Abstract
1. The effects of centrally acting drugs on the uptake of 3H-γ-aminobutyric acid (GABA) by slices of rat cerebral cortex have been studied.
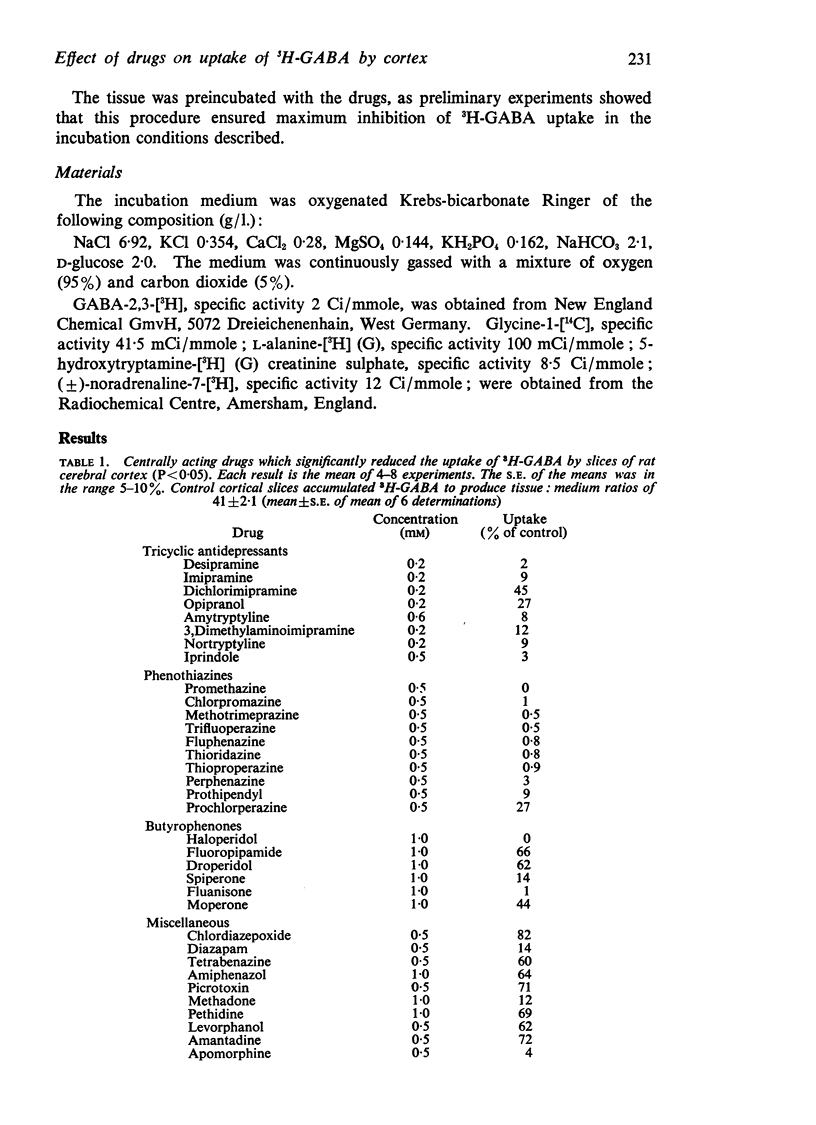
2. Many centrally acting drugs at concentrations of 0·1-1·0 mM significantly inhibited the uptake of 3H-GABA by cortical slices, but the only classes of drugs in which all members consistently produced inhibition of uptake were the phenothiazines, tricyclic antidepressants, and butyrophenones.
3. The receptor blocking drugs; phentolamine, propranolol, thymoxamine, mepyramine, and diphenhydramine at concentrations of 0·5-1 mM also significantly reduced the uptake of 3H-GABA. However, atropine, hexamethonium and (+)-tubocurarine had little effect on the uptake of 3H-GABA by cortical slices.
4. Centrally acting drugs, which did not significantly inhibit 3H-GABA uptake, included barbiturates, local anaesthetics, hallucinogens, monoamine oxidase inhibitors, anticonvulsants, and convulsants (except picrotoxin).
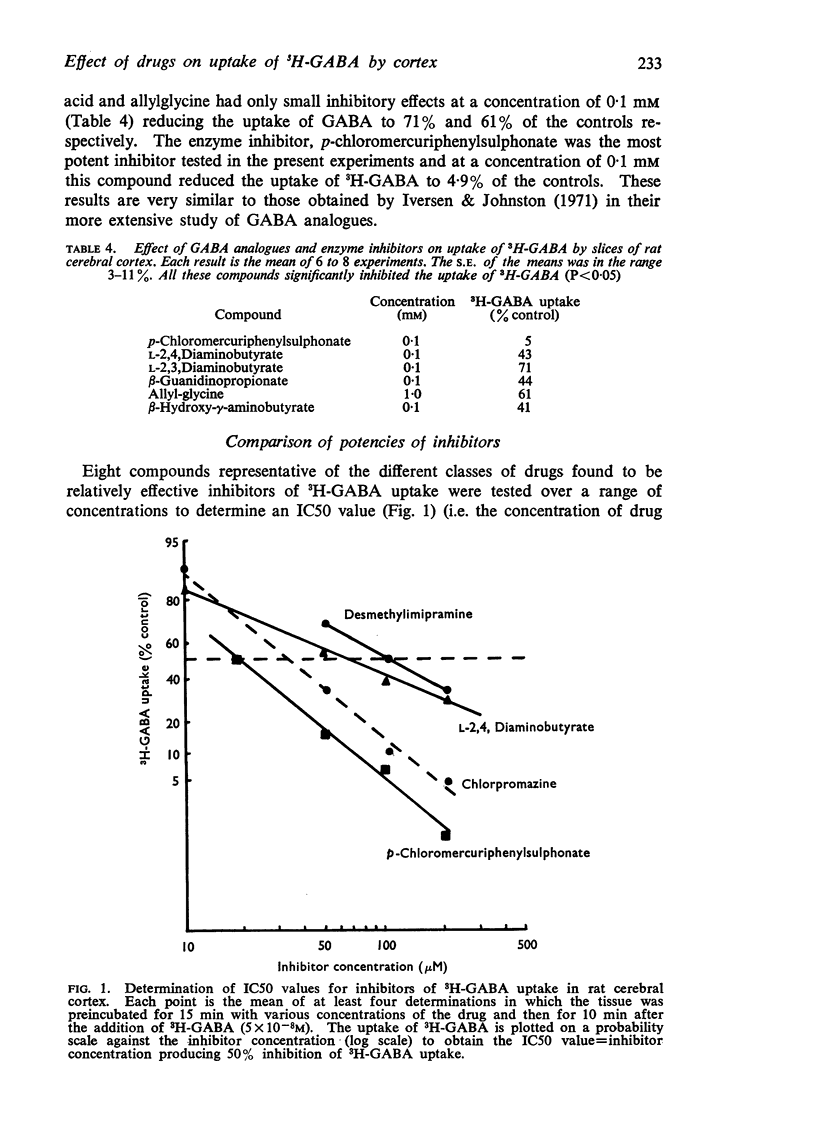
5. Chlorpromazine, prochlorperazine, L-2,4,diaminobutyric acid, desmethylimipramine, and iprindole inhibited the uptake of 3H-GABA by 50% (IC50) at concentrations of 30-100 μM. The most potent inhibitor of 3H-GABA uptake was p-chloromercuriphenylsulphonate (IC50 = 18 μM).
6. With the exception of L-2,4,diaminobutyric acid, an outstanding characteristic of these drugs was their complete lack of specificity. Thus at the IC50 for GABA, p-chloromercuriphenylsulphonate, chlorpromazine, prochlorperazine, iprindole, desmethylimipramine, apomorphine and diphenylhydramine also inhibited the uptake of radioactive glycine, alanine, noradrenaline, and 5-hydroxytryptamine. The uptake of the latter two compounds was often inhibited to a greater extent than GABA, glycine and alanine.
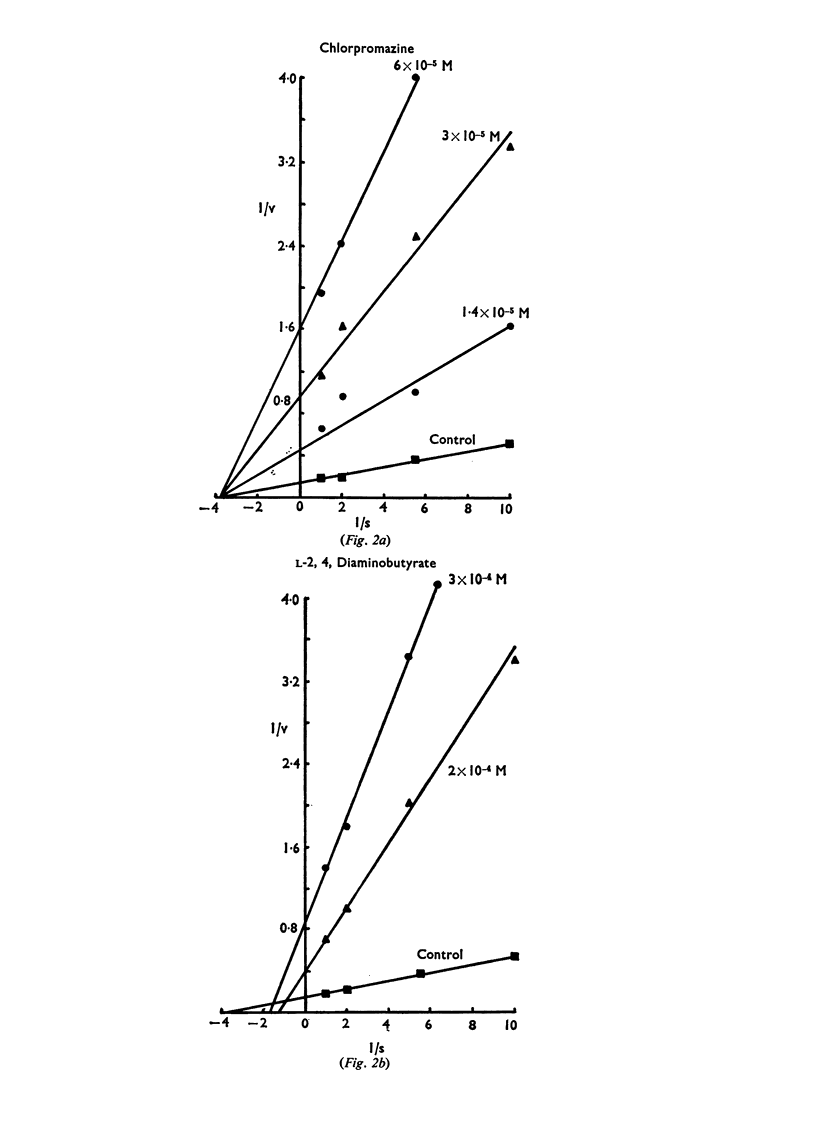
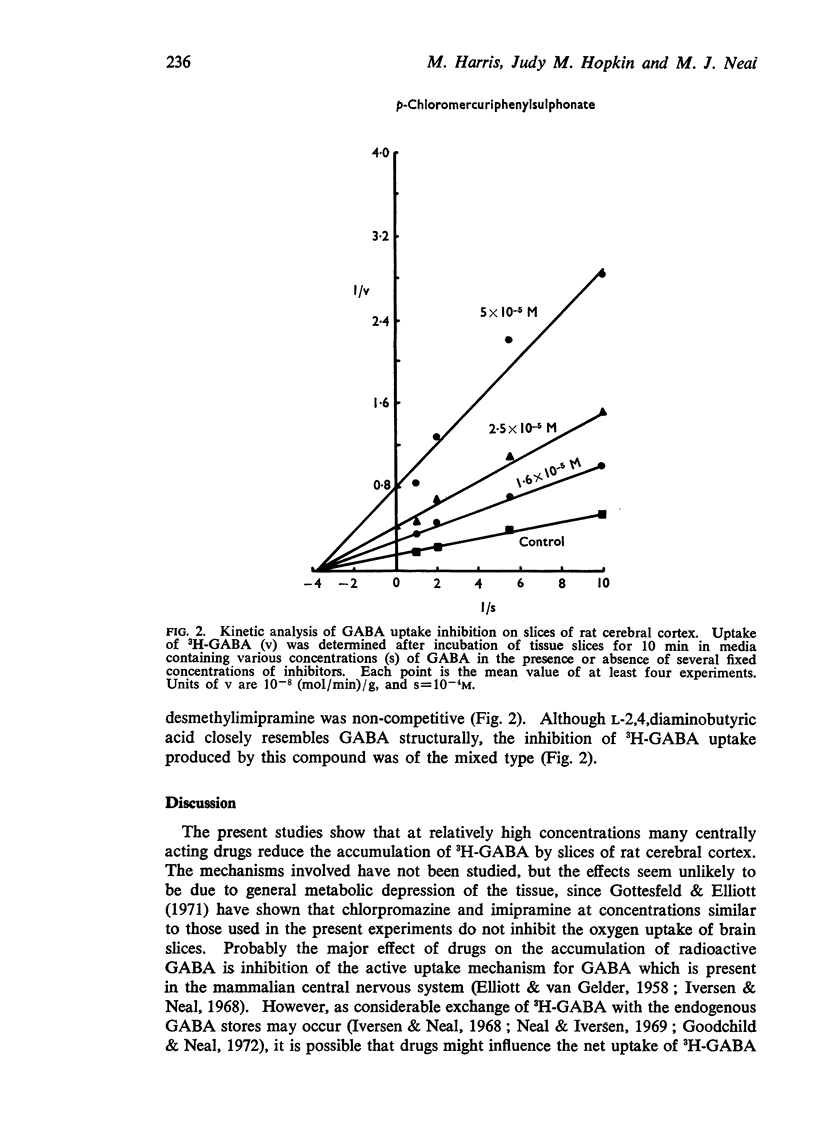
7. Kinetic analysis indicated that the inhibition of 3H-GABA by p-chloromercuriphenylsulphonate, chlorpromazine, and desmethylimipramine was noncompetitive. L-2,4,Diaminobutyric acid reduced the uptake of 3H-GABA by a `mixed' type of inhibition.
8. The present results do not support the suggestion that some centrally acting drugs may produce their effects by reducing the uptake of GABA in the brain after its release from inhibitory nerve terminals. Conceivably, the design of compounds which interfere effectively with the mechanisms of GABA operated synapses may lead to the introduction of whole new groups of centrally acting drugs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. E., Iversen L. L. Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography. Nature. 1971 Feb 26;229(5287):628–630. doi: 10.1038/229628a0. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960 Sep;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Johnston G. A. The inactivation of extracellularly administered amino acids in the feline spinal cord. Exp Brain Res. 1970 Jun 25;10(5):447–462. doi: 10.1007/BF00234262. [DOI] [PubMed] [Google Scholar]
- ELLIOTT K. A., VAN GELDER N. M. Occlusion and metabolism of gamma-aminobutyric acid by brain tissue. J Neurochem. 1958 Oct;3(1):28–40. doi: 10.1111/j.1471-4159.1958.tb12606.x. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Falck B. Autoradiography of some suspected neurotransmitter substances: GABA glycine, glutamic acid, histamine, dopamine, and L-dopa. Brain Res. 1971 Oct 8;33(1):157–172. doi: 10.1016/0006-8993(71)90314-3. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z., Elliott K. A. Factors that affect the binding and uptake of gaba by brain tissue. J Neurochem. 1971 May;18(5):683–690. doi: 10.1111/j.1471-4159.1971.tb11998.x. [DOI] [PubMed] [Google Scholar]
- Harris M., Hopkin J., Neal M. J. Proceedings: Effect of centrally acting drugs on the uptake of gamma-aminobutyric acid by the brain. Br J Pharmacol. 1972 Feb;44(2):339P–340P. [PMC free article] [PubMed] [Google Scholar]
- Hebb C. CNS at the cellular level: identity of transmitter agents. Annu Rev Physiol. 1970;32:165–192. doi: 10.1146/annurev.ph.32.030170.001121. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A. Cellular localization of labeled gamma-aminobutyric acid (3H-GABA) in rat cerebellar cortex: an autoradiographic study. Brain Res. 1970 Sep 16;22(3):391–396. doi: 10.1016/0006-8993(70)90480-4. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Johnston G. A. GABA uptake in rat central nervous system: comparison of uptake in slices and homogenates and the effects of some inhibitors. J Neurochem. 1971 Oct;18(10):1939–1950. doi: 10.1111/j.1471-4159.1971.tb09600.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kravitz E. A. The metabolism of gamma-aminobutyric acid (GABA) in the lobster nervous system--uptake of GABA in the nerve-muscle preparations. J Neurochem. 1968 Jul;15(7):609–620. doi: 10.1111/j.1471-4159.1968.tb08960.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Iversen L. L. Glycine uptake in rat central nervous system slices and homogenates: evidence for different uptake systems in spinal cord and cerebral cortex. J Neurochem. 1971 Oct;18(10):1951–1961. doi: 10.1111/j.1471-4159.1971.tb09601.x. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Autoradiographic localization of 3 H-GABA in rat retina. Nat New Biol. 1972 Feb 16;235(59):217–218. doi: 10.1038/newbio235217a0. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Subcellular distribution of endogenous and (3H) gamma-aminobutyric acid in rat cerebral cortex. J Neurochem. 1969 Aug;16(8):1245–1252. doi: 10.1111/j.1471-4159.1969.tb05972.x. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Pickles H. G. Uptake of 14C glycine by spinal cord. Nature. 1969 May 17;222(5194):679–680. doi: 10.1038/222679a0. [DOI] [PubMed] [Google Scholar]
- Neal M. J. The uptake of [14C]glycine by slices of mammalian spinal cord. J Physiol. 1971 May;215(1):103–117. doi: 10.1113/jphysiol.1971.sp009460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K., Ito M., Ochi R., Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp Brain Res. 1967;4(1):43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Ross S. B., Renyi A. L., Ogren S. O. A comparison of the inhibitory activities of iprindole and imipramine on the uptake of 5-hydroxytryptamine and noradrenaline in brain slices. Life Sci I. 1971 Nov 15;10(22):1267–1277. doi: 10.1016/0024-3205(71)90325-0. [DOI] [PubMed] [Google Scholar]
- SANO K., ROBERTS E. Binding of gama-aminobutyric acid by mouse brain preparations. Biochem Pharmacol. 1963 May;12:489–502. doi: 10.1016/0006-2952(63)90228-4. [DOI] [PubMed] [Google Scholar]
- Shaskan E. G., Snyder S. H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970 Nov;175(2):404–418. [PubMed] [Google Scholar]


