Abstract
1 In order to establish the origin of the increased efflux of radioactivity caused by electrical stimulation of cerebral cortical slices which had been incubated with [3H]-choline, labelled choline and acetylcholine (ACh) collected by superfusion were separated by gold precipitation.
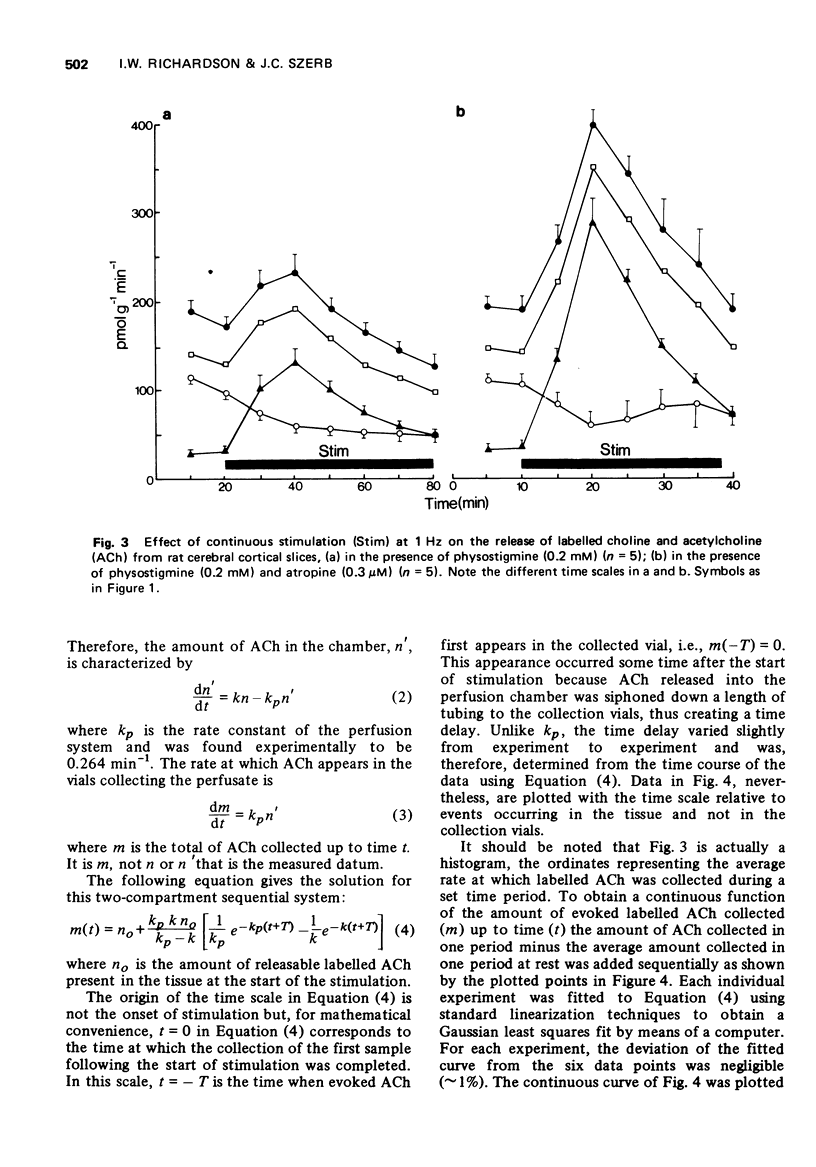
2 In the presence of physostigmine electrical stimulation (1 Hz, 10 min) increased the release of only [3H]-ACh which was greatly enhanced by the addition of atropine.
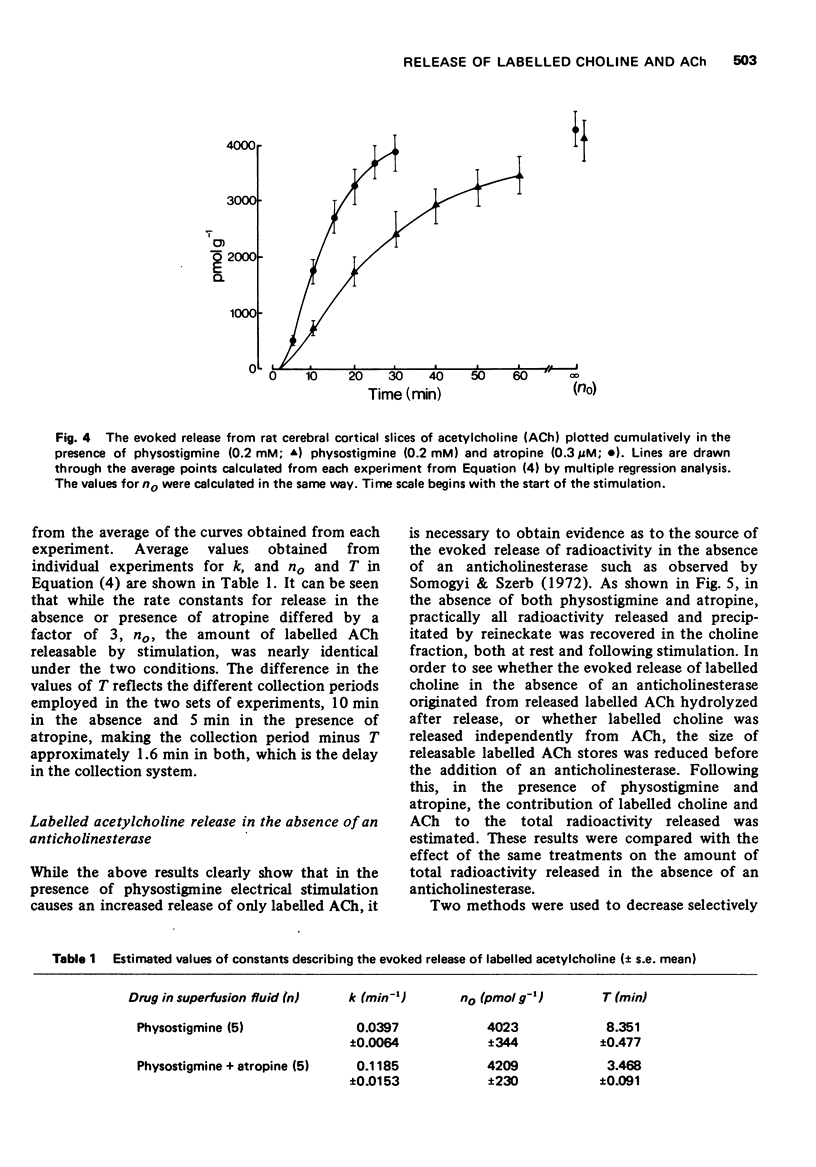
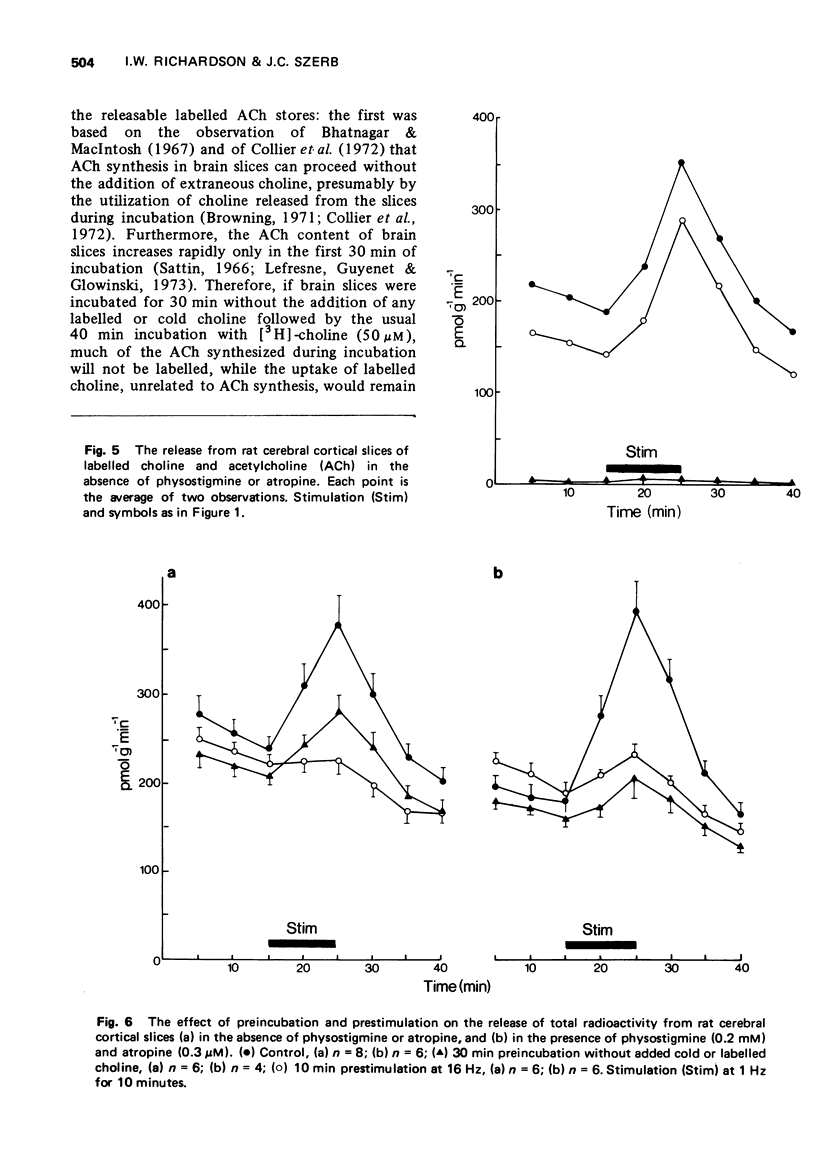
3 Continuous stimulation in the presence of physostigmine resulted in an evoked release of [3H]-ACh which declined asymptotically. This evoked release appeared to follow first-order kinetics with a rate constant which remained stable over the course of prolonged stimulation.
4 The rate constant for the evoked release of [3H]-ACh with 1 Hz stimulation was three times greater in the presence of physostigmine and atropine than in the presence of physostigmine alone, while the size of the store from which [3H]-ACh was released was nearly identical under these two conditions.
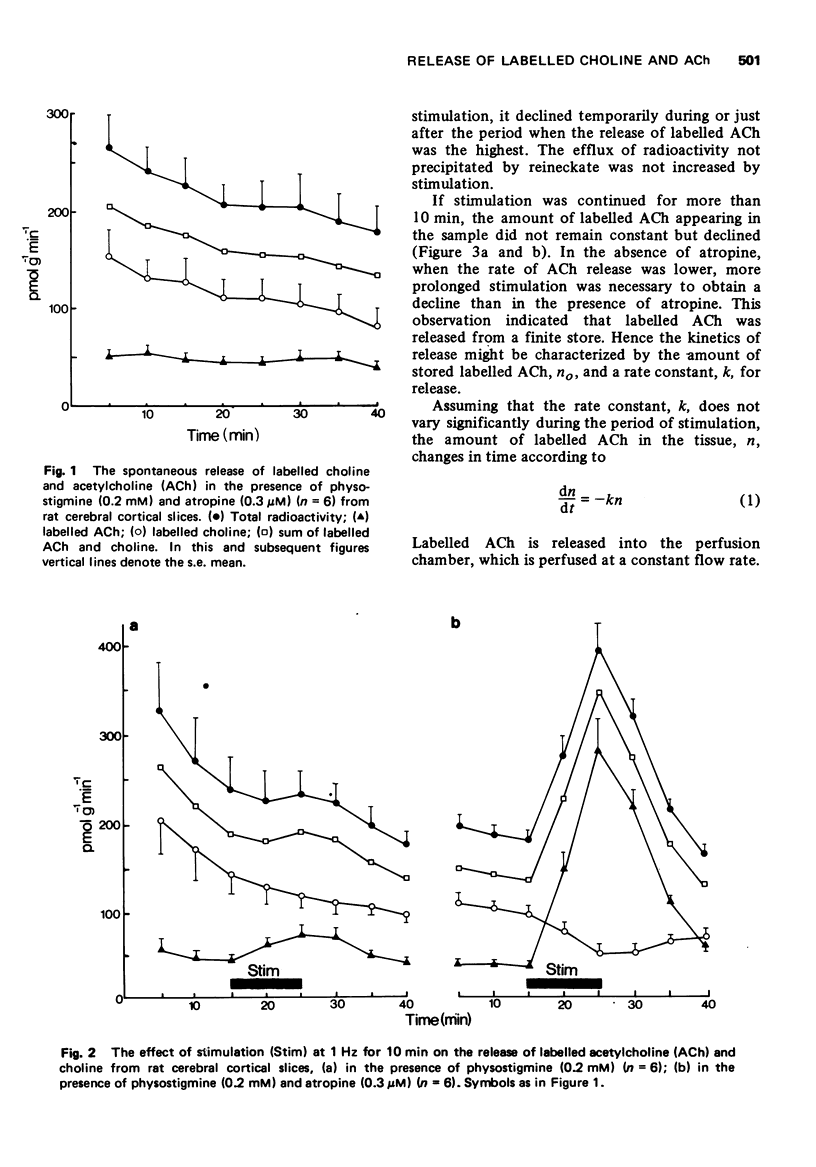
5 In the absence of physostigmine and atropine, stimulation caused the appearance of only [3H]-choline in the samples.
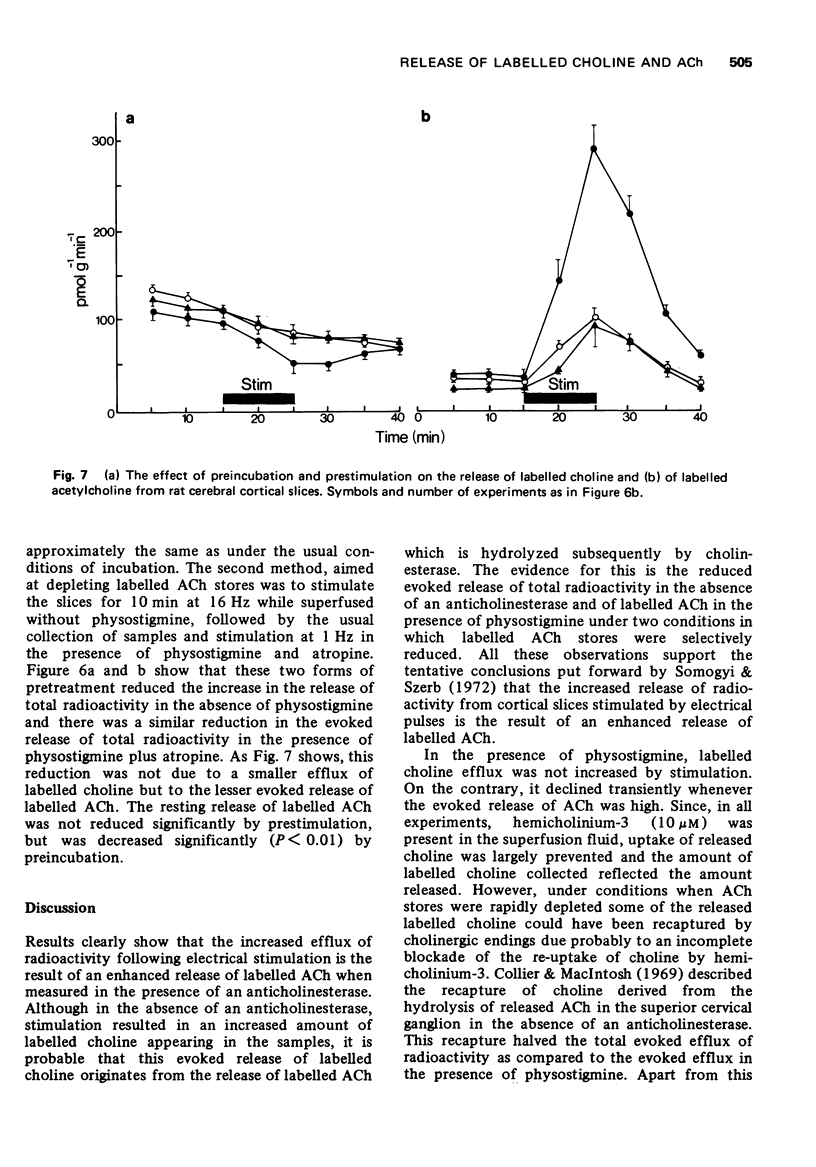
6 Reduction of [3H]-ACh stores before the application of physostigmine resulted in a reduced evoked release of total radioactivity, both in the absence or presence of physostigmine and atropine, and decreased the evoked release of [3H]-ACh without affecting the release of [3H]-choline.
7 Results suggest that electrical stimulation of cortical slices which had been incubated with [3H]-choline causes the release of only [3H]-ACh, both in the presence or absence of an anticholinesterase. The evoked increase in the efflux of total radioactivity is therefore a good measure of the release of [3H]-ACh.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatnagar S. P., MacIntosh F. C. Effects of quaternary bases and inorganic cations on acetylcholine synthesis in nervous tissue. Can J Physiol Pharmacol. 1967 Mar;45(2):249–268. doi: 10.1139/y67-028. [DOI] [PubMed] [Google Scholar]
- Bourdois P. S., Mitchell J. F., Somogyi G. T., Szerb J. C. The output per stimulus of acetylcholine from cerebral cortical slices in the presence or absence of cholinesterase inhibition. Br J Pharmacol. 1974 Dec;52(4):509–517. doi: 10.1111/j.1476-5381.1974.tb09718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning E. T. Free choline formation by cerebral cortical slices from rat brain. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1586–1590. doi: 10.1016/0006-291x(71)90202-6. [DOI] [PubMed] [Google Scholar]
- Collier B., Katz H. S. The synthesis, turnover and release of surplus acetylcholine in a sympathetic ganglion. J Physiol. 1971 May;214(3):537–552. doi: 10.1113/jphysiol.1971.sp009447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier B., Poon P., Salehmoghaddam S. The formation of choline and of acetylcholine by brain in vitro. J Neurochem. 1972 Jan;19(1):51–60. doi: 10.1111/j.1471-4159.1972.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Cooke W. J., Robinson J. D. Factors influencing choline movements in rat brain slices. Biochem Pharmacol. 1971 Sep;20(9):2355–2366. doi: 10.1016/0006-2952(71)90235-8. [DOI] [PubMed] [Google Scholar]
- Lefresne P., Guyenet P., Glowinski J. Acetylcholine synthesis from (2- 14 C)pyruvate in rat striatal slices. J Neurochem. 1973 Apr;20(4):1083–1097. doi: 10.1111/j.1471-4159.1973.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Molenarr P. C., Polak R. L., Nickolson V. J. Subcellular localization of newly-formed (3H)acetylcholine in rat cerebral cortex in vitro. J Neurochem. 1973 Sep;21(3):667–678. doi: 10.1111/j.1471-4159.1973.tb06011.x. [DOI] [PubMed] [Google Scholar]
- Sattin A. The synthesis and storage of acetylcholine in the striatum. J Neurochem. 1966 Jun;13(6):515–524. doi: 10.1111/j.1471-4159.1966.tb09866.x. [DOI] [PubMed] [Google Scholar]
- Szerb J. C., Somogyi G. T. Depression of acetylcholine release from cerebral cortical slices by cholinesterase inhibition and by oxotremorine. Nat New Biol. 1973 Jan 24;241(108):121–122. doi: 10.1038/newbio241121a0. [DOI] [PubMed] [Google Scholar]


