Short abstract
The biologically active metabolite of vitamin D3, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], acts through vitamin D receptors, which were found in rheumatoid tissues in the present study. IL-1β-activated rheumatoid synovial fibroblasts and human articular chondrocytes were shown to respond differently to exposure to 1α,25(OH)2D3, which has different effects on the regulatory pathways of specific matrix metalloproteinases and prostaglandin E2.
Keywords: 1α,25-dihydroxyvitamin D3; matrix metalloproteinase; prostaglandin E2; rheumatoid arthritis
Abstract
Introduction:
1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], the biologically active metabolite of vitamin D3, acts through an intracellular vitamin D receptor (VDR) and has several immunostimulatory effects. Animal studies have shown that production of some matrix metalloproteinases (MMPs) may be upregulated in rat chondrocytes by administration of 1α,25(OH)2D3; and cell cultures have suggested that 1α,25(OH)2D3 may affect chondrocytic function. Discoordinate regulation by vitamin D of MMP-1 and MMP-9 in human mononuclear phagocytes has also been reported. These data suggest that vitamin D may regulate MMP expression in tissues where VDRs are expressed. Production of 1α,25(OH)2D3 within synovial fluids of arthritic joints has been shown and VDRs have been found in rheumatoid synovial tissues and at sites of cartilage erosion. The physiological function of 1α,25(OH)2D3 at these sites remains obscure. MMPs play a major role in cartilage breakdown in the rheumatoid joint and are produced locally by several cell types under strict control by regulatory factors. As 1α,25(OH)2D3 modulates the production of specific MMPs and is produced within the rheumatoid joint, the present study investigates its effects on MMP and prostaglandin E2 (PGE2) production in two cell types known to express chondrolytic enzymes.
Aims:
To investigate VDR expression in rheumatoid tissues and to examine the effects of 1α,25-dihydroxyvitamin D3 on cultured rheumatoid synovial fibroblasts (RSFs) and human articular chondrocytes (HACs) with respect to MMP and PGE2 production.
Methods:
Rheumatoid synovial tissues were obtained from arthroplasty procedures on patients with late-stage rheumatoid arthritis; normal articular cartilage was obtained from lower limb amputations. Samples were embedded in paraffin, and examined for presence of VDRs by immunolocalisation using a biotinylated antibody and alkaline-phosphatase-conjugated avidin-biotin complex system. Cultured synovial fibroblasts and chondrocytes were treated with either 1α,25(OH)2D3, or interleukin (IL)-1β or both. Conditioned medium was assayed for MMP and PGE2 by enzyme-linked immunosorbent assay (ELISA), and the results were normalised relative to control values.
Results:
The rheumatoid synovial tissue specimens (n = 18) immunostained for VDRs showed positive staining but at variable distributions and in no observable pattern. VDR-positive cells were also observed in association with some cartilage-pannus junctions (the rheumatoid lesion). MMP production by RSFs in monolayer culture was not affected by treatment with 1α,25(OH)2D3 alone, but when added simultaneously with IL-1β the stimulation by IL-1β was reduced from expected levels by up to 50%. In contrast, 1α,25(OH)2D3 had a slight stimulatory effect on basal production of MMPs 1 and 3 by monolayer cultures of HACs, but stimulation of MMP-1 by IL-1β was not affected by the simultaneous addition of 1α,25(OH)2D3 whilst MMP-3 production was enhanced (Table 1). The production of PGE2 by RSFs was unaffected by 1α,25(OH)2D3 addition, but when added concomitantly with IL-1β the expected IL-1 β-stimulated increase was reduced to almost basal levels. In contrast, IL-1β stimulation of PGE2 in HACs was not affected by the simultaneous addition of 1α,25(OH)2D3 (Table 2). Pretreatment of RSFs with 1α,25(OH)2D3 for 1 h made no significant difference to IL-1β-induced stimulation of PGE2, but incubation for 16 h suppressed the expected increase in PGE2 to control values. This effect was also noted when 1α,25(OH)2D3 was removed after the 16h and the IL-1 added alone. Thus it appears that 1α,25(OH)2D3 does not interfere with the IL-1β receptor, but reduces the capacity of RSFs to elaborate PGE2 after IL-1β induction.
Discussion:
Cells within the rheumatoid lesion which expressed VDR were fibroblasts, macrophages, lymphocytes and endothelial cells. These cells are thought to be involved in the degradative processes associated with rheumatoid arthritis (RA), thus providing evidence of a functional role of 1α,25(OH)2D3 in RA. MMPs may play important roles in the chondrolytic processes of the rheumatoid lesion and are known to be produced by both fibroblasts and chondrocytes. The 1α,25(OH)2D3 had little effect on basal MMP production by RSFs, although more pronounced differences were noted when IL-1β-stimulated cells were treated with 1α,25(OH)2D3, with the RSF and HAC showing quite disparate responses. These opposite effects may be relevant to the processes of joint destruction, especially cartilage loss, as the ability of 1α,25(OH)2D3 to potentiate MMP-1 and MMP-3 expression by 'activated' chondrocytes might facilitate intrinsic cartilage chondrolysis in vivo. By contrast, the MMP-suppressive effects observed for 1α,25(OH)2D3 treatment of 'activated' synovial fibroblasts might reduce extrinsic chondrolysis and also matrix degradation within the synovial tissue. Prostaglandins have a role in the immune response and inflammatory processes associated with RA. The 1α,25(OH)2D3 had little effect on basal PGE2 production by RSF, but the enhanced PGE2 production observed following IL-1β stimulation of these cells was markedly suppressed by the concomitant addition of 1α,25(OH)2D3. As with MMP production, there are disparate effects of 1α,25(OH)2D3 on IL-1β stimulated PGE2 production by the two cell types; 1α,25(OH)2D3 added concomitantly with IL-1β had no effect on PGE2 production by HACs. In summary, the presence of VDRs in the rheumatoid lesion demonstrates that 1α,25(OH)2D3 may have a functional role in the joint disease process. 1α,25(OH)2D3 does not appear to directly affect MMP or PGE2 production but does modulate cytokine-induced production.
Table 1.
Comparative effects of 1 α,25-dihydroxyvitamin D3 (1 α,25D3) on interleukin (IL)-1-stimulated matrix metalloproteinase (MMP)-1 and MMP-3 production by rheumatoid synovial fibroblasts and human articular chondrocytes in vivo
| Fibroblasts | Chondrocytes | |||
| MMP-1 | MMP-3 | MMP-1 | MMP-3 | |
| Control | 1 | 1 | 1 | 1 |
| + 1α,25D3 | 1.03 ± 0.27 | 2.07 ± 0.35 | 1.38 ± 0.19 | 1.59 ± 0.22 |
| + IL-1 | 31.09 ± 4.97 | 31.28 ± 8.49 | 3.45 ± 0.49 | 9.05 ± 0.62 |
| + IL-1 + 1α,25D3 | 15.55 ± 5.86 | 11.84 ± 2.82 | 3.71 ± 0.53 | 11.11 ± 0.31 |
Data given are normalized relative to control values and are expressed ± SEM for three cultures of each cell type.
Table 2.
Comparative effects of 1α,25-dihydroxyvitamin D3 (1α,25D3) on Interleukin (IL)-1-stimulated prostaglandin E2 production by rheumatoid synovial fibroblasts and human articular chondrocyte in vivo
| Fibroblasts | Chondrocytes | |
| Control | 1 | 1 |
| + 1α,25D3 | 1.23 ± 0.16 | 1.35 ± 0.25 |
| + IL-1 | 7.07 ± 1.09 | 3.7 ± 1.05 |
| + IL-1 + 1α,25D3 | 1.61 ± 0.7 | 4.23 ± 1.10 |
Data given are normalized relative to control values and are expressed ± SEM for three cultures of each cell type.
Introduction
The biologically active metabolite of vitamin D3, 1α,25-dihydroxyvitaminD3 [1α,25(OH)2D3], acts through an intracellular receptor [vitamin D receptor (VDR)] and has a main role in the regulation of calcium and phosphorus metabolism [1]. It also has several immunomodulatory actions such as its effect on the differentiation and proliferation of T lymphocytes, and the regulation of immunoglobulin production by B lymphocytes [2,3,4].1α,25(OH)2D3 may affect chondrocytic function, such as proteoglycan and collagen synthesis [5]; and animal studies have shown that the production of some matrix metalloproteinases (MMPs), namely interstitial collagenase (MMP-1), stromelysin (MMP-3) and 72-kDa gelatinase (MMP-2), may be upregulated in rat chondrocytes by administration of the metabolite [6]. Discoordinate regulation by vitamin D of MMP-1 and MMP-9 in human mononuclear phagocytes has also been reported [7]. Together these data have suggested that vitamin D can regulate MMP expression in tissues or pathologies where receptors for the hormone are expressed.
The kidney is recognized as the primary source of 1α,25(OH)2D3, producing the metabolite via 1-hydroxylation of 25-hydroxyvitamin D3-[1]. However, the local production of 1α,25(OH)2D3 within synovial fluids of arthritic joints, especially the macrophage component, has recently been indicated [8,9]; and receptors for vitamin D have also been demonstrated in rheumatoid synovial tissues and at sites of cartilage erosion [10]. Such studies have demonstrated a local source of 1α,25(OH)2D3 within the rheumatoid joint, but its regulation and physiological functions at this site remain obscure.
MMPs are reputed to play a major role in cartilage breakdown in the rheumatoid joint and are produced locally by several cell types, but especially by synovial fibroblasts and articular chondrocytes [11,12,13,14,15,16]. MMP production and release is microenvironmental in nature and is tightly regulated by several factors, including the proinflammatory cytokines tumour necrosis factor-α and interleukin (IL)-1β [17]. Because 1α,25(OH)2D3 has been shown to modulate the production of specific MMPs and is produced within the rheumatoid joint, the present study was designed to investigate the effects of 1α,25(OH)2D3 on MMP and prostaglandin E2 (PGE2) production by rheumatoid synovial fibroblasts (RSFs) and human articular chondrocytes (HACs), cell types known to express chondrolytic enzymes both in vitro and in vivo.
Methods
Tissue samples
Samples of rheumatoid synovial tissue, cartilage and cartilage-pannus junction were obtained from arthroplasty procedures performed on patients with classic late-stage rheumatoid arthritis. Normal articular cartilage samples were obtained from lower limb amputations. Samples were fixed in Carnoy's fixative at 20°C for 2 h, embedded in paraffin wax and 5 μm sections cut. Tissue sections were dewaxed, rehydrated and examined for the presence of VDR.
Immunolocalization of vitamin D receptors
Tissue sections were treated with 2N HCl at 37°C for 30 min, this being the antigen retrieval procedure recommended by the supplier of the primary antibody. Nonimmune rabbit serum at 10% (vol : vol) in TRIS-buffered saline was applied to the sections for 20 min at 20°C before incubation with the primary antibody. Rat monoclonal antibody to chick VDR (Biogenex, San Remo, USA), which is known to cross-react with human VDR, was applied to the sections for 2 h at 20°C after dilution 1 : 40 in TRIS-buffered saline. After 3 × 10 min washing in TRIS-buffered saline, biotinylated rabbit anti-rat immunoglobulin G (DAKO, Glostrup, Denmark) diluted 1 : 200 in TRIS-buffered saline was applied to the sections for 45 min at 20°C. After further washing in TRIS-buffered saline, alkaline phosphatase-conjugated ABC (Avidin-biotin complex system; DAKO) was applied to the sections for 45min at 20°C, diluted as instructed by the supplier. After further washing the alkaline phosphatase was developed using new fuchsin substrate to give a red colour. Sections were lightly counterstained using Harris's haematoxylin or toluidine blue. Non-immune rat immunoglobulin G was substituted for the primary antibody at similar concentrations on control tissue sections [10].
Cell cultures
Rheumatoid synovial tissue and human articular cartilage were enzymically digested to provide synovial fibroblast and chondrocyte cultures as previously described [18,19]. Cells were grown in Dulbecco's Modified Eagle's Medium + 10% (vol : vol) foetal calf serum, harvested and seeded into 12-well culture dishes (Nunc, Gibco, UK) Triplicate wells of confluent cell cultures in Dulbecco's Modified Eagle's Medium + 2% foetal calf serum were treated with 1α,25(OH)2D3 (10-8 mol/l), IL-1β (0.05 ng/ml), or IL-1β + 1α,25(OH)2D3 (0.05 ng/ml and 10-8 mol/l, respectively) and incubated for 48 h at 37°C. The conditioned medium was collected and assayed for MMP-1, MMP-2, MMP-3 and MMP-9, and PGE2 using enzyme-linked immunosorbent assay (ELISA) methodology. Cell numbers per well were counted at the end of each experiment after 70% ethanol fixation and toluidine blue staining.
Enzyme linked immunosorbent assays
ELISA methodology was used to determine protein levels of MMP-1 (collagenase 1), MMP-3 (stromelysin) and MMP-9 (gelatinase B) as previously described [20]. MMP-2 (Gelatinase A) was measured using ELISA kits purchased from The Binding Site (Birmingham, UK); and PGE2 was measured using an ELISA assay kit purchased from R & D Systems Europe, Ltd (Abingdon,UK).
All ELISA results were initially calculated in ng or pg protein/ml culture medium/106 cells per 48 h. Three different cultures of both RSFs and HACs were examined, but the capacities of each cell type to produce the MMPs and PGE2 varied between the individual cultures. Therefore, the data from each culture was 'normalized' relative to control values, and the data sets from the three cultures of each cell type were subsequently pooled. This provided an evaluation that showed qualitative similarities for the RSFs and HACs, but demonstrated differences in 1α,25(OH)2D3 responses by each of these two cell types.
Results
Demonstration of the vitamin D receptor in rheumatoid tissues in vivo
Specimens of rheumatoid synovial tissue (n = 18) immunostained for VDR were shown to have variable distributions of the receptor. All specimens showed some positive staining, but this could be less than 5% or as much as 70% of the total cell population. Different cell types within the synovial specimens were shown to express the receptor, including macrophages, endothelial cells, lymphocytes and fibroblastic cells, but no regular pattern was observed. Cells with fibroblastic morphology immunostained for VDR are shown in Figure 1a. Chondrocytes within articular cartilage from rheumatoid joints also expressed the receptor in six out of 10 specimens (Fig. 1b), this being a much higher frequency compared with the one in 10 specimens of normal articular cartilage from nonarthritic joints (data not shown). VDR-positive cells were also observed in association with some cartilage–pannus junctions, described here as the rheumatoid lesion (Fig. 1c).
Figure 1.
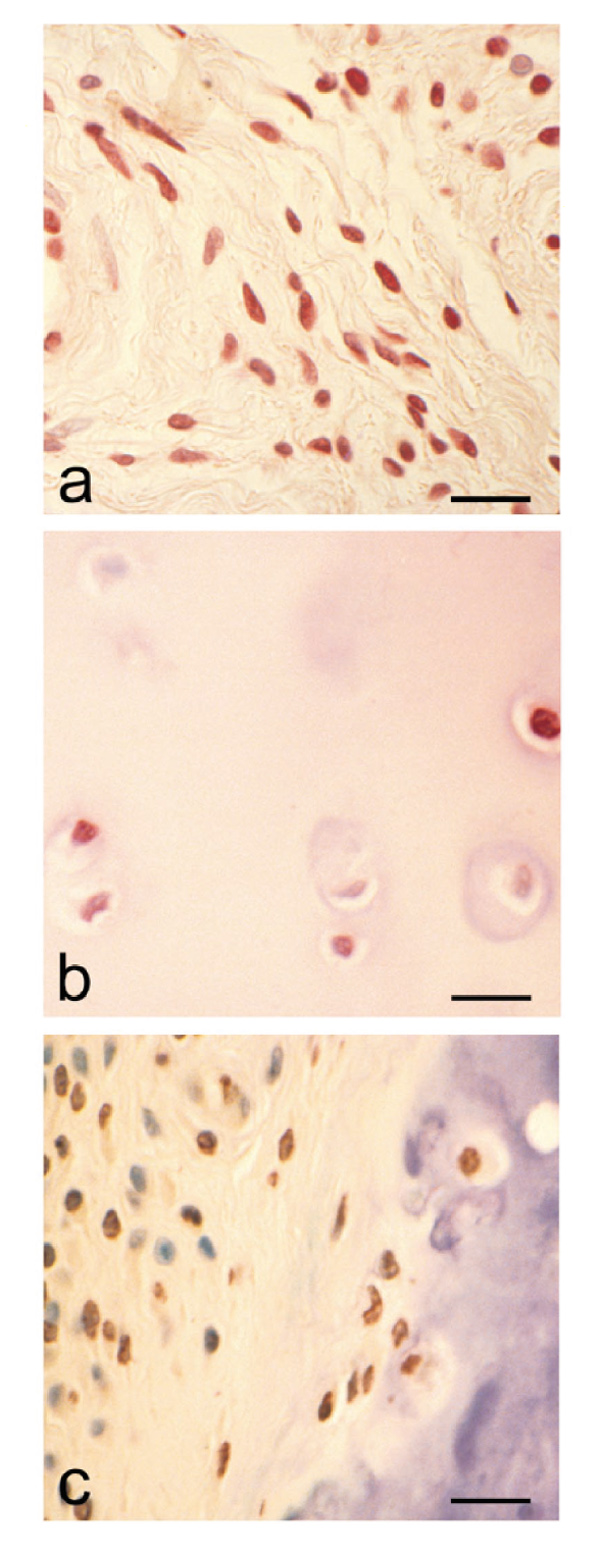
Immunolocalization of the vitamin D receptor (VDR) in rheumatoid tissues. (a) Immunolocalization of VDR in rheumatoid synovium. Note positive red immunostaining of fibroblastic cells. (Counterstain Harris's haematoxylin; bar = 25 μm.) (b) Demonstration of VDR in cartilage from a rheumatoid joint. Note both positive and negative chondrocytes. (Counterstain Harris's haematoxylin; bar = 20 μm.) (c) VDR immunolocalization at the cartilage-pannus junction; cells within both pannus tissue and cartilage can be seen to be expressing the receptor. (Counterstain toluidine blue; bar = 25 μm.)
Effects of 1α,25-dihydroxyvitamin D3 on matrix metalloproteinase production by rheumatoid synovial fibroblasts
1α,25(OH)2D3 alone had no effect on basal MMP production by RSFs in monolayer culture, but the simultaneous addition of 1α,25(OH)2D3 with IL-1β reduced the expected stimulation of MMP-1, MMP-3 and MMP-9 by up to 50% (Fig. 2: P = 0.096, 0.009 and 0.01, for IL-1β versus IL-1β + 1α,25(OH)2D3 for MMP-1, MMP-3 and MMP-9, respectively, by Student's t-test). MMP-2 production was not affected by either IL-1β or IL-1β + 1α,25(OH)2D3 (data not shown), an observation that is in accord with the constitutive nature of MMP-2 expression [21].
Figure 2.
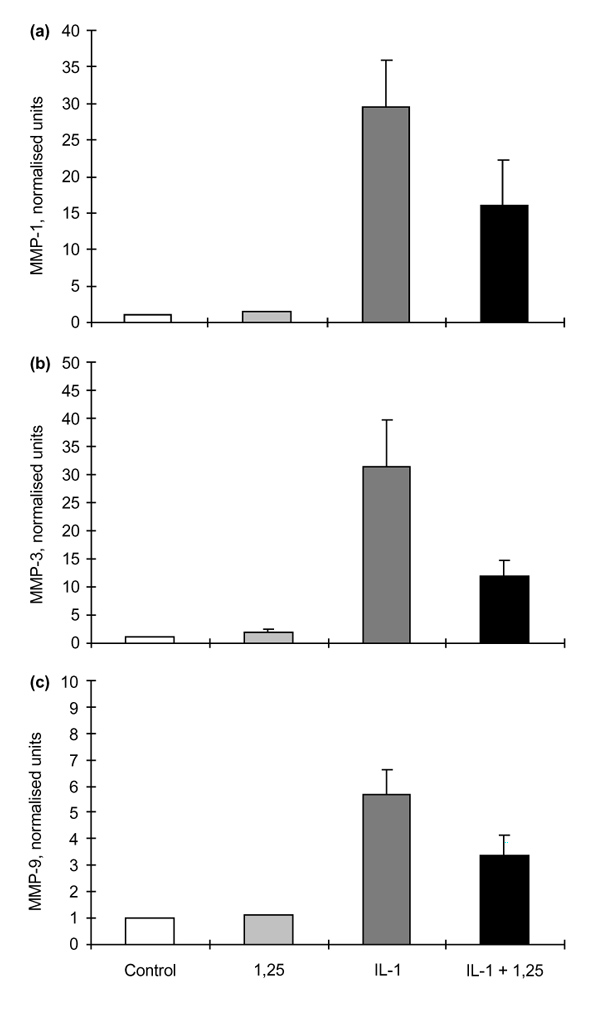
The effects of 1α,25-hydroxyvitamin D3 (1,25) on matrix metalloproteinase (MMP)-1, MMP-3 and MMP-9 production by rheumatoid synovial fibroblasts (RSFs) after 48 h incubation. (a) MMP-1 production by RSFs (n = 3) showing normalized values for control; + 1,25 (10-8 mol/l); + interleukin (IL)-1β (0.05 ng/ml); and + IL-1β and 1,25 (0.05 ng/ml and 10-8 mol/l, respectively). Before normalization, control values for MMP-1 were in the range 50–200 ng/ml culture medium/106 cells per 48 h. (b) MMP-3 production by RSF (n = 3) showing normalized values for control; + 1,25; + IL-1β; and + IL-1β and 1,25. Before normalization, control values for MMP-3 were in the range 10–40 ng/ml culture medium/106 cells per 48 h. (c) MMP-9 production by RSFs (n = 3) showing normalized values for control; + 1,25; + IL-1β; and +IL-1β and 1,25. Before normalization, control values for MMP-9 were in the range 10-50 ng/ml culture medium/106 cells per 48 h. Values are shown as means ± SEM.
Effects of 1α,25-dihydroxyvitamin D3 on matrix metalloproteinase production by human articular chondrocytes
In contrast to the data for RSFs, 1α,25(OH)2D3 had a slight stimulatory effect on basal production of MMP-1 and MMP-3 by monolayer cultures of HAC (Fig. 3: P = 0.098 and 0.002, for control versus 1α,25(OH)2D3, for MMP-1 and MMP-3, respectively, by Student's t-test). When stimulated with IL-1β MMP-1 and MMP-3 production was increased, and although simultaneous addition of 1α,25(OH)2D3 had no effect on the stimulation of the MMP-1 enzyme, MMP-3 production was further enhanced (Fig 3b: P = 0.008, by Students t-test). MMP-9 and MMP-2 were not produced in measurable quantities by these HAC cultures, either with or without IL-1β stimulation.
Figure 3.
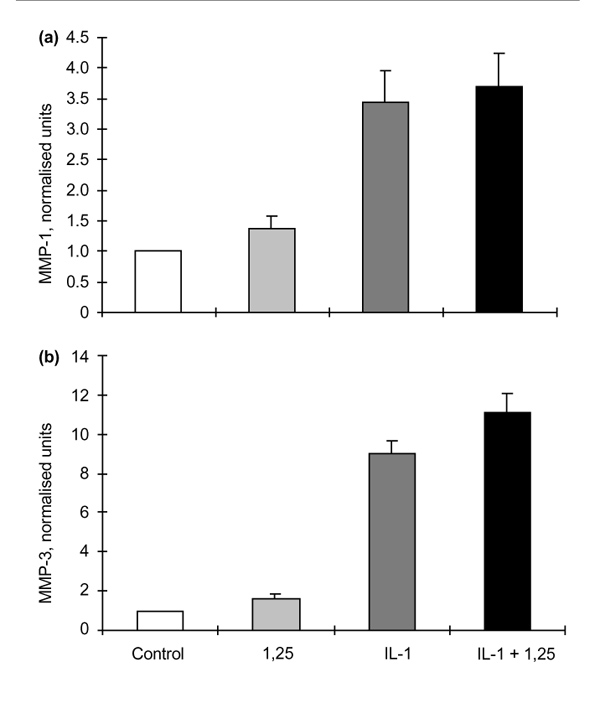
The effects of 1α,25-hydroxyvitamin D3 (1,25) on matrix metalloproteinase (MMP)-1 and MMP-3 production by human articular chondrocytes (HACs) after 48 h incubation. (a) MMP-1 production by HAC (n = 3) showing normalized data for control; + 1,25 (10-8 mol/l); + interleukin (IL)-1β (0.05 ng/ml); and + IL-1β and 1,25 (0.05 ng/ml and 10-8 mol/l, respectively). Before normalization, control values for MMP-1 were in the range 50–150 ng/ml culture medium/106 cells per 48 h. (b) MMP-3 production by HAC (n = 3) showing normalized data for control; +1,25; + IL-1β; and + IL-1β and 1,25. Before normalization, control values for MMP-3 were in the range 10–40 ng/ml culture medium/106 cells per 48 h. Values are shown as means ± SEM.
Effects of 1α,25-dihydroxyvitamin D3 on prostaglandin E2 production by rheumatoid synovial fibroblasts and human articular chondrocytes
PGE2 production by RSFs was unaffected by the addition of 1α,25(OH)2D3 alone. Treatment of RSFs with IL-1β upregulated the production of PGE2, but the addition of 1α,25(OH)2D3 together with IL-1β reduced the expected stimulation of PGE2 almost to control values (Fig. 4a:P = 0.014, for IL-1β versus IL-1β + 1α,25(OH)2D3, by Student's t-test).
Treatment of HACs with IL-1β also increased the production of PGE2, but in contrast to the effects noted for RSFs this IL-1-stimulation of PGE2 was not affected by the concomitant addition of 1α,25(OH)2D3 (Fig. 4b).
Figure 4.
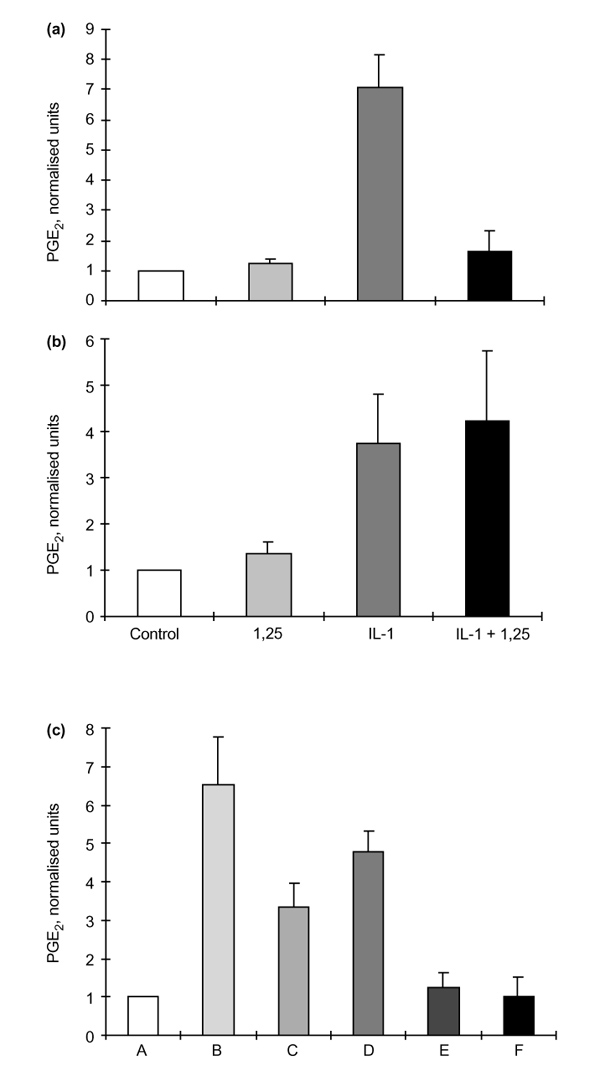
The effects of 1α,25-hydroxyvitamin D3 (1,25) on prostaglandin E2 (PGE2) production by rheumatoid synovial fibroblasts (RSFs) and human articular chondrocytes (HACs) after 48 h incubation. (a)PGE2production by RSFs (n = 3) showing normalized data for control; + 1,25 (10-8 mol/l); + interleukin (IL)-1β (0.05 ng/ml); and + IL-1β and 1,25 (0.05 ng/ml and 10-8 mol/l, respectively). Before normalization, control values for PGE2 production by RSFs were in the range 500–2000 pg/ml culture medium/106 cells per 48 h. (b) PGE2 production by HACs (n = 3) showing normalized data for control; + 1,25; + IL-1β; and + IL-1β and 1,25. Before normalization control values for PGE2 production by HAC were in the range 100–300 pg/ml culture medium/106 cells per 48 h. (c) Normalized data for PGE2production by IL-1β-stimulated RSFs after preincubation with 1α,25(OH)2D3, as follows: A, control; B, +IL-1β; C, 1,25 + IL-1β; D, 1 h preincubation with 1,25, then + IL-1β and 1,25; E, 16 h preincubation with 1,25, then IL-1β + 1,25; F, 16 h preincubation with 1,25, then IL-1β alone. Values are shown as means ± SEM.
To examine the possibility that 1α,25(OH)2D3 might obscure or interact with the IL-1β receptor of RSFs the latter were pretreated with 1α,25(OH)2D3 before incubation with IL-1β. Figure 4c shows that a 1-h preincubation with 1α,25(OH)2D3 followed by IL-1β was not significantly different from the two factors added together, but preincubation with 1α,25(OH)2D3 for 16 h suppressed the expected increase in PGE2 production to control values. This effect was noted even when the 1α,25(OH)2D3 was removed after the 16 h and IL-1β then added alone (Fig.4c, data column F). Thus, rather than directly interfering with the IL-1β receptor, it appears that 1α,25(OH)2D3 reduces the capacity of the RSFs to elaborate PGE2 (and probably the MMPs shown in Fig. 2) after IL-1β induction.
Discussion
The cell types within the rheumatoid lesion which were observed to express VDR included chondrocytes, fibroblasts, macrophages, lymphocytes and endothelial cells. These cells are all purported to be involved either directly or indirectly in the degradative processes associated with rheumatoid arthritis, possibly via their MMP and prostanoid production, or via the production of mediators responsible for inflammation and induction of proteinase expression by other cell types. Thus, the demonstration of VDR within the rheumatoid lesion provides support for a functional role of 1α,25(OH)2D3 in rheumatoid arthritis.
MMPs are considered to play important roles in the chondrolytic processes of the rheumatoid lesion [14,15,17]. These enzymes are known to be produced by both fibroblasts and chondrocytes, but little has been reported in the literature regarding a relationship between 1α,25(OH)2D3and MMP production or its regulation, and most of the data to date have been obtained from animal studies or immortalized cell lines [5,6,7]. 1α,25(OH)2D3 had little effect on basal MMP production by RSFs and marginally increased the basal production of MMP-1 and MMP-3 by chondrocytes. More pronounced differences were noted when IL-1β-stimulated or activated cells were treated with 1α,25(OH)2D3, the RSFs and HACs showing quite disparate responses. These opposite effects may be of relevance to the processes of joint destruction, especially cartilage loss, because the ability of 1α,25(OH)2D3 to potentiate MMP-1 and MMP-3 expression by 'activated' chondrocytes might facilitate intrinsic cartilage chondrolysis in vivo. By contrast, the MMP-suppressive effects observed for 1α,25(OH)2D3 treatment of 'activated' synovial fibroblasts might reduce extrinsic chondrolysis and also matrix degradation within the synovial tissue. We recognize that the present study is somewhat restricted to the 1α,25(OH)2D3 effects on MMP-1 and MMP-3 production. Although these are prominent and well characterized MMPs, there are many other enzymes in this family, together with plasminogen activators and other proteinases, which have not been examined. From the disparate effects of 1α,25(OH)2D3 on the RSFs and HACs it would seem that further studies on the 1α,25(OH)2D3-modified proteinase phenotypes of these cells are warranted.
Prostaglandins are primary mediators of inflammation and have important roles in the immune response and the inflammatory processes associated with rheumatoid arthritis, and PGE2 has been implicated in the potentiation of MMP production by some cell cultures [22,23]. 1α,25(OH)2D3 had little effect on basal PGE2 production by RSFs, but the enhanced PGE2 production observed following IL-1β stimulation of these cells was markedly suppressed by the concomitant addition of 1α,25(OH)2D3. By contrast, the increased PGE2 production observed for IL-1β-treated HACs was unaffected by the simultaneous addition of 1α,25(OH)2D3. Thus, as with MMP production, 1α,25(OH)2D3 has disparate effects on IL-1β-stimulated PGE2 production by these two cell types. Different responses by RSFs and HACs to the same ligand have been noted before; for example, IL-1β treatment was shown to stimulate glycosaminoglycan synthesis by RSFs, but inhibited its production by chondrocytes [24].
In summary, the immunolocalization of VDR in the rheumatoid lesion has demonstrated that the metabolite 1α,25(OH)2D3might have a functional role in the degradative and inflammatory processes of joint disease. Whereas 1α,25(OH)2D3 does not appear directly to affect the MMP or prostanoid production by unstimulated RSFs or HACs in vitro, it was shown to modulate the cytokine-induced MMP and PGE2 production by these two cell cultures. The recognized immunomodulatory properties of 1α,25(OH)2D3 could well be important in rheumatoid tissues, in which the inflammatory response is a characteristic feature. The transient, local manifestations of cartilage and matrix-degrading activity [25] could be modified by 1α,25(OH)2D3 if the cells present express VDR and the metabolite is produced locally. This study has demonstrated that most rheumatoid synovial specimens were expressing VDR at the time of surgery, and that IL-1β-'activated' synovial fibroblasts and chondrocytes in vitro showed significant and different responses to 1α,25(OH)2D3 exposure with regard to MMP and PGE2 production. Such observations suggest that 1α,25(OH)2D3 contributes indirectly rather than directly to MMP regulation via its action on other mediators or their signalling pathways, in accord with its recognized multifunctional and immunomodulatory properties [1,7].
Acknowledgments
Acknowledgments
We thank consultant orthopaedic surgeons T Dunningham (Tameside Hospital, Manchester) and M Morris (Devonshire Royal Hospital, Buxton) for the supply of rheumatoid tissues, Professor A J Freemont for help with supply of normal cartilage, and Professor E Barbara Mawer for her support and advice. This work was supported by grant No. WO541 from the Arthritis Research Campaign, UK.
References
- Norman AW, Roth J, Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors and biological response (calcium binding proteins). Endocr Rev. 1982;3:331–336. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Suda T. The role of 1α25dihydroxyvitamin D3 in myeloid cell differentiation. Proc Soc Exp Biol Med. 1989;191:214–220. doi: 10.3181/00379727-191-42911. [DOI] [PubMed] [Google Scholar]
- Lemire JM. Immunomodulatory actions of 1,25dihydroxyvitamin D3. J Ster Biochem Mol Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- Lemire JM. Immunomodulatory role of 1,25dihydroxyvitmain D3. J Cell Biochem. 1992;49:26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Kelly CM, von Deck M, Lian JB. Effect of 1,25dihydroxyvitamin D3 on induction of chondrocyte maturation in culture: extracellular matrix gene expression and morphology. Endocrinology. 1990;126:1599–1609. doi: 10.1210/endo-126-3-1599. [DOI] [PubMed] [Google Scholar]
- Dean DD, Schwartz Z, Schmitz J, et al. Vitamin D regulation of metalloproteinase activity in matrix vesicles. Conn Tiss Res. 1996;35:385–390. doi: 10.3109/03008209609029208. [DOI] [PubMed] [Google Scholar]
- Lacraz S, Dayer J-M, Nocod I, Welgus HG. 1,25dihydroxyvitamin D3 dissociates production of interstitial collagenase and 92kDa gelatinase in human mononuclear phagocytes. J Biol Chem. 1994;269:6485–6490. [PubMed] [Google Scholar]
- Mawer EB, Hayes ME, Still PE, et al. Evidence for non-renal synthesis of 1,25-dihydroxyvitamin D in patients with inflammatory arthritis. J Bone Miner Res. 1991;6:733–739. doi: 10.1002/jbmr.5650060711. [DOI] [PubMed] [Google Scholar]
- Hayes ME, Denton J, Freemont AJ, Mawer EB. Synthesis of the active metabolite of vitamin D, 1,25(OH)2D3, by synovial fluid macrophages in arthritic diseases. Ann Rheum Dis. 1989;48:723–729. doi: 10.1136/ard.48.9.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow LC, Smith SJ, Mawer EB, Woolley DE. Vitamin D receptors in the rheumatoid lesion: expression by chondrocytes, macrophages and synoviocytes. Ann Rheum Dis. 1999;58:118–121. doi: 10.1136/ard.58.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DE, Harris Ed , Jr, Mainardi CL, Brinckerhoff CE. Collagenase immunolocalisation in cultures of rheumatoid synovial cells. Science. 1978;200:773–775. doi: 10.1126/science.205952. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Hibbs MS, Amento EP. Constitutive expression of a 92-kDa gelatinase by rheumatoid synovial fibroblasts by inflammatory cytokines. J Clin Invest. 1991;88:1656–1662. doi: 10.1172/JCI115480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow LC, Lees M, Woolley DE. Comparative studies of collagenase and stromelysin 1 expression by rheumatoid synoviocytes in vitro . Virchows Arch B: Cell Pathol . 1995;425:569–576. doi: 10.1007/BF00199344. [DOI] [PubMed] [Google Scholar]
- Okada Y, Taakeuchi N, Tomita K, Nakanishi I, Nagase H. Immunolocalisation of matrix metalloproteinase-3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989;48:645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravellese EM, Darling JM, Ladd AL, Katz JN, Glimcher LH. In situ hybridisation studies of stromelysin and collagenase mRNA expression in rheumatoid synovium. Arthritis Rheum. 1991;34:1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Woolley DE. Comparative immunolocalisation studies of collagenase 1 and collagenase 3 production in the rheumatoid lesion, and by human chondrocytes and synoviocytes in vitro . . Br J Rheumatol. 1998;37:64–70. doi: 10.1093/rheumatology/37.1.64. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H. Matrix metalloproteinases: a review. . Crit Rev Oral Biol. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Dayer JM, Krane SSM, Russell RGG, Robinson DR. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci USA. 1976;73:945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meats JE, McGuire MK, Russell RGG. Human synovium releases a factor which stimulates chondrocyte production of PGE and plasminogen activator. Nature. 1980;286:891–892. doi: 10.1038/286891a0. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Harper N, Dunningham T, et al. Effects of induced mast cell activation on prostaglandin E and metalloproteinase production by rheumatoid synovial tissue in vitro . Ann Rheum Dis. 1998;57:25–32. doi: 10.1136/ard.57.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Morodomi T, Enghild JJ, et al. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts: purification and activation of the precursor and enzymatic properties. Eur J Biochem. 1990;194:721–730. doi: 10.1111/j.1432-1033.1990.tb19462.x. [DOI] [PubMed] [Google Scholar]
- Goodwin JS. Are prostaglandins proinflammatory, antiinflammatory, both or neither? J Rheumatol. 1991;18 (suppl 28):26–29. [PubMed] [Google Scholar]
- Dayer J-M, Goldring SR, Robinson DR, Krane SM. Cell-cell interactions and collagenase production. Collagenase in Normal and Pathological Connective Tissues. Edited by Woolley DE, Evanson JM. Chichester, UK: John Wiley & Sons; 1980. pp. 83–104.
- Yaron I, Meyer FA, Dayer J-M, Bleiberg I, Yaron M. Some recombinant human cytokines stimulate glycosaminoglycan synthesis in human synovial fibroblast cultures and inhibit it in human articular cartilage cultures. Arthritis Rheum. 1989;32:173–176. doi: 10.1002/anr.1780320210. [DOI] [PubMed] [Google Scholar]
- Woolley DE, Tetlow LC. Observations on the microenvironmental nature of cartilage degradation in rheumatoid arthritis. Ann Rheum Dis. 1997;56:151–161. doi: 10.1136/ard.56.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]


