Abstract
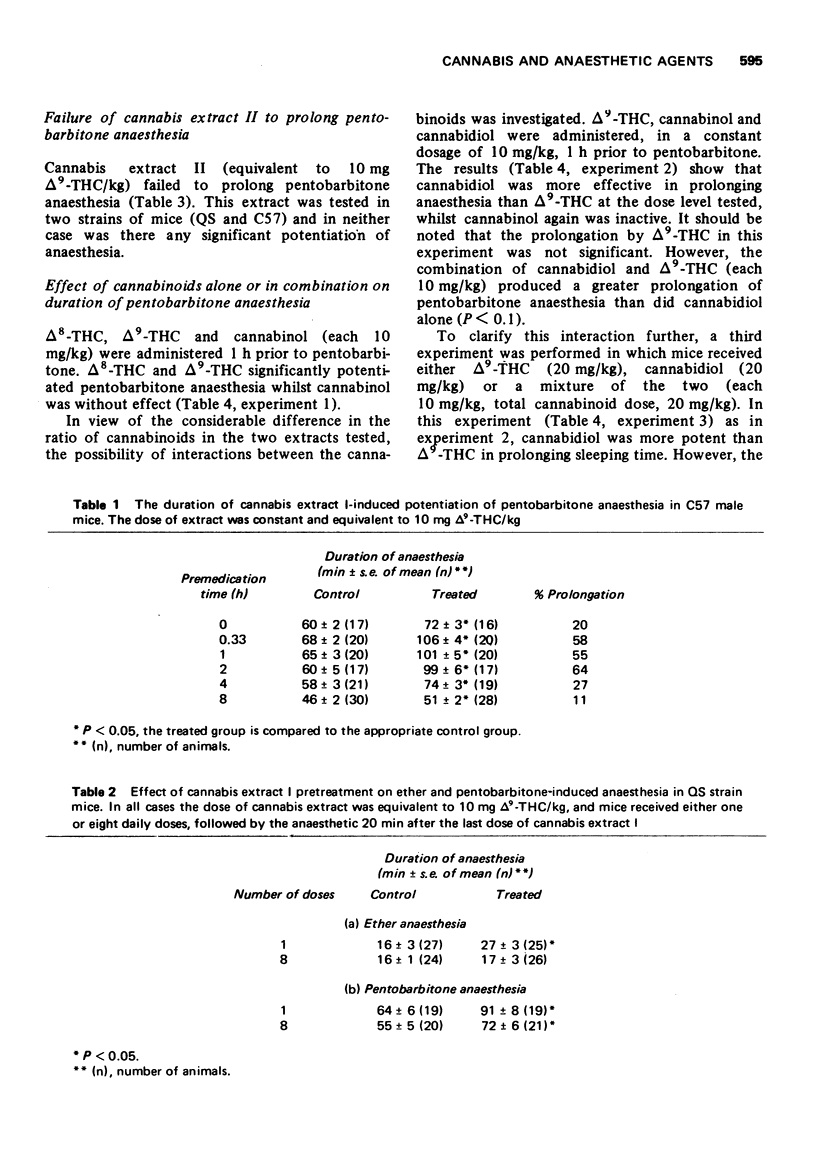
1 A cannabis extract (I) (in a concentration equivalent to 10 mg Δ9-tetrahydro-cannabinol(THC)/kg) prolonged pentobarbitone anaesthesia in mice maximally 20 min to 2 h after medication. The effect was still significant after 8 h, but less than at 2 hours.
2 The cannabis extract (I) (equivalent to 10 mg Δ9-THC/kg) prolonged both pentobarbitone and ether anaesthesia in mice when administered 20 min before the anaesthetic. After eight consecutive daily doses of cannabis, the pentobarbitone anaesthesia was still significantly longer than a control group, while ether anaesthesia was not significantly prolonged.
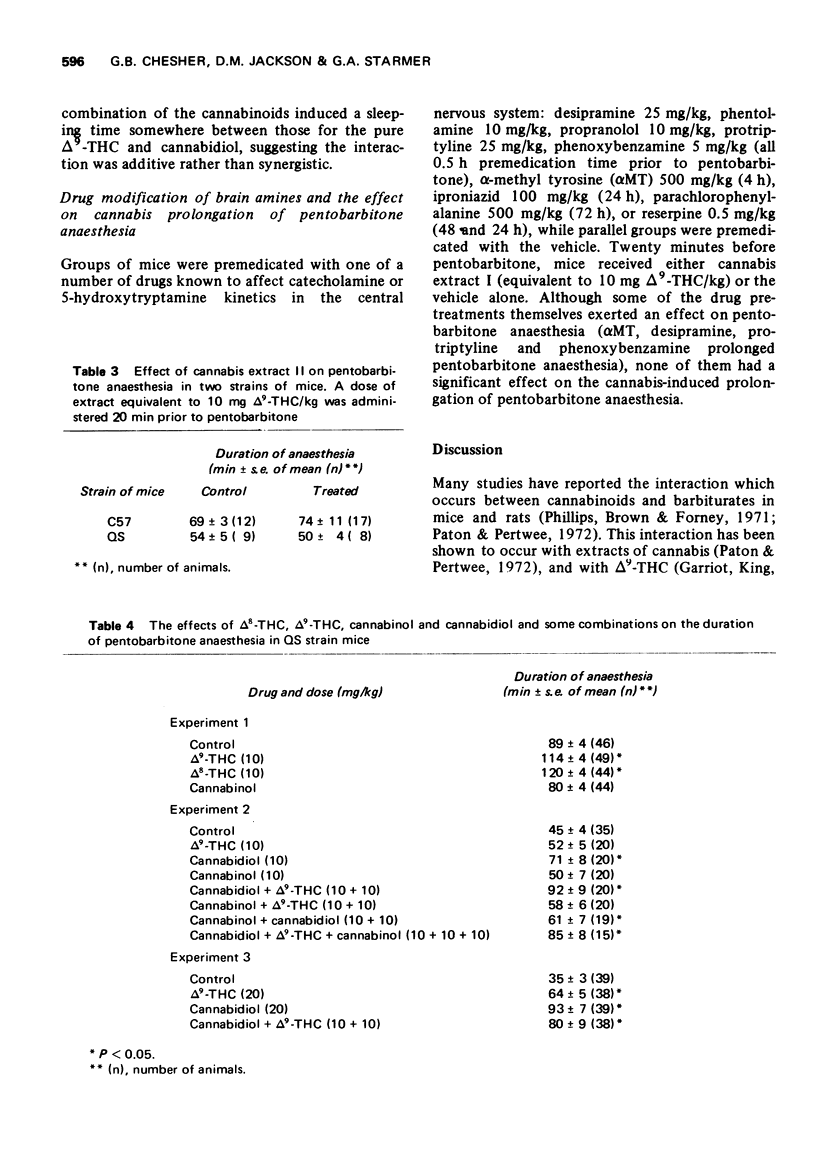
3 A second cannabis extract (II) with a different ratio of cannabinoids (also administered in dosage equivalent to 10 mg Δ9-THC/kg) failed to affect pentobarbitone anaesthesia in mice. This extract presented about 4% the dose of cannabidiol as extract I.
4 Δ8-THC, Δ9-THC and cannabidiol prolonged pentobarbitone anaesthesia with cannabidiol being generally more active than Δ9-THC. Cannabinol (10 mg/kg) was inactive.
5 The effects of cannabidiol and Δ9-THC were found to be additive, and there was a consistent trend for cannabinol to reduce the effectiveness of Δ9-THC and cannabidiol when given in combination.
6 Premedication with phenoxybenzamine, phentolamine, propranolol, iproniazid, protriptyline, desipramine, reserpine, α-methyl tyrosine or parachlorophenylalanine did not affect the extract I-induced prolongation of pentobarbitone anaesthesia.
7 It is concluded that cannabis may affect pentobarbitone and ether anaesthesia in mice at least partially by a direct depressant effect, and that the cannabis-induced prolongation of anaesthesia is probably unrelated to any effect on central 5-hydroxytryptamine or catecholamine neurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOSE B. C., SAIFI A. Q., BHAGWAT A. W. Effect of cannabis indica on hexobarbital sleeping time and tissue respiration of rat brain. Arch Int Pharmacodyn Ther. 1963 Feb 1;141:520–524. [PubMed] [Google Scholar]
- BOSE B. C., SAIFI A. Q., BHAGWAT A. W. OBSERVATIONS ON THE PHARMACOLOGICAL ACTIONS OF CANNABIS INDICA. II. Arch Int Pharmacodyn Ther. 1964 Jan 1;147:285–290. [PubMed] [Google Scholar]
- Carlsson A., Fuxe K., Hamberger B., Lindqvist M. Biochemical and histochemical studies on the effects of imipramine-like drugs and (+)-amphetamine on central and peripheral catecholamine neurons. Acta Physiol Scand. 1966 Jul-Aug;67(3):481–497. doi: 10.1111/j.1748-1716.1966.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Christensen H. D., Freudenthal R. I., Gidley J. T., Rosenfeld R., Boegli G., Testino L., Brine D. R., Pitt C. G., Wall M. E. Activity of delta8- and delta9-tetrahydrocannabinol and related compounds in the mouse. Science. 1971 Apr 9;172(3979):165–167. doi: 10.1126/science.172.3979.165. [DOI] [PubMed] [Google Scholar]
- Corrodi H., Hanson L. C. Central effects of an inhibitor of tyrosine hydroxylation. Psychopharmacologia. 1966;10(2):116–125. doi: 10.1007/BF00455973. [DOI] [PubMed] [Google Scholar]
- Garriott J. C., King L. J., Forney R. B., Hughes F. W. Effects of some tetrahydrocannabinols on hexobarbital sleeping time and amphetamine induced hyperactivity in mice. Life Sci. 1967 Oct 1;6(19):2119–2128. doi: 10.1016/0024-3205(67)90232-9. [DOI] [PubMed] [Google Scholar]
- Gill E. W., Paton W. D., Pertwee R. G. Preliminary experiments on the chemistry and pharmacology of cannabis. Nature. 1970 Oct 10;228(5267):134–136. doi: 10.1038/228134a0. [DOI] [PubMed] [Google Scholar]
- Holtzman D., Lovell R. A., Jaffe J. H., Freedman D. X. 1-delta9-tetrahydrocannabinol: neurochemical and behavioral effects in the mouse. Science. 1969 Mar 28;163(3874):1464–1467. doi: 10.1126/science.163.3874.1464. [DOI] [PubMed] [Google Scholar]
- Koe B. K., Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966 Dec;154(3):499–516. [PubMed] [Google Scholar]
- Krantz J. C., Jr, Berger H. J., Welch B. L. Blockade of (-)-trans- 9 -tetrahydrocannabinol depressant effect by cannabinol in mice. Am J Pharm Sci Support Public Health. 1971 Sep-Oct;143(5):149–152. [PubMed] [Google Scholar]
- Kubena R. K., Barry H., 3rd Interactions of delta-tetrahydrocannabinol with barbiturates and methamphetamine. J Pharmacol Exp Ther. 1970 May;173(1):94–100. [PubMed] [Google Scholar]
- Paton W. D., Pertwee R. G. Effect of cannabis and certain of its constituents on pentobarbitone sleeping time and phenazone metabolism. Br J Pharmacol. 1972 Feb;44(2):250–261. [PMC free article] [PubMed] [Google Scholar]
- Phillips R. N., Brown D. J., Forney R. B. Enhancement of depressant properties of alcohol or barbiturate in combination with aqueous suspended delta 9-tetrahydrocannabinol in rats. J Forensic Sci. 1971 Apr;16(2):152–161. [PubMed] [Google Scholar]
- Ross S. B., Renyi A. L. Inhibition of the uptake of tritiated catecholamines by antidepressant and related agents. Eur J Pharmacol. 1967 Dec;2(3):181–186. doi: 10.1016/0014-2999(67)90084-2. [DOI] [PubMed] [Google Scholar]
- SPECTOR S., SJOERDSMA A., UDENFRIEND S. BLOCKADE OF ENDOGENOUS NOREPINEPHRINE SYNTHESIS BY ALPHA-METHYL-TYROSINE, AN INHIBITOR OF TYROSINE HYDROXYLASE. J Pharmacol Exp Ther. 1965 Jan;147:86–95. [PubMed] [Google Scholar]
- WHITTLE B. A. THE USE OF CHANGES IN CAPILLARY PERMEABILITY IN MICE TO DISTINGUISH BETWEEN NARCOTIC AND NONNARCOTIC ALALGESICS. Br J Pharmacol Chemother. 1964 Apr;22:246–253. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


