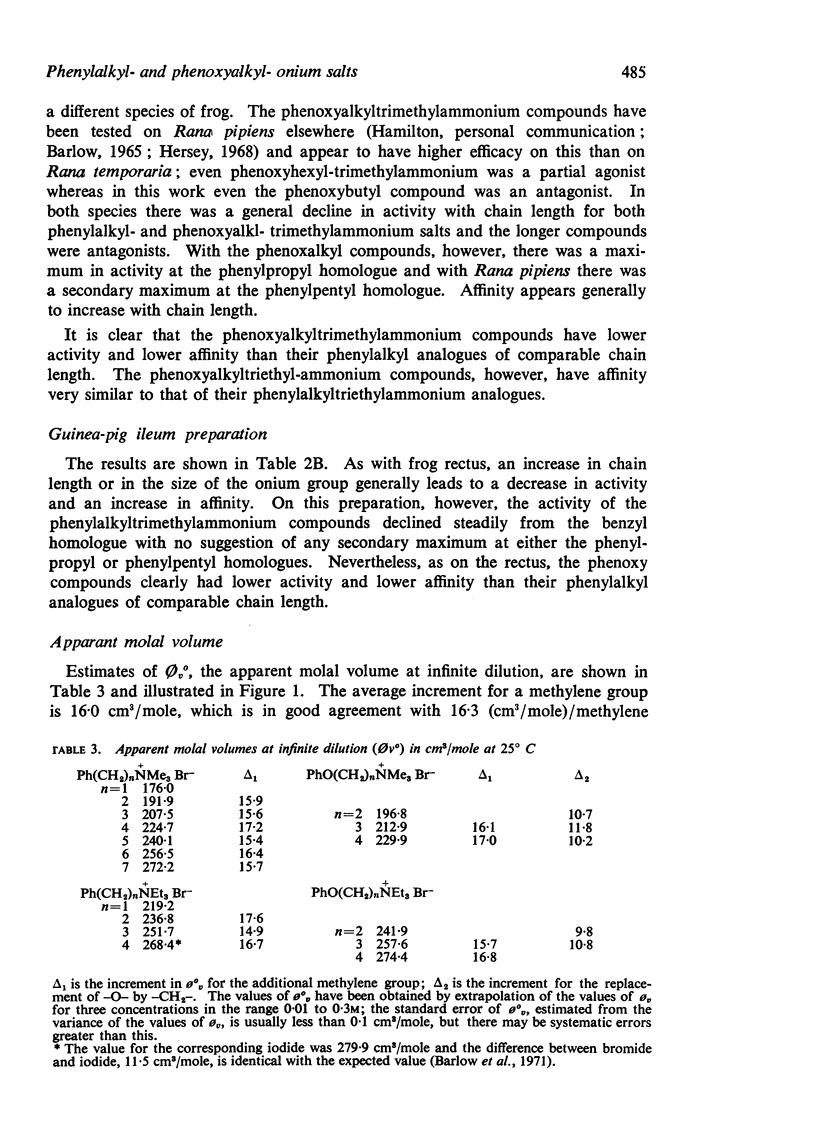
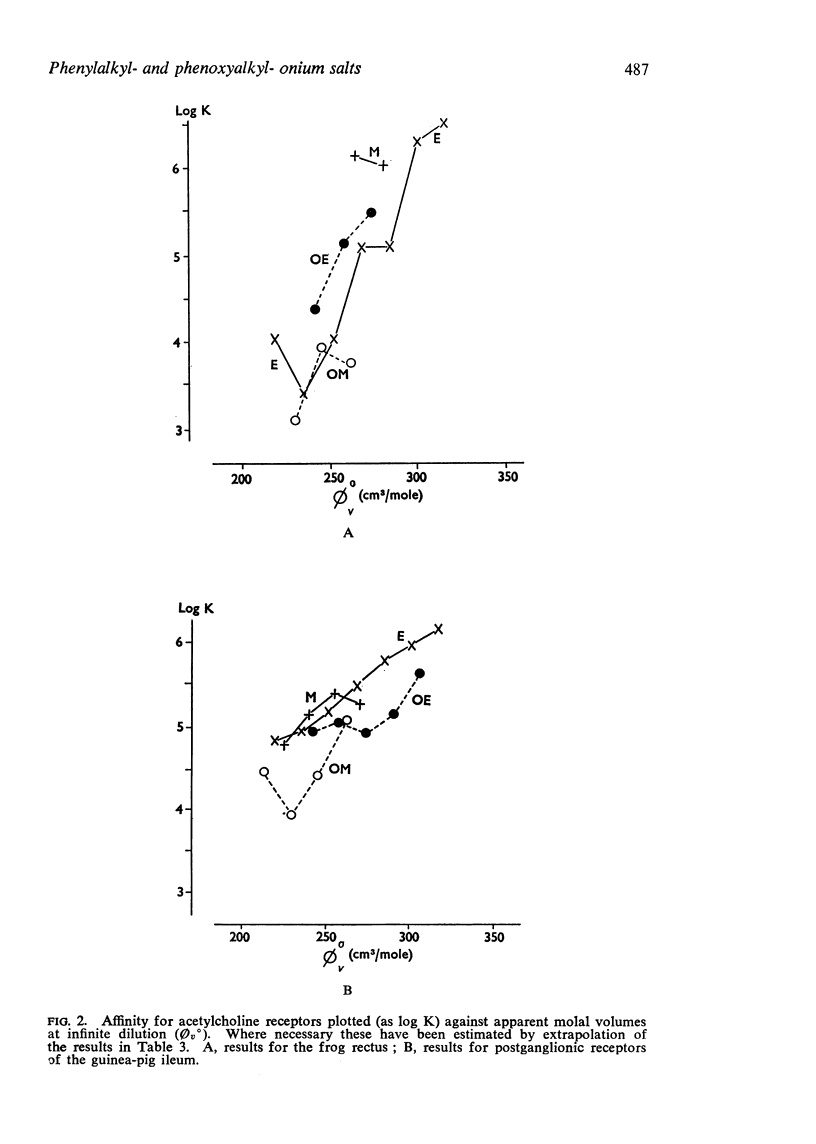
2. The apparent molal volumes at infinite dilution of some of the compounds have been measured from estimates of density made with an Anton Paar precision density meter.
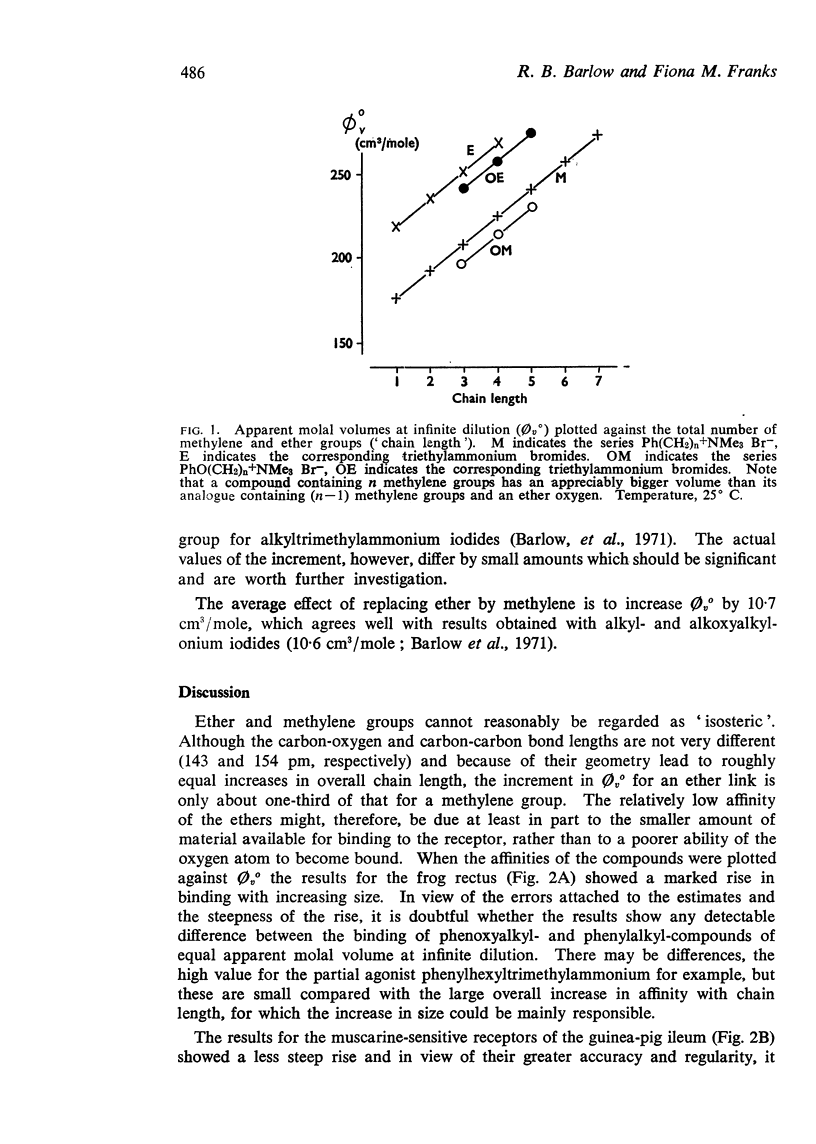
3. An ether oxygen occupies at infinite dilution in water only about one-third of the volume occupied by a methylene group.
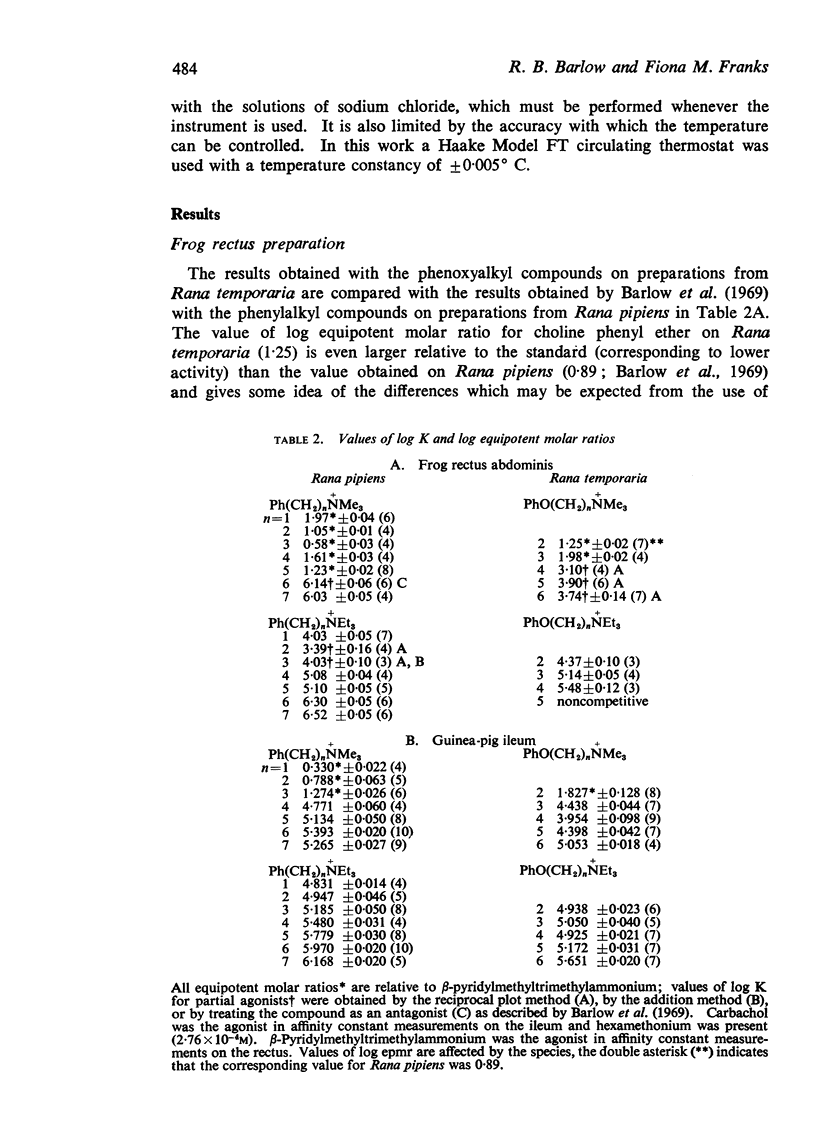
4. The replacement of methylene by ether oxygen reduces nicotine-like activity and affinity for nicotine-sensitive receptors. The reduction in affinity may be partly due to the decrease in size.
5. The replacement of methylene by ether oxygen reduces activity and affinity at muscarine-sensitive receptors but in some compounds there is a bigger reduction in affinity than would be expected simply from the reduction in size.
6. It is suggested that the affinity of these compounds largely depends on hydrophobic bonding and that effects on water structure in the environment of the receptor may also be involved in the actions of agonists.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson F. B., Barlow R. B., Mustafa M. G., Stephenson R. P. Relationships between chemical structure and affinity for acetylcholine receptors. Br J Pharmacol. 1969 Sep;37(1):207–233. doi: 10.1111/j.1476-5381.1969.tb09539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW R. B., SCOTT K. A., STEPHENSON R. P. AN ATTEMPT TO STUDY THE EFFECTS OF CHEMICAL STRUCTURE ON THE AFFINITY AND EFFICACY OF COMPOUNDS RELATED TO ACETYLCHOLINE. Br J Pharmacol Chemother. 1963 Dec;21:509–522. doi: 10.1111/j.1476-5381.1963.tb02019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Franks F. M., Pearson J. D. A comparison of the affinities of antagonists for acetylcholine receptors in the ileum, bronchial muscle and iris of the guinea-pig. Br J Pharmacol. 1972 Oct;46(2):300–312. doi: 10.1111/j.1476-5381.1972.tb06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Lowe B. M., Pearson J. D., Rendall H. M., Thompson G. M. Ion size and activity at acetylcholine receptors. Mol Pharmacol. 1971 Jul;7(4):357–366. [PubMed] [Google Scholar]
- Barlow R. B., Scott N. C., Stephenson R. P. The affinity and efficacy of onium salts on the frog rectus abdominis. Br J Pharmacol Chemother. 1967 Sep;31(1):188–196. doi: 10.1111/j.1476-5381.1967.tb01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow R. B., Thompson G. M., Scott N. C. The affinity and activity of compounds related to nicotine on the rectus abdominis muscle of the frog (Rana pipiens). Br J Pharmacol. 1969 Nov;37(3):555–584. doi: 10.1111/j.1476-5381.1969.tb08496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. R., Dawes P. M., Williams S. G. Relationship between the molecular conformation of choline aryl ethers and nicotine-like stimulant activity. Br J Pharmacol Chemother. 1968 Jan;32(1):113–126. doi: 10.1111/j.1476-5381.1968.tb00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEY P. On relationships between structure and nicotine-like stimulant activity in choline esters and ethers. Br J Pharmacol Chemother. 1952 Mar;7(1):117–129. doi: 10.1111/j.1476-5381.1952.tb00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


