Abstract
We have identified expression of T-cell receptor γ chain (TCRγ) mRNA in human prostate and have shown that it originates from epithelial cells of the prostate and not from infiltrating T-lymphocytes. In contrast, the T-cell receptor δ chain (TCRδ) gene is silent in human prostate. The major TCRγ transcript in prostate has a different size than the transcript expressed in thymus, spleen, and blood leukocytes. It is expressed in normal prostate epithelium, adenocarcinoma of the prostate, and the prostatic adenocarcinoma cell line LNCaP. The RNA originates from an unrearranged TCRγ locus, and it is initiated within the intronic sequence directly upstream of the Jγ1.2 gene segment. The prostate-specific TCRγ transcript consists of the Jγ1.2 and Cγ1 gene segments, and it has an untranslated sequence including a polyadenylation signal and poly(A) sequence at the 3′end. The finding that prostate epithelial cells express a high level of a transcript from a gene that was thought to by exclusively expressed by T-lymphocytes is highly unexpected.
The database of expressed sequence tags (dbEST) is a division of GenBank that contains sequence data and other information on single-pass cDNA sequences, or expressed sequence tags (ESTs). The sequences in dbEST (http://www.ncbi.nlm.nih.gov/dbEST) originate from cDNA libraries, each of which is usually prepared from a particular cell type, organ, or tumor. We have previously described how the dbEST database can be used to identify genes that are specifically and highly expressed in the human prostate (1). In clustering of human prostate ESTs, we found many ESTs representing the untranslated 3′end of the T-cell receptor γ chain (TCRγ) but not ESTs representing the T-cell receptor δ chain (TCRδ), α chain (TCRα), or β chain (TCRβ). ESTs representing the TCRγ transcript were found both in normal prostate and prostate cancer libraries. The prostate expression was unexpected, leaving two possibilities open. The TCRγ transcript expressed in the prostate could originate from infiltrating γδ T-lymphocytes, or it could originate from other cell types in the prostate.
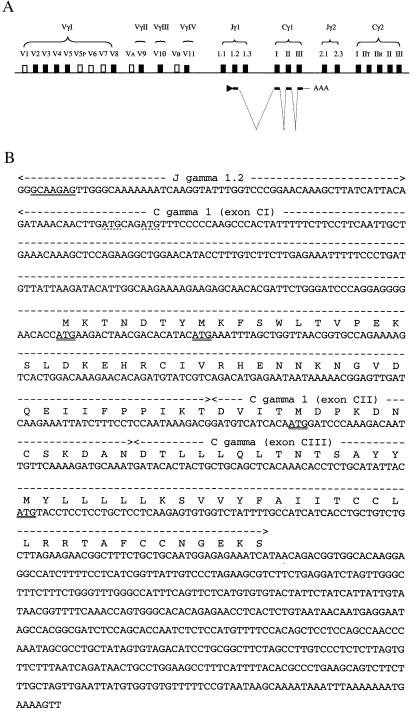
The human TCRγ locus is located on chromosome 7, p15-p14 (2, 3). The TCRγ gene is composed of variable (V), joining (J), and constant (C) gene segments that undergo a series of rearrangements to form functionally active genes in mature γδ T-lymphocytes. In the human germline, 14 Vγ gene segments have been identified and divided into four separate subgroups based on sequence homology (4, 5). There are five Jγ gene segments organized in two subgroups (Jγ1 and Jγ2) and two different Cγ genes (Cγ1 and Cγ2). The TCRγ gene is normally coexpressed together with TCRδ in γδ T-lymphocytes. Expression of TCR genes has so far been limited to cells from lymphoid tissues.
In this paper, we focused our studies on the nature of the full-length prostate TCRγ transcript and on determining whether the transcript originates from prostate cells or from γδ T-lymphocytes infiltrating the human prostate. We show that prostate-specific TCRγ transcripts are expressed in epithelial cells within the acinar ducts of the prostate as well as in prostate cancer and the prostatic adenocarcinoma cell line LNCaP. The predominant transcript is a TCR Jγ1.2-Cγ1 transcript that originates from an unrearranged TCRγ locus.
MATERIALS AND METHODS
RNA Dot Blot and Northern Blot Hybridizations.
RNA dot blot (RNA master blot, CLONTECH) and Northern blot analysis (MTN, CLONTECH) were performed on a variety of human tissues. Northern blot analysis also was performed on mRNA from prostate adenocarcinoma cell lines LNCaP and PC-3 (American Type Culture Collection). Isolation of poly(A) RNA was carried out by using the FastTrack kit (Invitrogen). RNA was electrophoresed on a 1% agarose gel and was transferred to nylon-based membranes (GeneScreen Plus, DuPont), according to established procedures (6). A cDNA probe specific for the untranslated 3′end [3′ untranslated region (3′UTR)] of the TCRγ transcript was made from EST plasmid ng79d11 (Genome Systems, St. Louis). A probe specific for the constant domain of the TCRγ transcript (TCR Cγ) was made from LNCaP cDNA, and a probe for the constant domain of the TCRδ transcript (TCR Cδ) was made from a TCRδ plasmid, donated by Ilan Kirch (Naval Hospital, Bethesda, MD). A human β-actin probe was used as a quantity control of the mRNA preparations. Probes were labeled with 32P by random primer extension (Lofstrand Laboratories, Gaithersburg, MD) to a specific activity of 1 μCi/ng (1 Ci = 37 GBq). The RNA membranes were blocked for 2 hours at 45°C in hybridization solution containing 50% formamide (Hybrisol I, Oncor, Gaithersburg, MD) and then were probed for 15 hours at 45°C with 20 μCi cDNA in 20 ml of hybridization solution. The membranes were washed twice for 15 minutes at room temperature in 2 × standard saline citrate (SSC)/0.1% SDS and twice for 20 minutes at 55–65°C in 0.1% SSC/0.1% SDS. The membranes were exposed to an imaging film (X-Omat, Kodak) at −80°C before development.
RNA in Situ Hybridization.
The TCRγ constant domain and the TCRγ untranslated 3′end nucleotide sequence is amplified by reverse transcriptase–PCR (RT-PCR) from LNCaP mRNA, were cloned into pBluescript II SK (+) (Stratagene), and were verified by DNA sequencing. Antisense and sense TCRγ 35S-riboprobes were made by T7 and T3 RNA polymerase, respectively. Paraffin blocks of eight archived cases of prostatic transurethral resection specimens from the National Cancer Institute were retrieved. Cases were selected that included both malignant and benign prostatic ducts. Average age of the cases was 69, and Gleason scores of the tumor ranged from 3 + 3 = 6/10 to 4 + 5 = 9/10. The blocks were processed on glass slides and were hybridized by using the riboprobes (Molecular Histology, Gaithersburg, MD). After hybridization, the slides were counterstained with hematoxylin and eosin and were examined by using a Zeiss Axiophot Microscope equipped with a variable condenser providing bright field and dark field.
RT-PCR Analysis.
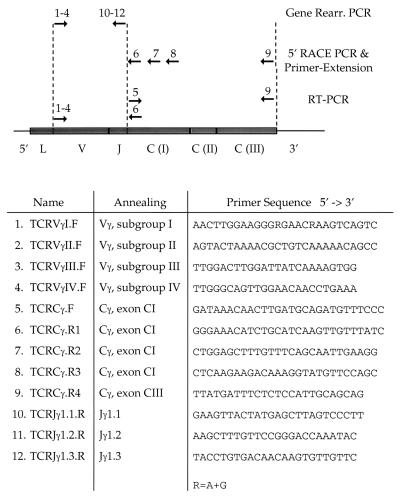
Single stranded cDNAs were prepared from 150–250 ng of LNCaP and PC-3 poly(A) mRNA, respectively, by using oligo(dT) priming (Amersham Pharmacia). PCR primers were designed to amplify different portions of the TCRγ transcript. To amplify cDNA only and not trace amounts of genomic DNA, which may be present in the mRNA preparations, primer pairs were always combined to generate PCR products spanning two or more exons. One PCR was set up to amplify either of the two TCRγ constant domain genes, Cγ1 or Cγ2, with a forward primer in exon CI (TCRCγ.F) and a reverse primer in exon CIII (TCRCγ.R4) (Table 1). Variable-to-constant domain-spanning PCRs were set up by using forward primers specific to each of the four subgroups of TCRγ variable gene segments (TCRVγI.F, TCRVγII.F, TCRVγIII.F, TCRVγIV.F) in combination with a reverse primer in the TCRγ constant gene segment (TCRCγ.R1) (Table 1). Wax-mediated, hot-start PCRs were conducted for 30 cycles by using high-fidelity PCR components (Expand, Boehringer-Mannheim). The PCR products were analyzed on 1.2% agarose gels with 0.5 μg/ml of ethidium bromide. Specific PCR products were gel purified (Qiagen, Chatsworth, CA), T/A cloned (Invitrogen), and sequenced on an automated capillary sequencer (Perkin–Elmer) by using Perkin–Elmer’s dRhodamine terminator cycle sequencing kit.
Table 1.
Primers (→) used for analysis of the prostate TCRγ transcript
Analysis of TCRγ VJ Gene Rearrangement.
Genomic DNA was prepared from 5 × 107 LNCaP cells according to established procedures (6). A set of 12 PCRs was performed, each with a forward primer from one of the four subgroup of Vγ gene segments (TCRVγI.F, TCRVγII.F, TCRVγIII.F, TCRVγIV.F) in combination with a reverse primer from one of the three Jγ1 gene segments (TCRJγ1.1.R, TCRJγ1.2.R, TCRJγ1.3.R) (Table 1). Hot start PCRs were conducted for 30 cycles by using 500 ng of genomic DNA, and the PCRs were examined on 1.2% agarose gels with 0.5 μg/ml of ethidium bromide. Human placenta DNA (CLONTECH) was used as a positive control of the primers, and PCR amplification of Jγ1.1 to Jγ1.2 genomic DNA was performed as a positive control of the template.
Primer-Extension Analysis of RNA.
The start point of the prostate TCRγ transcript was determined by primer-extension analysis of LNCaP mRNA. mRNA (5 μg) was mixed with 0.08 pmol of 32P-end labeled TCRCγ.R2 primer, annealing 48–75 nucleotides from the 5′end of Cγ1. The analysis was carried out by using 20 units of Moloney Murine Leukemia Virus (MMLV)–reverse transcriptase (Superscript, GIBCO/BRL), according to established procedure (7). The sample was electrophoresed on a 6% polyacrylamide-urea DNA sequencing gel in parallel with a 32P-end labeled molecular weight marker (MspI-digested pBR322, Lofstrand Laboratories). After electrophoresis, the gel was blotted to Whatman paper and was dried and subjected to autoradiography.
5′ Rapid Amplification of cDNA Ends (RACE) PCR Analysis.
Double-stranded cDNA was made from 500 ng of LNCaP poly(A) mRNA by using the Marathon cDNA amplification kit (CLONTECH) and 25 pmol of the TCRγ gene-specific primer (TCRCγ.R3) (Table 1). Marathon-adapters then were ligated to the ends of the synthesized cDNA. Rapid amplification of the 5′-cDNA ends (5′-RACE) PCR was conducted by using a gene-specific primer (TCRCγ.R2) (Table 1), annealing upstream of the primer used for reverse transcription, and an adapter-specific primer. Hot start conditions were applied (Advantage, CLONTECH), and the PCR products were analyzed and cloned as described for the RT-PCR analyses. DNA from the 5′-RACE PCR analytic gel was transferred to a nylon membrane, and a 32P-end labeled primer (TCRCγ.R1) (Table 1) hybridizing further upstream was applied to identify possible bands not detected by ethidium bromide/UV-light.
In Vitro Transcription-Coupled Translation.
The complete prostate TCRγ transcript, as obtained by RT-PCR and 5′-RACE PCR, was amplified by RT-PCR, was cloned into pBluescript II SK (+) (Stratagene), and was sequenced and examined in an in vitro transcription-coupled translation system, using T7 RNA polymerase and wheat germ extract (TNT, Promega). 35S-Met (ICN) was incorporated in the reaction for visualization of translated products. The reaction was analyzed under reducing condition on a polyacrylamide gel (16.5% Tris/Tricine, Bio-Rad) together with a pre-stained marker (GIBCO/BRL). The gel was dried and subjected to autoradiography.
RESULTS
Prostate ESTs Representing TCRγ Were Identified by Database Analysis.
We have identified 23 TCRγ ESTs, from 20 cDNA clones, derived from six tumor and two normal prostate cDNA libraries. The TCRγ composite sequence from assembly of prostate ESTs has 76 nucleotides of TCRγ constant domain sequence and 448 nucleotides of untranslated 3′ region sequence and poly(A) sequence. By alignment of the prostate ESTs to mature TCRγ transcripts from cell lines established from peripheral blood T-lymphocytes (GenBank accession nos. M16768, M16804, and M30894), we found that the prostate EST composite sequence is identical to the TCRγ transcript from peripheral blood T-lymphocytes. The dbEST database analysis indicates that the TCRγ gene is highly transcribed in human prostate.
Expression of TCRγ (3′UTR) in Human Prostate Verified by RNA Dot Blot.
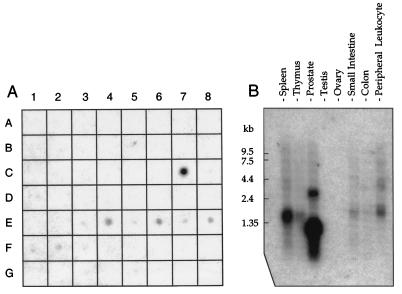
To analyze the transcriptional activity TCRγ gene in human prostate, a cDNA probe from the untranslated 3′end (3′UTR) of the TCRγ transcript was assayed on mRNA from 50 different human tissues (Fig. 1A). We verified that normal prostate (position C7) expresses TCRγ mRNA, and we further observed that prostate has by far the strongest expression of all tissues represented on the dot blot membrane. TCRγ gene expression also was found in small intestine (E3), spleen (E4), thymus (E5), peripheral leukocyte (E6), lymph node (E7), bone marrow (E8), and lung (F2).
Figure 1.
Hybridization analysis of TCRγ mRNA expression. (A) Multiple tissue dot blot showing differential expression of human TCRγ. Positive tissues are prostate (C7), small intestine (E3), spleen (E4), thymus (E5), peripheral leukocyte (E6), lymph node (E7), bone marrow (E8), and lung (F2). (B) Northern blot showing TCRγ transcript sizes in normal tissues. Two TCRγ transcripts expressed in prostate are 1.1 and 2.8 kb whereas the predominant transcript in spleen, thymus, and peripheral blood leukocytes is 1.5 kb. The film was exposed for 20 hours.
Northern Blot Shows Two Size-Specific TCRγ Transcripts in Human Prostate.
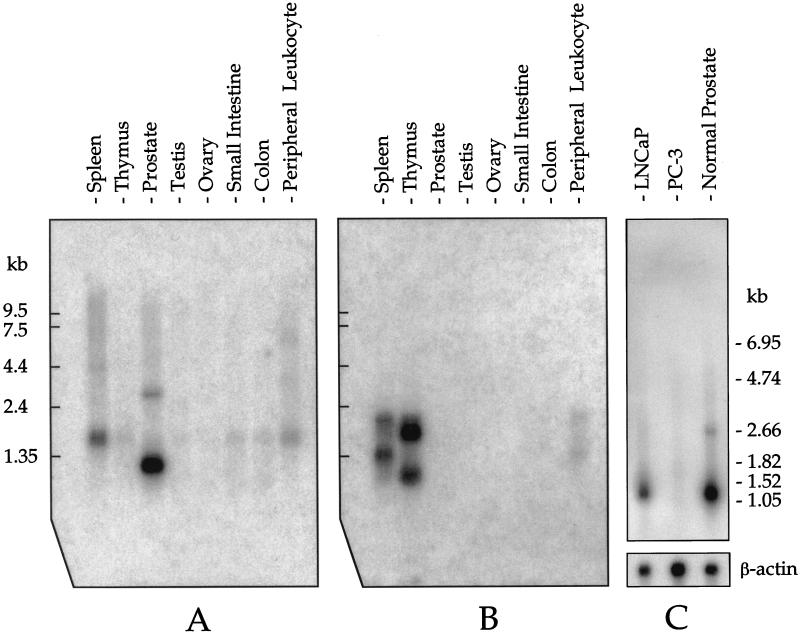
Northern blot hybridization using the 3′UTR probe revealed that prostate has two TCRγ transcripts of ≈1.1 and 2.8 kilobases (kb) (Fig. 1B, lane 3) whereas the predominant transcript in spleen, thymus, small intestine, and blood leukocytes is 1.5 kb. A transcript size of 1.5 kb is consistent with TCRγ mRNA from γδ T-lymphocytes [GenBank accession nos. M16768, M16804 (8), and M30894 (9)]. Because the database analysis indicated that a constant domain of TCRγ is part of the prostate transcript, we also used a TCRγ constant domain probe (TCR Cγ). We found the same 1.1- and 2.8-kb bands in the prostate (Fig. 2A, lane 3).
Figure 2.
Northern blot analysis of TCR γδ expression. (A) A TCRγ constant domain (TCR Cγ) cDNA probe shows the 1.1-and 2.8-b prostate-specific transcripts (compare with Fig. 1B). The film was exposed for 20 hours. (B) A TCRδ constant domain (TCR Cδ) cDNA probe reveals that TCRδ mRNA is not expressed in prostate whereas expression is seen in spleen, thymus, and peripheral blood leukocytes. The film was exposed for 50 hours. (C) A TCR Cγ cDNA probe shows that the LNCaP cell line expresses TCRγ whereas the PC-3 cell line does not. The film was exposed for 20 hours. Human β-actin mRNA expression was analyzed as a control.
Prostate Cells Expressing TCRγ Do Not Express TCRδ or CD3 Transcripts.
TCR γ chain protein is normally coexpressed with the TCR δ chain protein. Because the TCRγ gene is transcriptionally active in human prostate, we went on to analyze the transcriptional activity of the TCRδ gene. The dbEST was analyzed (http://www.ncbi.nlm.nih.gov/BLAST) by using the TCRδ transcript nucleotide sequence. ESTs from prostate cDNA libraries did not match any part of the TCR δ chain transcript. Furthermore, Northern blot analysis did not detect any prostate expression of TCRδ mRNA (Fig. 2B, lane 3). We conclude that the TCRδ gene is silent in prostate. As expected, TCRδ transcripts are expressed in spleen, thymus, and blood leukocytes (Fig. 2B).
LNCaP Cells, but not PC-3 Cells, Express the Prostate-Specific TCRγ Transcripts.
Given that TCRγ mRNA is expressed in normal prostate, we next analyzed whether it is also expressed in prostate cancer. The prostate-specific 1.1-kb transcript was found in mRNA preparations from LNCaP but not in mRNA preparations from PC-3 (Fig. 2C). The prostate-specific 2.8-kb transcript, expressed in normal prostate, is also present in LNCaP, although to a much lesser degree.
RNA in Situ Hybridization Shows TCRγ Expression in Prostate Epithelial Cells.
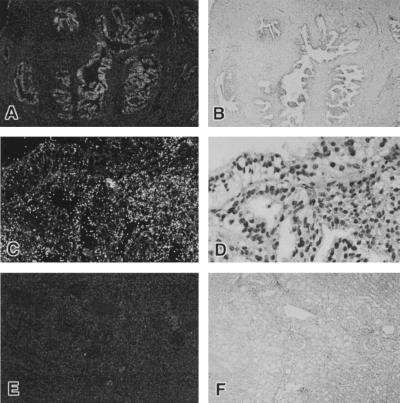
The prostate consists of acinar glandular tissue with variable and mixed population of simple duct lining epithelial cells, ranging to complex hyperplastic ducts in the glandular compartments. These compartments are tightly connected to smooth muscle cells, fibroblasts, and other cell types in the prostate stroma. To determine the cellular localization of the human prostate TCRγ expression, RNA in situ hybridization was carried out with TCR(Cγ-3′UTR) sense and antisense riboprobes. We found that TCRγ mRNA is highly expressed in epithelial cells within the acinar ducts of the prostate whereas stromal cells and other cell types in the prostate are negative (Fig. 3 A and C). TCRγ expression also was detected in hyperplastic and neoplastic areas of the prostate. The expression in benign and neoplastic acinar epithelium is comparable (data not shown). TCRγ expression could not be observed in human kidney tissue (Fig. 3E) or in human brain (data not shown).
Figure 3.
RNA in situ hybridization on paraffin-embedded tissue sections using a TCRγ (Cγ1–3′UTR) anti-sense, 35S-labeled riboprobe. The left panel photos are from dark field microscopy whereas the corresponding right panel photos are from in bright field microscopy. The bright grains shown in pictures taken in dark field are signals of RNA hybridization. (A) Prostate tissues from a 67-year-old man showing positive acinar epithelial cells and negative stromal cells (×5). (B) Bright field of A. (C) Higher magnification (×40) showing positive areas in the lower right corner. (D) Bright field of C. (E) Kidney tissues showing no RNA hybridization (×5). (F) Bright field of E.
The Prostate TCRγ Transcript Contains Cγ1 but not any VJγ Genes.
After we had established the TCR γδ expression profile in the prostate, we went on to characterize the predominant, 1.1-kb, prostate-specific TCRγ transcript. The LNCaP cell line was used for the characterization because one cannot exclude the possibility of mRNA contamination from infiltrating T-cells in the mRNA preparations extracted from bulk prostate tissue. We knew from database analysis that the 3′end sequence of the prostate TCRγ transcript is identical to that from peripheral blood leukocytes and that the location of the polyadenylation signal is identical. Therefore, the difference in transcript size between prostate and leukocytes is attributable to sequence differences upstream of the stretch identified by the prostate ESTs. An RT-PCR set up to amplify the constant domain portion of the TCRγ transcript identified the TCRCγ1 gene. The slightly larger TCRCγ2 is not expressed in LNCaP. Variable domain (Vγ)- to constant domain (Cγ)-spanning RT-PCRs did not yield any product, indicating that Vγ is not part of the prostate-specific TCRγ transcript.
LNCaP Has Not Undergone VJ Gene Rearrangement in the TCRγ Locus.
Because RT-PCRs intending to amplify the variable domain of TCRγ did not yield any product, we next analyzed the TCRγ locus. During the development of γδ T-cells, the TCR loci undergo V(D)J gene rearrangements to bring together the gene segments that make up the variable domain of the receptor. To address whether LNCaP cells have undergone TCRγ VJ gene rearrangement, PCRs were carried out on genomic DNA by using combinations of TCRVγ and TCRJγ primers, to cover every possible rearrangement (see Materials and Methods). None of the primer combinations yielded any PCR product, showing that LNCaP cells have not undergone VJ gene rearrangement of the TCRγ locus. The fact that TCRγ VJ rearrangement has not taken place in prostate epithelial cells shows that the prostate expression is different from that of mature γδ T-lymphocytes.
Prostate Epithelial Cells Express a TCR (JC)γ Transcript.
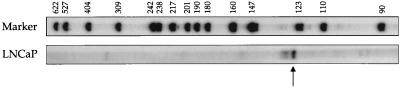
Because the identified prostate TCRγ transcripts consist of Cγ but not of any Vγ gene segment, we next analyzed what sequence is upstream of Cγ1. RNA primer-extension and 5′RACE PCR were carried out to obtain the starting point of transcription. The primer-extension experiment conducted on LNCaP mRNA showed a predominant band of ≈128 nucleotides with minor bands in the 130–135 nucleotide area (Fig. 4). Because the reverse transcription started 75 bases from the 5′end of Cγ1 (see Materials and Methods), the transcript has ≈53 nucleotides upstream of Cγ1. The 5′ RACE PCR conducted on LNCaP cDNA revealed one specific PCR product. The amplified product was found to contain a Jγ1.2 gene segment, correctly spliced to the Cγ1 gene segments. A number of clones isolated by RACE PCR were sequenced. They initiated close to the start site defined by the primer extension experiment. A somewhat variable starting point of transcription is consistent with the identification of minor bands slightly larger than the predominant one in the primer-extension experiment. An illustration of how the prostate TCRγ is transcribed and spliced is shown in Fig. 5A. The nucleotide sequence of the TCRγ transcript, as obtained from LNCaP, is shown in Fig. 5B. The composite sequence is 1,020 ± 3 nucleotides long. It contains ≈53 bases from the Jγ1.2 gene segment, 519 bases of Cγ1, followed by 448 bases of untranslated sequence containing a polyadenylation signal and poly(A) sequence at the 3′end.
Figure 4.
Primer-extension of LNCaP mRNA. The reverse primer anneals in the constant domain of TCRγ, starting 75 nucleotides from the 5′end of Cγ1. The reverse transcription stopped at ≈128 nucleotides, indicated by the arrow, revealing that the transcript is initiated ≈53 nucleotides upstream of Cγ1. The lane with TCRγ reverse transcription of LNCaP was exposed for 72 hours whereas the marker lane was exposed for 8 hours.
Figure 5.
The prostate TCRγ transcript. (A) Illustration on how the prostate TCRγ is transcribed and spliced. The transcript consists of a Jγ1.2 segment, the three exons of Cγ1, followed by untranslated sequence. (B) Nucleotide sequence of the TCRγ transcript as obtained from LNCaP cDNA. The starting point of transcription (underlined) is within the 10 first nucleotides of the Jγ1.2 segment. The four translational initiation codons (ATG) in the original TCRγ reading frame are double underlined. The sequence data is available from the European Molecular Biology Laboratory/GenBank/DNA Data Base in Japan under accession no. AF151103.
In Vitro Translation of the Prostate-Specific TCRγ Transcript.
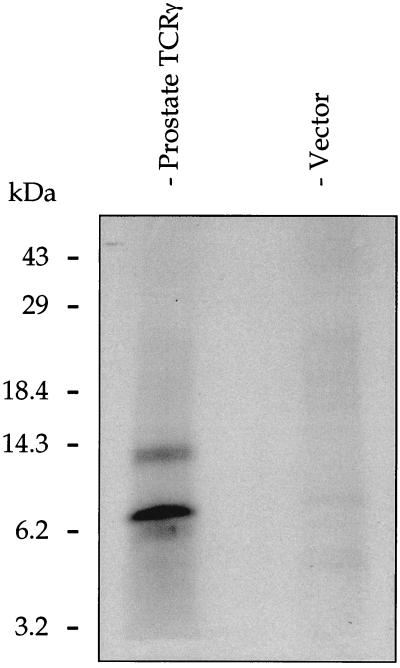
The prostate transcript has four translational initiation codons (ATG) in the original TCRγ reading frame that are double underlined in Fig. 5B. Calculated protein sizes for the four different start points are 12.8, 12.0, 6.7, and 3.2 kDa, respectively. To analyze the translational activity of the prostate transcript, in vitro transcription-coupled translation was carried out by using full-length prostate TCRγ cDNA. Two proteins of ≈8 and 13 kDa were obtained (Fig. 6, lane 1). Negative control reactions did not yield any protein product.
Figure 6.
In vitro transcription-coupled translation analysis of the prostate TCRγ. Two proteins with estimated sizes of 8 and 13 kDa were obtained (lane 1). Negative control reactions using the empty vector (lane 2) did not yield any protein product.
DISCUSSION
Specific Expression of TCRγ Transcripts in Epithelial Cells of the Prostate.
We have identified expression of T-cell receptor γ chain (TCRγ) mRNA in human prostate and have shown that it originates from epithelial cells of the prostate and not from infiltrating γδ T-lymphocytes. We also demonstrate that the T-cell receptor δ chain (TCRδ) gene is silent in prostate. TCRγ mRNA is expressed in epithelial cells within the acinar ducts of the prostate as well as in prostate cancer. Two TCRγ transcripts of 1.1 and 2.8 kb are present in human prostate. They are different in size compared with the 1.5-kb TCRγ transcript found in spleen, thymus, and peripheral blood leukocytes. The TCRγδ mRNA expression profile suggests that the transcription in prostate does not follow the usual pathway of γδ T-lymphocytes. The prostate TCRγ expression was initially discovered by analysis of the publicly available EST database. Our results show that EST clustering is a powerful tool to identify novel and unexpected gene expression. The prostate ESTs representing the TCRγ transcript are all from cDNA libraries made from cells isolated by laser capture microdissection (10). The fact that the TCRγ transcripts proved to originate from prostate epithelial cells and not from infiltrating γδ T-lymphocytes verifies that microdissection is a valuable technique to procure pure cell subpopulations from specific microscopic regions of tissues.
The Prostate TCR(JC)γ Transcript.
The prostatic adenocarcinoma cell line, LNCaP, which was isolated from a lymph node metastasis (11), expresses readily detectable levels of the 1.1-kb prostate-specific TCRγ transcript. The expression in LNCaP cells shows that the transcript originates from epithelial cells and that it can be carried on during the development of a prostatic malignancy. The LNCaP transcript consists of ≈53 bases of the Jγ1.2 gene segment, the three Cγ1 exons, and the untranslated sequence followed by poly(A) sequence. The prostate transcript is different from the mature T-lymphocyte transcript in that it lacks a Vγ gene segment and that it is initiated within the intronic sequence directly upstream of Jγ1.2 (data not shown). The promoter driving the prostate TCRγ transcript and its mechanism of activation in prostate epithelial cells are under investigation. The 2.8-kb prostate-specific TCRγ transcript is very faint in LNCaP, and the 5′ RACE PCR experiment did not retrieve any product consistent with a 2.8-kb transcript. Therefore, the 2.8-kb transcript needs further study.
Comparison with TCR (JC)γ Transcripts in T-Lymphocytes.
Many studies have shown that it is possible to detect TCR gene transcription before, or concomitant with, the onset of V(D)J rearrangement in hematopoietic cells (12–15). The TCRγ gene has been reported to be transcriptionally active in murine bone marrow-resident T-lymphocyte precursor cells with unrearranged γ loci, resulting in sterile TCR Cγ transcripts (12). In addition, expression of unrearranged TCR Vγ transcripts also have been reported during ontogeny (16). Sterile transcription of TCR and Ig gene segments has so far been limited to cells from the lymphoid lineages (17). Furthermore, activation of germline transcription at nearly all TCR and Ig loci temporally correlates with activation of locus recombination (15–18). We have shown, by independent experiments using genomic DNA and cDNA, that recombination has not taken place of the TCRγ locus of prostate epithelial cells. Therefore, the expression of the TCR (JC)γ transcript in prostate epithelium does not correlate with recombination, and it may serve a different function than the sterile transcripts observed in T-lymphocyte precursor cells.
The Possibility of a Novel Prostate-Specific Protein in the TCRγ Locus.
The prostate TCRγ transcript is highly expressed, and we hypothesize that there is an underlying, biologically important reason. The fact that VJ gene rearrangement has not taken place in the TCRγ locus of prostate epithelial cells excludes the possibility that a mature TCR γ chain protein is made. We also exclude the possibility that a TCRγ constant domain protein is made without the TCRγ variable domain because no translational initiation codon (ATG) is found upstream of Cγ. In TCR γ chain proteins, a Jγ segment encodes 16–20 amino acids of the variable domain whereas the major part of the variable domain is encoded by one of the Vγ segments. Unless the amino acids encoded by a Jγ segment are combined with amino acids encoded by a Vγ gene segment, they cannot function as a TCR in MHC recognition. This raises the possibility of a novel prostate-specific protein, encoded from within Cγ. Our hypothesis is that one of the ATG codons in the original TCRγ reading frame initiates translation, although a different reading frame or a less frequently used initiation codon may be used. The in vitro transcription-coupled translation experiment using prostate TCRγ cDNA reveals that the transcript is fully functional. Two proteins were obtained. The 13-kDa protein most likely originates from the first double underlined ATG in Fig. 5B, which yields a calculated protein size of 12.8 kDa. The 8-kDa protein could originate from the third double underlined ATG, calculated size of 6.7 kDa, or from the first initiation codon in the second reading frame. We are currently investigating the nature of the protein encoded by the prostate-specific TCRγ transcript. In conclusion, the fact that prostate epithelial cells, or that any non-lymphoid-derived cell type, express high level of a transcript from a gene that was thought to be exclusively expressed by cells from the lymphoid lineage, is a highly unexpected discovery.
Acknowledgments
We thank Drs. Donna Peehl, Alfred Johnson, Michael Emmert-Buck, and Mark Raffeld for helpful comments and discussions. Magnus Essand is sponsored in part by the Swedish Cancer Society.
ABBREVIATIONS
- EST
expressed sequence tag
- dbEST
database of ESTs
- TCRγ
T-cell receptor γ chain
- UTR
untranslated region
- RT
reverse transcriptase
- RACE
rapid amplification of cDNA ends
- kb
kilobase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF151103).
References
- 1.Vasmatzis G, Essand M, Brinkmann U, Lee B K, Pastan I. Proc Natl Acad Sci USA. 1998;95:300–304. doi: 10.1073/pnas.95.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabbitts T H, Lefranc M-P, Stinson M A, Sims J E, Schroder J, Steinmetz M, Spurr N L, Solomon E, Goodfellow P N. EMBO J. 1985;4:1461–1465. doi: 10.1002/j.1460-2075.1985.tb03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensmana M, Mattei M G, Lefranc M-P. Cytogenet Cell Genet. 1991;56:31–32. doi: 10.1159/000133040. [DOI] [PubMed] [Google Scholar]
- 4.Lefranc M-P, Chuchana P, Dariavach P, Nguyen C, Huck S, Brockly F, Jordan B, Lefranc G. Eur J Immunol. 1989;19:989–994. doi: 10.1002/eji.1830190606. [DOI] [PubMed] [Google Scholar]
- 5.Lefranc M-P, Rabbitts T H. Res Immunol. 1990;141:565–577. doi: 10.1016/0923-2494(90)90058-7. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1995. [Google Scholar]
- 7.George C P, Kadonaga J T. A Laboratory Guide to RNA: Isolation, Analysis and Synthesis. 1996. , ed. P. A. Krieg, (Wiley–Liss, New York), pp. 133–139. [Google Scholar]
- 8.Krangel M S, Band H, Hata S, McLean J, Brenner M B. Science. 1987;237:64–67. doi: 10.1126/science.2955517. [DOI] [PubMed] [Google Scholar]
- 9.Littman D R, Newton M, Crommie D, Ang S L, Seidman J G, Gettner S N, Weiss A. Nature (London) 1987;326:85–88. doi: 10.1038/326085a0. [DOI] [PubMed] [Google Scholar]
- 10.Emmert-Buck M R, Bonner R F, Smith P D, Chuaqui R F, Zhuang Z, Goldstein S R, Weiss R A, Liotta L A. Science. 1996;274:998–1001. doi: 10.1126/science.274.5289.998. [DOI] [PubMed] [Google Scholar]
- 11.Horoszewicz J S, Leong S S, Kawinski E, Karr J P, Rosenthal H, Chu T M, Mirand E A, Murphy G P. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 12.Wang T G, Lybarger L, Soloff R, Dempsey D, Chervenak R. Mol Immunol. 1996;33:957–964. doi: 10.1016/s0161-5890(96)00048-x. [DOI] [PubMed] [Google Scholar]
- 13.Shimamura M, Ohta S. Eur J Immunol. 1995;25:1541–1546. doi: 10.1002/eji.1830250611. [DOI] [PubMed] [Google Scholar]
- 14.Villey I, Quartier P, Selz F, de Villartay J P. Eur J Immunol. 1997;27:1619–1625. doi: 10.1002/eji.1830270705. [DOI] [PubMed] [Google Scholar]
- 15.Sikes M L, Gomez R J, Song J, Oltz E M. J Immunol. 1998;161:1399–1405. [PubMed] [Google Scholar]
- 16.Goldman J P, Spencer D M, Raulet D H. J Exp Med. 1993;177:729–739. doi: 10.1084/jem.177.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauzurica P, Krangel M S. J Exp Med. 1994;179:1913–1921. doi: 10.1084/jem.179.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sleckman B P, Gorman J R, Alt F W. Annu Rev Immunol. 1996;14:459–481. doi: 10.1146/annurev.immunol.14.1.459. [DOI] [PubMed] [Google Scholar]