Abstract
Septal infusions of glucose exacerbate memory deficits produced by co-infusions of drugs that increase γ-aminobutyric acid (GABA)A receptor activity. To further understand the interaction between glucose and GABA, this experiment tested whether glucose would also potentiate spatial working memory deficits produced by septal infusions of the GABAB receptor agonist baclofen. Fifteen minutes prior to assessing spontaneous alternation (SA), male Sprague–Dawley derived rats were given septal infusions of vehicle, glucose (33 nmol), baclofen (0.1 nmol), or glucose combined with baclofen in one solution. Septal co-infusions of glucose with baclofen, at doses that individually had no effect, significantly impaired SA. Thus, the memory-impairing effects of glucose are observed with either GABAA or GABAB receptor ligands. This raises the possibility that glucose may impair memory by increasing synaptic levels of GABA and subsequent activation of these different receptor subtypes. These effects of glucose could contribute to the memory-impairing effects of hyperglycemia.
Keywords: Septum, Glucose, GABA, Spontaneous alternation, Memory
Extensive evidence indicates that modest increases in blood glucose levels enhance memory (Korol & Gold, 1998; Messier, 2004). More limited evidence suggests that increases in blood glucose levels can also produce memory deficits. For example, memory deficits are associated with streptozotocin-induced experimental diabetes (Gispen & Biessels, 2000) and diabetes in humans (Allen, Frier, & Strachan, 2004). Although diabetes is a complex disorder and memory deficits likely result from a number of causes, degree of hyperglycemia is often the best predictor of the memory dysfunction associated with diabetes (e.g., Reaven, Thompson, Nahum, & Haskins, 1990; U’Ren, Riddle, Lezak, & Bennington-Davis, 1990). Moreover, some studies have shown that correcting glycemic levels with oral hypoglycemic agents reverses memory deficits in diabetic patients (Gradman, Laws, Thompson, & Reaven, 1993; Meneilly, Cheung, Tessier, Yakura, & Tuokko, 1993). These latter findings suggest that the memory deficits may not result completely from chronic processes, and that hyperglycemia in particular may be a contributing cause. There are also a few reports indicating that acute administration of glucose impairs memory in normal subjects. For example, in rats, Gold, Vogt, and Hall (1986) showed that a single postraining systemic injection of glucose impaired inhibitory avoidance retention and Rodriguez, Horne, Mondragon, and Phelps (1994) showed a similar effect in an active avoidance task. Likewise, Craft and colleagues showed in humans that acute administration of glucose can impair memory (Craft et al., 1993; Craft, Murphy, & Wemstrom, 1994).
One mechanism that may contribute to these contrasting positive and negative effects of glucose is the neurotransmitter systems that are engaged at the time of glucose elevation. For example, infusions of glucose into the medial septum (MS) reverse spontaneous alternation (SA) deficits induced by morphine, galanin, or lemakalim (Ragozzino, Parker, & Gold, 1992; Stefani & Gold, 1998; Stefani, Nicholson, & Gold, 1999). In contrast to these positive effects, glucose produces deficits when co-infused into the MS with GABA agonists. Specifically, septal co-infusions of sub- effective doses of glucose with the GABAA agonist/partial GABAC agonist muscimol or the benzodiazepine (BZD) GABAA receptor modulator chlordiazepoxide (CDP) impair SA performance (Krebs & Parent, 2004; Shah & Parent, 2004). GABAA and GABAB receptors are present in the MS (Bowery, Hudson, & Price, 1987; Gao, Hornung, & Fritschy, 1995). GABA-C receptors are predominantly located in areas involved in vision (Alakuijala, Palgi, Wegelius, Schmidt, & Enz, 2005), and to the best of our knowledge, there are no reports indicating that GABA-C receptors are present in the medial septum of the adult rat. As a result, the findings with muscimol and CDP suggest that the impairing interaction between glucose and GABA involves the GABAA receptor subtype.
The findings with the GABA receptor ligands raise the possibility that the impairing effects of hyperglycemia could involve an influence of glucose on the GABA neurotransmitter system. This interaction between glucose and GABA could occur at the level of the GABAA receptor or could involve an influence of glucose on synaptic levels of GABA. The latter possibility is supported by extensive data indicating that glucose can increase GABA levels, including brain extracellular levels measured with in vivo microdialysis (Amoroso, Schmid-Antomarchi, Fosset, & Lardunski, 1990; During, Leone, Davis, Kerr, & Sherwin, 1995; Fink & Gothert, 1993). If the memory-impairing effects of glucose involve an increase in extracellular GABA levels, then glucose should also potentiate the memory-impairing effects of a GABAB receptor agonist. In contrast, if the memory-impairing effects of glucose involve a specific influence of glucose on GABAA receptor function, then glucose should not potentiate memory deficits produced by a GABAB receptor agonist. To test this hypothesis, we examined the effects of co-infusing glucose with the GABAB receptor agonist baclofen into the MS on SA. The purpose of Experiment 1 was to determine the dose–response properties of septal infusions of baclofen on SA. The goal of Experiment 2 was to test whether septal infusions of glucose would potentiate the memory-impairing effects of baclofen as it does with muscimol and CDP. Specifically, we determined whether combining glucose with the highest sub-effective dose of baclofen would produce SA deficits.
Fifty-eight (n = 6–16 per group; Experiment 1) and 36 (n = 6–15 per group; Experiment 2) male Sprague–Dawley derived rats weighing 200–250 g upon arrival (Charles River, Wilmington, MA) were used. The rats were housed individually on a 12 h light–dark cycle (lights on at 7:00 a.m.) with food and water ad libitum. The Georgia State University Institutional Animal Care and Use Committee (IACUC) approved all procedures involving rats.
At least 1 week after arrival, the rats were anesthetized with 3% isoflurane gas delivered in medical grade oxygen at 1000 mL/min. Anesthesia was maintained with 2% insoflurane gas in 500 mL/min oxygen. Rats were given injections of atropine (0.4 mg/kg, IP; Henry Schein, Denver, PA) and penicillin (1500 units, IM, Crystiben), and stereotaxic surgical procedures (David Kopf Instruments, Tujunga, CA) were used to implant one 22-gauge stainless-steel guide cannula (Plastics One, Roanoke, VA). The cannula was aimed at the MS (10° angle to avoid the sagittal sinus; 0.5 mm anterior [AP] and 0.9 mm lateral [ML] to bregma, 4.9 mm ventral to dura [DV]). The angle of the cannula was counterbalanced across hemispheres. After surgery, the rats were given an injection of 0.9 % sterile saline (3.0 cc, SC) and kept under a warm lamp until recovery from anesthesia.
Behavioral testing occurred at least 1 week after surgery. In Experiment 1, rats were given septal infusions of vehicle (phosphate-buffered saline [PBS], 0.5 μL, 0.5 μL/min, pH = 7.4), or (±) baclofen (0.05, 0.1, 1.0, 2.0, or 3.0 nmol; Sigma). In Experiment 2, rats were given septal infusions of vehicle, glucose (33 nmol), (±) baclofen (0.1 nmol), or glucose co-administered with baclofen in one solution. The dose of glucose was selected based on previous experiments showing that this dose alone does not affect memory, but produces deficits when co-infused with sub-effective doses of muscimol or CDP (Krebs & Parent, 2004; Shah & Parent, 2003, 2004). The 0.1 nmol dose of baclofen was selected on the basis of the results of Experiment 1 showing that this was the highest dose that did not affect SA (i.e., maximum sub-effective dose).
Fifteen minutes after the infusions, SA performance was assessed. SA is a behavior that is dependent on the septohippocampal system, and it is assumed to be a measure of spatial working memory (Lalonde, 2002). This assumption is supported by the finding that SA is impaired by removing directional cues or by increasing the interval between arm choices (Dember, 1989; Lalonde, 2002). SA was assessed by placing each rat in a Y-maze composed of three equally spaced arms (60°; 60 cm long × 17.5 cm high). The floor of each arm was composed of stainless steel (3.5 cm wide) and the top (14 cm wide) was covered with a translucent plexiglass lid. The rats were placed in the same starting arm of the Y-maze and allowed to explore the maze for 8 min. The sequence and number of arms the rats entered were recorded. The maze was cleaned with 70% EtOH after each rat was tested. The number of arms each rat entered was used as a measure of activity. A percent alternation score was computed for all rats that entered at least 10 arms. An alternation was defined as entering three different arms consecutively. The percent alternation score was computed by dividing the number of alternations each rat made by the number of arms entered minus two (i.e., the number of alternations possible in a three-armed maze) and then multiplying that resulting quotient by 100.
After behavioral testing, the rats were euthanized with an overdose of sodium pentobarbital (400 mg/kg, ip) and perfused intracardially with 0.9% saline followed by 10% formalin. Their brains were removed and stored in 10% formalin for at least 2 days before they were sectioned (60 μm) on a cryostat (Leica CM 3050 S). The sections were stained with thionin and an unbiased observer determined the cannulae placement using a light microscope (Olympus BX41). Acceptable MS cannulae placement was defined as an injection tip located within the MS, but not within the lateral septum. The approximate locations of septal infusions are shown in Fig. 1. The data were expressed as means and standard errors of the mean (SEM) and analyzed using a one-way univariate analysis of variance and Tukey HSD post hoc tests. An alpha level of 0.05 was used as the criterion for statistical significance.
Fig. 1.
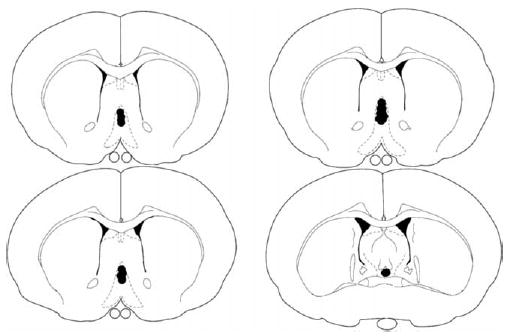
Schematic illustration of coronal sections of the rat brain showing the approximate location of MS infusion sites. Atlas plates were adapted from Paxinos and Watson, 1986).
The results of the Experiment 1 indicated that septal infusions of the GABAB receptor agonist baclofen significantly impaired SA performance in a dose-dependent manner [F(5,57) = 5.222; p < .01; see Fig. 2]. Specifically, the percent alternation scores of rats given the 1.0, 2.0, and 3.0 nmol doses of baclofen were significantly lower than those of rats given vehicle (p < 0.05). There were no differences between the SA scores of rats given vehicle and those given the lower doses of (±) baclofen (0.05 and 0.1 nmol; p > .05).
Fig. 2.
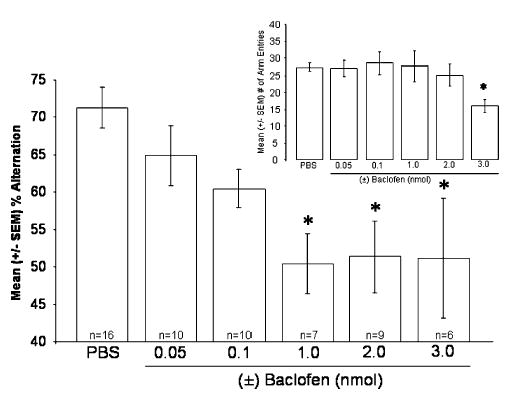
Effects of septal infusions of the GABAB receptor agonist baclofen on mean percent alternation scores (±SEM). Septal infusions of baclofen decreased percent alternation scores in a dose-dependent manner (* p < 0.05 vs. vehicle). Inset: Effects of the same treatments on mean number of arm entries (±SEM). Septal infusions of the highest dose of baclofen (3 nmol) decreased the number of arms the rats entered in the maze (*p < 0.05 vs. vehicle).
There was a tendency for the septal infusions of baclofen to decrease the number of arms the rats entered in the maze [F(5,57) = 2.295; p= .059; see Fig. 2 inset]. The post hoc analyses indicated that this tendency was due to the fact that the rats given the highest 3.0 nmol dose entered fewer arms than did those given vehicle (p < .05; see Fig. 2). The other doses did not affect the number of arms the rats entered in the maze (p > .05 vs. vehicle).
Importantly, the results of Experiment 2 showed that septal co-infusions of glucose with baclofen significantly affected SA performance [F(3,35) = 5.16; p < .01; see Fig. 3]. The SA scores of rats given septal co-infusions of baclofen with glucose were significantly lower than those of rats given infusions of vehicle alone (p < 0.01). Glucose and baclofen did not affect SA when administered separately. That is, there were no differences between the SA scores of rats given infusions of vehicle, glucose alone, or baclofen alone (p > 0.05). The impairing interaction between glucose and baclofen on SA was not likely due to an effect on activity levels, because the infusions did not significantly affect the number of arms the rat entered in the maze [F(3,35) = 1.06; p > .05; see Fig. 3 inset].
Fig. 3.
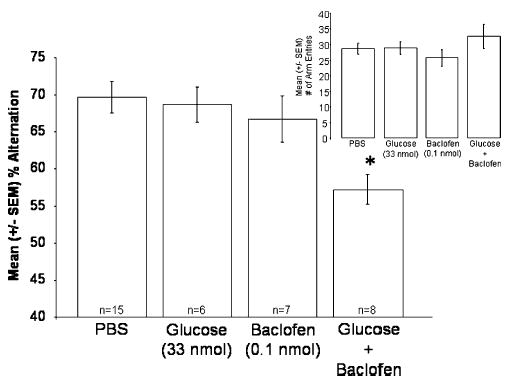
Effects of septal co-infusions of glucose with baclofen on mean percent alternation scores (±SEM). Septal infusions of glucose combined with baclofen, at doses that had no effect when infused alone, decreased percent alternation scores (* p < 0.01 vs. vehicle rats). Inset: The same manipulations did not affect the mean number of arm entries (±SEM).
The present findings demonstrate that septal infusions of the GABAB agonist baclofen significantly impair SA performance in a dose-dependent manner. These findings are congruent with previous research showing that septal GABAB receptor activation impairs spatial working memory in the radial arm maze (Stackman & Walsh, 1994). More importantly, the data show that septal co-infusions of glucose with a sub-effective dose of baclofen impair SA. These findings are consistent with past findings showing that septal infusions of glucose produce memory deficits hen co-infused with the GABAA agonist, partial GABAC agonist muscimol or the BZD agonist CDP (Krebs & Parent, 2004; Shah & Parent, 2003, 2004). Thus, these findings show that the impairing effects of glucose are not restricted to the GABAA receptor subtype. The finding that glucose potentiates the memory-impairing effects of drugs that influence either the GABAA or GABAB receptor is important given the differences between these receptor subtypes. The GABAA receptor is typically located on post-synaptic sites and is linked to a chloride-gated ionophore. In contrast, the GABAB receptor can be located on both pre- and post-synaptic sites and is coupled to calcium or potassium channels via g-proteins and second messenger systems (Martin & Olsen, 2000). One parsimonious hypothesis that can account for the fact that co-infusions of glucose with either a GABAA or GABAB receptor agonist impairs memory is that glucose is influencing the neurotransmitter that binds to these different receptor subtypes. For instance, glucose could increase septal GABA release, which would, in turn, summate with the effects of the sub-effective doses of either GABA agonist to produce memory deficits. This hypothesis is supported by extensive evidence showing that glucose can indeed increase GABA levels in other brain regions (Amoroso et al., 1990; During et al., 1995; Fink & Gothert, 1993). There are several mechanisms through which glucose could increase synaptic GABA levels. For example, glucose serves as a precursor for GABA biosynthesis and may also regulate the activity of glutamic acid decarboxylase (GAD), the enzyme that regulates GABA synthesis (Martin & Tobin, 2000). In other regions of the brain, such as the substantia nigra, glucose increases GABA release by inhibiting ATP-sensitive potassium channels (K+ATP; Amoroso et al., 1990; During et al., 1995). It is unclear, though, how K+ATP channels could participate in the negative effects of glucose on memory, given previous research showing that K+ATP channels likely participate in the memory-enhancing effects of elevating glucose in the MS (Stefani & Gold, 1998; Stefani et al., 1999). In cortex, glucose increases GABA release through a process that involves hyperosmolarity (Fink & Gothert, 1993). However, we have shown that hyperosmolarity is not involved in the memory-impairing effects of glucose, because equiosmolar concentrations of other sugars do not mimic the memory-impairing interaction between glucose and the GABA receptor agonist muscimol (Shah & Parent, 2003, 2004).
In summary, the present findings show that septal co-infusions of glucose with the GABAB receptor agonist baclofen, at doses that individually have no effect, significantly impair SA, a measure of spatial working memory. These findings add to the growing body of evidence showing that there is a synergistic, memory-impairing interaction between glucose elevation in the MS and septal GABA receptor activation. Combined with previous evidence, these findings raise the possibility that the negative effects of hyperglycemia on memory may involve an increase in GABA release. Future research should be aimed at determining whether elevating glucose in the septum does indeed increase GABA levels, and whether drugs that impair GABA synthesis or release also prevent the memory-impairing effects of glucose.
Acknowledgments
This research was supported in part by grants from NINDS-NIDDK-JDF (RO1NS41173-02) and the Center for Behavioral Neuroscience (STC Program of the National Science Foundation under Agreement No. IBN-9876754).
References
- Alakuijala A, Palgi M, Wegelius K, Schmidt M, Enz R, et al. GABA receptor ρ subunit expression in the developing rat brain. Developmental Brain Research. 2005;154:15–23. doi: 10.1016/j.devbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Allen KV, Frier BM, Strachan MW. The relationship between type 2 diabetes and cognitive function: longitudinal studies and their methodological limitations. European Journal of Pharmacology. 2004;490:167–175. doi: 10.1016/j.ejphar.2004.02.054. [DOI] [PubMed] [Google Scholar]
- Amoroso S, Schmid-Antomarchi H, Fosset M, Lardunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247(4944):852–854. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987;20:365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Craft S, Dagogo-Jack S, Wiethop BV, Murphy C, Nevins RT, Fleischman S, et al. *Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: A longitudinal study. Behavioral Neuroscience. 1993;107:926–940. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- Craft S, Murphy C, Wemstrom J. Glucose effects and glucose regulation in memory and nonmemory tasks: The influence of age, sex, regulation, and glucoregulatory response. Psychobiology. 1994;22:95–105. [Google Scholar]
- Dember WN. Spontaneous alternation behavior. New York: Springer-Verlag; 1989. [Google Scholar]
- During MJ, Leone P, Davis KE, Kerr D, Sherwin RS. Glucose modulates rat substantia nigra GABA release in vivo via ATP-sensitive potassium channels. Journal of Clinical Investigation. 1995;95 (5):2403–2408. doi: 10.1172/JCI117935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Gothert M. High D-glucose concentrations increase GABA release but inhibit release of norepinephrine and 5-hydroxy-troptamine in rat cerebral cortex. Brain Research. 1993;618(2):220–226. doi: 10.1016/0006-8993(93)91269-x. [DOI] [PubMed] [Google Scholar]
- Gao B, Hornung JP, Fritschy JM. Identification of distinct GABAA-receptor subtypes in cholinergic and parvalbumin-positive neurons of the rat and marmoset medial septum-diagonal band complex. Neuroscience. 1995;65(1):101–117. doi: 10.1016/0306-4522(94)00480-s. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends in Neurosciences. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Gold PE, Vogt J, Hall JL. Glucose effects on memory: Behavioral and pharmacological characteristics. Behavioral and Neural Biology. 1986;46:145–155. doi: 10.1016/s0163-1047(86)90626-6. [DOI] [PubMed] [Google Scholar]
- Gradman TJ, Laws A, Thompson LW, Reaven GM. Verbal learning and/or memory improves with glycemic control in older subjects with non-insulin-dependent diabetes mellitus. Journal of the American Geriatric Society. 1993;41:1305–1312. doi: 10.1111/j.1532-5415.1993.tb06480.x. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Glucose, memory, and aging. The American Journal of Clinical Nutrition. 1998;67(4):764S–771S. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- Krebs DL, Parent MB. Septal co-infusions of glucose and the benzodiazepine chlordiazepoxide impair spontaneous alternation. San Diego, CA: Society for Neuroscience Abstracts; 2004. [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neuroscience and Biobehavioral Reviews. 2002;26(4):764S–771S. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Martin DL, Olsen RW, editors. GABA in the nervous system: The view at fifty years. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2000. [Google Scholar]
- Martin DL, Tobin AJ. Mechanisms controlling GABA synthesis and degradation in the brain. In: Martin DL, Olsen RW, editors. GABA in the nervous system: The view at fifty years. Philadelphia, PA: Lippincott, Williams, & Wilkins; 2000. pp. 25–41. [Google Scholar]
- Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. Journal of Gerontology. 1993;48:M117–M121. doi: 10.1093/geronj/48.4.m117. [DOI] [PubMed] [Google Scholar]
- Messier C. Glucose improvement of memory: A review. European Journal of Pharmacology. 2004;490(1–3):33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Springer-Verlag; 1986. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Parker ME, Gold PE. Spontaneous alternation and inhibitory avoidance impairments with morphine injections into the medial septum. Attenuation by glucose administration. Brain Research. 1992;597(2):241–249. doi: 10.1016/0006-8993(92)91480-3. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Thompson LW, Nahum D, Haskins E. Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;13:16–21. doi: 10.2337/diacare.13.1.16. [DOI] [PubMed] [Google Scholar]
- Rodriguez WA, Horne A, Mondragon AN, Phelps DD. Comparable dose–response functions for the effects of glucose and fructose on memory. Behavioral and Neural Biology. 1994;61:162–169. doi: 10.1016/s0163-1047(05)80070-6. [DOI] [PubMed] [Google Scholar]
- Shah AA, Parent MB. Septal infusions of glucose or pyruvate, but not fructose, produce avoidance deficits when co-infused with the GABA agonist muscimol. Neurobiology of Learning and Memory. 2003;79 (3):243–251. doi: 10.1016/s1074-7427(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Shah AA, Parent MB. Septal infusions of glucose or pyruvate with muscimol impair spontaneous alternation. Brain Research. 2004;996(2):250–256. doi: 10.1016/j.brainres.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Walsh TJ. Baclofen produces dose-related working memory impairments after intraseptal injection. Behavioral Neural Biology. 1994;61(2):181–185. doi: 10.1016/s0163-1047(05)80073-1. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Gold PE. Intra-septal injections of glucose and glibenclamide attenuate galanin-induced spontaneous alternation performance deficits in the rat. Brain Research. 1998;813(1):50–56. doi: 10.1016/s0006-8993(98)00876-2. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Nicholson GM, Gold PE. ATP-sensitive potassium channel blockade enhances spontaneous alternation performance in the rat: A potential mechanism for glucose-mediated memory enhancement. Neuroscience. 1999;93(2):557–563. doi: 10.1016/s0306-4522(99)00128-1. [DOI] [PubMed] [Google Scholar]
- U’Ren RC, Riddle MC, Lezak MD, Bennington-Davis M. The mental efficiency of the elderly person with type II diabetes mellitus. Journal of the American Geriatric Society. 1990;38:505–510. doi: 10.1111/j.1532-5415.1990.tb02398.x. [DOI] [PubMed] [Google Scholar]


