Abstract
DNA molecules containing unmethylated CpG-dinucleotides in particular base contexts (“CpG motifs”) are excellent adjuvants in rodents, but their effects on human cells have been less clear. Dendritic cells (DCs) form the link between the innate and the acquired immune system and may influence the balance between T helper 1 (Th1) and Th2 immune responses. We evaluated the effects of CpG oligodeoxynucleotides alone or in combination with granulocyte–macrophage colony-stimulating factor (GMCSF) on different classes of purified human DCs. For primary dendritic precursor cells isolated from human blood, CpG oligonucleotides alone were superior to GMCSF in promoting survival and maturation (CD83 expression) as well as expression of class II MHC and the costimulatory molecules CD40, CD54, and CD86 of DCs. Both CD4-positive and CD4-negative peripheral blood dendritic precursor cells responded to CpG DNA which synergized with GMCSF but these DCs showed little response to lipopolysaccharide (LPS). In contrast, monocyte-derived DCs did not respond to CpG, but they were highly sensitive to LPS, suggesting an inverse correlation between CpG and LPS sensitivity in different subsets of DCs. Compared with GMCSF, CpG-treated peripheral blood DCs showed enhanced functional activity in the mixed lymphocyte reaction and induced T cells to secrete increased levels of Th1 cytokines. These findings demonstrate the ability of specific CpG motifs to strongly activate certain subsets of human DCs to promote Th1-like immune responses, and support the use of CpG DNA-based trials for immunotherapy against cancer, allergy, and infectious diseases.
Keywords: adjuvant, immunotherapy, therapeutic DNA, oligodeoxynucleotide
The vertebrate immune system has the ability to recognize the presence of bacterial DNA on the basis of recognition of so-called CpG motifs, unmethylated cytidine-guanosine dinucleotides within a specific pattern of flanking bases (1). It is known from the literature that CpG oligonucleotides are excellent adjuvants in murine models. CpG DNA is as potent as the complete Freund’s adjuvant regarding the induction of B cell and T cell responses, but it is less toxic and it induces a T helper 1 (Th1) response (2, 3). Alum, the adjuvant that is used routinely in human vaccination, induces the less favorable Th2 response. CpG is more effective than alum, and the combination of CpG and alum shows synergy in mice (4, 5). CpG oligonucleotides enhance the efficacy of immunization with tumor antigen in a murine B cell lymphoma model (2), induce antigen-specific cytotoxic T cell responses (5, 6), and have utility in the immunotherapy of allergy, infectious disease, and cancer disease models (7–9). Furthermore, the presence of immunostimulatory DNA sequences in plasmids has been shown to be required for effective intradermal gene immunization (10).
Dendritic cells (DCs) form the link between the innate and the acquired immune system by presenting antigens and by their expression of pattern recognition receptors that detect microbial molecules in their local environment. The use of DCs as a cellular adjuvant is a promising approach in immunotherapy of infectious disease and cancer. Numerous animal models demonstrate conclusively that ex vivo generated DCs pulsed with protein antigen are useful for the immunotherapy of infectious diseases and cancer (11–16). Initial clinical studies indicate that tumor antigen-pulsed DCs can be effective in the immunotherapy of cancer patients (17–20).
Three different protocols, each using a different cell of origin, are in use to obtain human DCs. These include (i) monocyte-derived DCs (21), (ii) stem cell-derived DCs (22), and (iii) isolation of DCs from peripheral blood (23). Recently, studies involving monocyte-derived DCs have attracted major attention. The incubation of purified CD14-positive monocytes with granulocyte–macrophage colony-stimulating factor (GMCSF) and IL-4 followed by maturation with pro-inflammatory cytokines or lipopolysaccharide (LPS) provides large numbers of DCs within 1 week. Because monocyte-derived DCs tend to dedifferentiate toward macrophages in the absence of IL-4 (24), DCs generated in this manner may not reflect the physiologic situation.
In the present study we hypothesize that much of the adjuvant activity of CpG is based on the direct activation of DCs by CpG. To test this hypothesis, we examined the effects of immunostimulatory CpG oligonucleotides and control oligonucleotides on monocyte-derived DCs, and on peripheral blood DCs isolated by immunomagnetic cell sorting. We found that peripheral blood DCs, but not monocyte-derived DCs, show remarkably strong responses to CpG-containing DNA which synergizes with GMCSF. Our results suggest that treatment with the combination of CpG and DCs, with or without GMCSF, may be a promising immunotherapeutic strategy.
MATERIALS AND METHODS
Oligodeoxynucleotides.
The optimal motif recognized by human immune cells is different from the optimal mouse motif. We tested a large number of oligonucleotides for their ability to activate human B cells and natural killer (NK) cells (unpublished work), and we selected particularly potent oligonucleotides as examples of a family of active CpG-containing oligonucleotides for use in the present study. The CpG oligonucleotides (Operon Technologies, Alameda, CA) used were 2006 (24-mer), 5′-TCGTCGTTTTGTCGTTTT- GTCGTT-3′, completely phosphorothioate-modified, and 2080 (20-mer), 5′-TCGTCGTTCCCCCCCCCCCC-3′, unmodified phosphodiester. The non-CpG control oligonucleotides used were 2117 (24-mer), 5′-TQGTQGTTTTGTQGT- TTTGTQGTT-3′, in which Q = 5-methylcytidine, completely phosphorothioate-modified, 2041 (24-mer), 5′-CTGGTCTTTCTGGTTTTTTTCTGG-3′, completely phosphorothioate-modified, and 2078 (20-mer), 5′-TGCTGCTTCCCCCCC- CCCCC-3′, unmodified phosphodiester. Oligonucleotides were diluted in TE (10 mM Tris⋅HCl/1 mM EDTA, pH 8) using pyrogen-free reagents. Phosphorothioate oligonucleotides (2006 and 2117) were added at a final concentration of 2 μg/ml. On the basis of preliminary experiments in which no effect was seen after a single addition, phosphodiester oligonucleotides were added at 0, 12, and 24 h at a concentration of 30 μg/ml.
Detection of Endotoxin.
The activity of LPS was measured by using the Limulus amebocyte lysate (LAL) assay [endotoxin units (EU)/ml]. The lower detection limit of the LAL assay in our hands was 0.03 EU/ml (LAL-assay BioWhittaker, Walkersville, MD). The LPS sample used in our studies (from Salmonella typhimurium, Sigma catalog no. L2262) had an activity of 4.35 ng/EU. No endotoxin could be detected in the oligonucleotides (<0.075 EU/mg).
Isolation of DCs.
DCs were isolated from peripheral blood by using the VARIOMACS technique (Miltenyi Biotec, Auburn, CA) and the technique previously described (23). Peripheral blood mononuclear cells (PBMC) were obtained from buffy coats of healthy blood donors (Elmer L. DeGowin Blood Center, University of Iowa) by Ficoll-Paque density gradient centrifugation (Histopaque-1077, Sigma) as described (25). PBMC were incubated with anti- CD3, CD14, CD16, CD19, and CD56 antibodies conjugated to colloidal superparamagnetic microbeads and passed over a depletion column in a strong magnetic field. Cells in the flow-through were incubated with a microbead-conjugated antibody to CD4 and passed over a positive selection column. Viability was determined by trypan blue exclusion (>95%). In select experiments, the CD4-negative fraction was also collected for study. Monocyte-derived DCs were generated by incubation of purified monocytes (obtained by immunomagnetic separation) in media containing GMCSF (800 units/ml, Genzyme, Boston) and IL-4 (500 units/ml, Genzyme). Fresh cytokines were added every other day. After 5 days, more than 95% of the cells were lineage-negative and expressed CD1a and high levels of MHC II. LPS or CpG oligonucleotides were added on day 5, and activation (CD86) was measured at day 7.
Cell Culture.
Cells were suspended in RPMI 1640 culture medium supplemented with 10% (vol/vol) heat-inactivated (56°C, 1 h) FCS (HyClone), 1.5 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (all from GIBCO/BRL) (complete medium). All compounds were purchased endotoxin-tested. Freshly prepared dendritic precursor cells (final concentration 3 × 105 cells per ml) were cultured in complete medium containing 800 units/ml GMCSF (1.25 × 104 units/mg; Genzyme), 10 ng/ml LPS (described above), or oligonucleotides as indicated. In some experiments, prostaglandin E2 (Sigma, dissolved in ethanol) was added from day 2 to day 3 (final concentration 10−7 M).
Surface Antigen Staining.
At the indicated time points, cells were harvested and surface antigen staining was performed as described (26). Monoclonal antibodies to HLA-DR (Immu-357), CD80 (MAB104), and CD83 (HB15A) were purchased from Immunotech (Marseille, France). All other antibodies were purchased from PharMingen (San Diego): mAbs to CD1a (HI149), CD3 (UCHT1), CD14 (M5E2), CD19 (B43), CD40 (5C3), CD54 (HA58), and CD86 [2331 (FUN-1)]. FITC-labeled IgG1,κ (MOPC-21) and phycoerythrin-labeled IgG2b,κ (27) were used to control for specific staining.
Flow Cytometry.
Flow cytometric data were acquired on a FACScan (Becton Dickinson Immunocytometry systems, San Jose, CA). Spectral overlap was corrected by appropriate compensation. Analysis was performed on viable cells within a morphologic gate [forward scattering (FSC), side scattering (SSC), >94% of cells MHC II positive and lineage marker negative]. Data were analyzed with the computer program flowjo (version 2.5.1, Tree Star, Stanford, CA).
Staining with 5-(and 6-)Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE).
CFSE (Molecular Probes) is a fluorescein-derived intracellular fluorescent label that is divided equally between daughter cells upon cell division. Staining of cells with CFSE allows both quantification and immunophenotyping (phycoerythrin-labeled antibodies) of proliferating cells in a mixed cell suspension. The technique is described in detail by Lyons et al. (27). Briefly, monocyte-depleted PBMC were washed twice in PBS, resuspended in PBS (1 × 107 cells per ml) containing CFSE at a final concentration of 5 μM, and incubated at 37°C for 10 min. Cells were washed three times with PBS and used as responder cells for the mixed lymphocyte reaction (MLR).
MLR.
A total of 100,000 CFSE-stained monocyte-depleted PBMC (responder cells) were added to each well (100 μl, 96-well plate, U-bottom). DCs or macrophages (stimulator cells) from a different donor were harvested, washed two times in PBS, resuspended in complete medium, and added to the responder cells at the indicated concentrations. After 5 days, cells were harvested, and CD3-positive cells (T cells) were analyzed for their CFSE content by flow cytometry. Proliferating cells show a lower CFSE staining than nonproliferating cells. IFN-γ and IL-5 were determined in the supernatants by ELISA (R & D Systems, Minneapolis).
Statistical Analysis.
Data are expressed as means ± SEM. Statistical significance of differences was determined by the unpaired two-tailed Student’s t test. Differences were considered statistically significant for P < 0.05. Statistical analyses were performed by using statview 4.51 software (Abacus Concepts, Calabasas, CA).
RESULTS
Generation and Characterization of Peripheral Blood DCs.
Immunomagnetic depletion of lineage-positive cells and subsequent positive selection of CD4-positive cells allows the isolation of dendritic precursor cells from peripheral blood (see Materials and Methods). We obtained 0.7 to 2.4 × 106 DCs from buffy coats (2.5 to 5 × 108 mononuclear cells). The purity of the DC preparation (MHC II bright, lineage marker negative) varied from 94% to 99%.
CpG as a Growth Factor for Dendritic Precursor Cells.
The presence of GMCSF promotes survival of freshly isolated dendritic precursor cells from peripheral blood. In the absence of GMCSF, dendritic precursor cells undergo apoptosis within 2 days. Freshly isolated cells were incubated in the presence of GMCSF or CpG oligonucleotides (2006, CpG phosphorothioate; 2080, CpG phosphodiester) for 48 h. Light microscopy revealed the formation of cell clusters in response to GMCSF and the CpG oligonucleotides within 1 day. Cell survival was improved in the presence of both CpG phosphorothioate and phosphodiester oligonucleotides (Fig. 1). A single addition of 2006 (2 μg/ml) to freshly prepared DCs was superior to GMCSF (800 units/ml) in promoting cell survival (74.3% ± 5.2% vs. 57.1% ± 2.3%). The combination of GMCSF and 2006 further increased the number of viable cells (81.0% ± 6.7%). In the presence of the control oligonucleotide 2117 (2 μg/ml) cell survival was low and comparable to the sample without GMCSF or CpG DNA (10.8% ± 5.2% and 7.4% ± 4.2%). The CpG phosphorothioate oligonucleotide (2006) was effective at a lower concentration (2 μg/ml) than the nuclease-sensitive phosphodiester oligonucleotide (30 μg/ml at 0, 12, and 24 h).
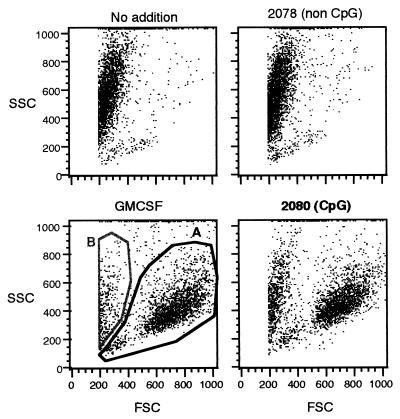
Figure 1.
CpG oligonucleotides promote survival of dendritic precursor cells. Freshly isolated dendritic precursor cells were incubated for 2 days with oligonucleotides or GMCSF (800 units/ml) and analyzed by flow cytometry. Viable cells were found in region A, nonviable cells in region B. Both the CpG oligonucleotide (2080 CpG phosphodiester oligonucleotide, 3 × 30 μg/ml) and GMCSF promote survival of dendritic precursor cells. The non-CpG control oligonucleotide (2078: identical to 2080 but GpCs instead of CpGs) showed no positive effect on cell survival. Results are representative for eight independent experiments.
CpG Induces Differentiation of Dendritic Precursor Cells.
Freshly isolated DCs have the appearance of medium-sized lymphocytes. After 2 days of incubation with GMCSF, the cells showed typical characteristics of DCs (CD3neg, CD14neg, CD19neg, CD4pos, MHC IIbright, CD54pos, CD80dim, CD86dim, CD40dim). Morphologically, they enlarged and exhibited sheet-like cell processes. Differentiation of dendritic precursor cells is reflected by the up-regulation of MHC II and an increase in cell size (FSC) and granularity (SSC). The addition of either GMCSF or the CpG oligonucleotide 2080 enhanced both granularity (SSC) and MHC II expression of DCs (Fig. 1). MHC II expression with CpG was higher than with GMCSF. Without addition of GMCSF or CpG oligonucleotide, only a small fraction of cells showed high granularity (SSC) and high expression of MHC II (Figs. 1 and 2, Upper Left) representing differentiated DCs.
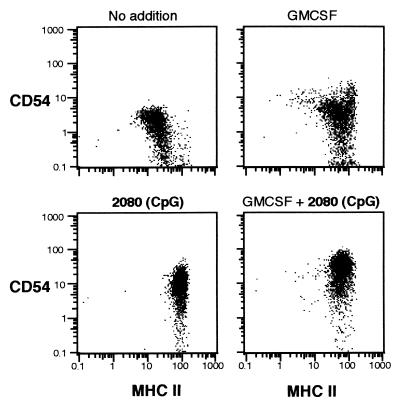
Figure 2.
CpG-induced expression of ICAM-1 and MHC II on dendritic precursor cells. Dendritic precursor cells were incubated for 48 h in the presence of GMCSF (800 units/ml) and phosphodiester oligonucleotides (2080: CpG; 2078: non-CpG, added at 0, 12, and 24 h; 30 μg/ml at each time point). Expression of MHC II and CD54 was examined by flow cytometry. Viable cells (2,500 per sample) were counted.
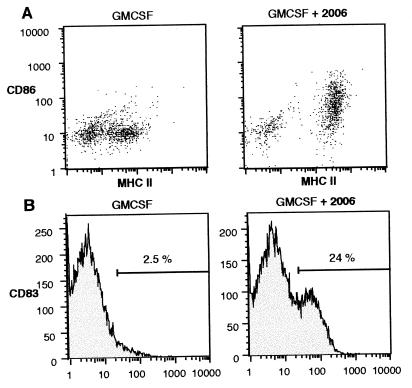
CpG Up-Regulates Costimulatory Molecules on Peripheral Blood DCs.
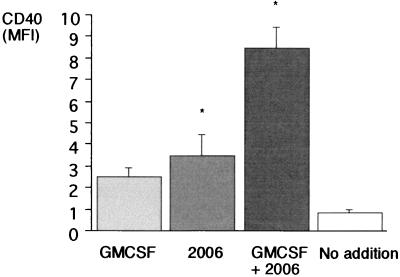
Functional activity of DCs requires the expression of costimulatory molecules. We examined the effect of CpG on the expression of the intercellular adhesion molecule 1 (ICAM-1, CD54) and the costimulatory surface molecules B7–2 (CD86) and CD40. We found that the expression of MHC II on DCs is not associated with the activation of DCs (CD54, Fig. 2). The CpG oligonucleotide 2006 (2 μg/ml) enhanced the expression of CD54 [mean fluorescence intensity (MFI) 25.0 vs. 7.0; P = 0.02, n = 5], CD86 (MFI 3.9 vs. 1.6; P = 0.01; n = 5), and CD40 (MFI 3.5 vs. 0.9; P = 0.04, n = 4; Fig. 3). The combination of GMCSF and 2006 showed a synergistic effect for CD86 and CD40 (MFI CD86: 7.0; P = 0.01; n = 5; CD40: 8.5; P < 0.01; n = 4).
Figure 3.
Activation of peripheral blood DCs. Dendritic precursor cells were incubated for 48 h in the presence of GMCSF (800 units/ml) and the CpG phosphorothioate oligonucleotide (2 μg/ml) as indicated. Expression of CD40 was examined (MFI, mean fluorescence intensity). Results represent the mean of four independent experiments; error bars indicate SEM. Statistical significance is indicated by ∗ (P < 0.05).
CpG specificity was confirmed with the control oligonucleotides 2117 (methylated version of 2006) and 2078 (similar to 2080 but CpG dinucleotide switched to GpC). In contrast to 2006, 2117 did not enhance expression of costimulatory molecules compared with GMCSF alone (MFI CD54: 31.9 ± 3.7 vs. 21.5 ± 0.9 vs. 19.4 ± 2; CD86: 3.5 ± 0.8 vs. 1.4 ± 0.2 vs. 1.3 ± 0.4; CD40: 7.2 ± 1.6 vs. 3.3 ± 0.3 vs. 2.2 ± 0.5; n = 2). Interestingly, LPS (10 ng/ml) showed no activation of dendritic precursor cells (MFI CD54: 21.6 ± 4.2; CD86: 1.2 ± 0.2; CD40: 2.2 ± 0.3). This result was surprising, because a 10-fold lower concentration of LPS (1 ng/ml) stimulates human CD14-positive monocytes to express CD54 and CD86, and to produce the proinflammatory cytokines tumor necrosis factor (TNF) and IL-6. TNF synthesis of monocytes can be found for LPS concentrations as low as 10 pg/ml, and 1 ng/ml already induces a maximal cytokine response (28).
CpG Induces Maturation of Peripheral Blood DCs.
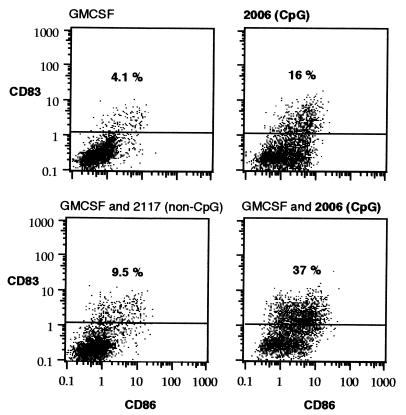
Expression of CD83 is characteristic for mature DCs. Peripheral blood DCs incubated with GMCSF contain only few CD83-positive cells (Fig. 4). The addition of LPS (10 ng/ml) or control oligonucleotide 2117 slightly increased the percentage of CD83-positive cells (from 4.1% to 8.6% and 9.7%, not shown). The addition of 2006 (2 μg/ml) increased the percentage of CD83-positive cells to 37%.
Figure 4.
CpG induces maturation (CD83 expression) of DCs. Freshly prepared peripheral blood DCs were incubated for 3 days with GMCSF (800 units/ml) and oligonucleotides (2006, CpG phosphorothioate; 2117, methylated 2006; 2 μg/ml). Values (%) represent the percentage of CD83-positive cells. Results are representative of four independent experiments.
CpG DNA Stimulates Dendritic Cell Development from CD4-Negative Peripheral Blood Precursors.
As shown earlier in Fig. 2, freshly prepared CD4-positive dendritic cell precursors express already high levels of MHC II, which is further enhanced in the presence of CpG DNA. Because CpG was found to function as a survival factor for DCs, we also examined the CD4-negative fraction of lineage marker-negative PBMC, which may contain CD4-negative dendritic precursor cells as well. This fraction contained a variable percentage (50–80%) of cells that expressed low amounts of MHC II and cells that were MHC II negative (Fig. 5). Although GMCSF alone did not influence the expression of MHC II by cells in this fraction, CpG DNA induced increased expression of MHC II as well as the expression of the costimulatory molecules CD86 (Fig. 5A), CD40, and CD54 (not shown). Surprisingly, CpG DNA alone was sufficient to induce CD83-positive mature DCs from these MHC II-low and CD4-negative dendritic precursor cells within 3 days (Fig. 5B).
Figure 5.
CpG induces MHC II, CD86, and CD83 on CD4-negative peripheral blood dendritic precursor cells. Lineage-positive cells and CD4-positive dendritic precursor cells were depleted from PBMC by immunomagnetic cell sorting. Remaining cells were incubated with GMCSF (800 units/ml) and the CpG oligonucleotide 2006 (2 μg/ml) as indicated. After 72 h, cells were stained with MHC II (differentiation marker), CD86 (costimulatory molecule), and CD83 (maturation marker) and examined by flow cytometry. (A) MHC II and CD86 expression is depicted. (B) Expression of CD83 on MHC II-positive cells is analyzed.
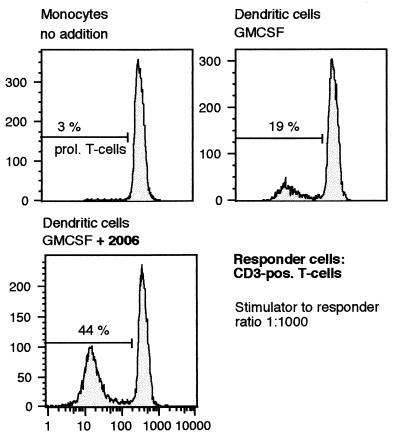
Functional Activity of Peripheral Blood DCs in Response to CpG.
We compared the functional activity of DCs by using the MLR. DCs and macrophages from the same donor were used as stimulator cells; monocyte-depleted lymphocytes from a different donor, as responder cells. The CFSE method (see Materials and Methods) was chosen to examine the proliferative response of T cells for the following reasons: (i) the degradation products of the oligonucleotides interfere with the standard [3H]thymidine proliferation assay; (ii) proliferating T cells can be distinguished from proliferating B cells by CD3 vs. CD19 staining (B cell proliferation is induced by low concentrations of CpG oligonucleotide independent of stimulator cells); and (iii) irradiation of stimulator cells is avoided. DCs incubated with both GMCSF and 2006 showed the highest stimulatory activity (Fig. 6). At a stimulator-to-responder ratio of 1:1000, DCs incubated with GMCSF and 2006 induced a higher T cell proliferation than did DCs incubated with GMCSF alone (43.1% ± 1.2% vs. 18.5% ± 0.8%, n = 2). The control oligonucleotide 2117 or LPS only slightly changed the stimulatory capacity of GMCSF-treated DCs (25% ± 1.0% and 20% ± 3.1%, not shown in Fig. 6). Monocytes did not stimulate T cell proliferation at a stimulator-to-responder ratio of 1:1000. In the presence of high numbers of DCs (stimulator-to-responder ratio 1:10), all dendritic cell preparations reached the maximum of 50% proliferative T cells, while activity of monocytes was still low (15.4% ± 0.4%). To examine the Th1 vs. Th2 pattern of cytokines, we measured the level of IFN-γ and IL-5 in the supernatants of the MLR. In the presence of DCs previously incubated with CpG DNA, the ratio of IFN-γ to IL-5 was higher than without CpG DNA (5.0 ± 0.8 vs. 2.8 ± 0.3), indicating CpG enhances the Th1 response. Prostaglandin E2 did not reverse the CpG effect regarding the Th1 response (4.8 ± 0.9). DCs generated from CD4-negative precursor cells were also functionally active in the MLR. CpG-treated cells were more active than were GMCSF-treated cells. The functional activity was further enhanced in the presence of prostaglandin E2 (10−7 M) (data not shown).
Figure 6.
Functional activity of CpG-treated peripheral blood DCs. DCs were incubated with GMCSF (800 units/ml) or the CpG oligonucleotide 2006 (2 μg/ml) as indicated. After 48 h, cells were harvested, and an allogeneic MLR was performed using viable DCs (trypan blue exclusion) or CD14-positive monocytes (immunomagnetic separation) from the same donor as stimulator cells. Lymphocytes (monocyte-depleted PBMC, CFSE-stained, see Materials and Methods) from a different donor were used as responder cells. After 5 days of coincubation, proliferation of CD3 (phycoerythrin)-positive T cells was determined by quantification of CFSE (proliferating cells show lower CFSE staining). Histograms for the stimulator-to-responder ratio 1:1,000 are shown. Data are representative for two independent experiments performed in duplicate.
Monocyte-Derived DCs Do Not Respond to CpG.
DCs can be obtained in large numbers by incubation of CD14-positive monocytes with GMCSF and IL-4 for 5 days. However, upon withdrawal of IL-4 these cells lose their dendritic cell characteristics and become CD14-positive macrophages (24). In a large series of experiments testing different conditions we found that monocyte-derived DCs are not activated by CpG motifs, but are highly sensitive to LPS or tumor necrosis factor (data not shown).
Ultrastructural Changes of Peripheral Blood DCs in Response to CpG.
With scanning electron microscopy, DCs cultivated in the presence of CpG DNA displayed a more irregular shape, longer veil processes, and more sheet-like projections than did cells cultivated with GMCSF alone. With transmission electron microscopy, CpG DNA-treated DCs showed high-density multilaminar and multivesicular intracytoplasmic bodies, and less heterochromatin in the nucleus (not shown).
DISCUSSION
There is evidence from mouse models that CpG DNA acts as a potent vaccine adjuvant for promoting Th1-like immune responses (2–7, 10, 29–32). In contrast, very little published data about CpG DNA effects on human cells are available (10, 28, 33), and no reports whether CpG DNA may activate human DCs, which is currently an area of great therapeutic interest, and where GMCSF has shown much promise. In the present report we demonstrate that CpG DNA is a more potent stimulus than GMCSF for inducing primary blood DC survival, differentiation, activation, maturation, and the functional ability to promote a Th1-like T cell response.
CpG DNA was superior to GMCSF in preserving in vitro survival of primary blood DCs and inducing differentiation, which was reflected by an increase in cell size, granularity, and MHC II expression. CpG treatment led to activation of DCs as represented by up-regulation of the costimulatory molecules ICAM-1 (CD54), B7–2 (CD86), and CD40 and to maturation indicated by expression of CD83 (34). The combination of CpG DNA and GMCSF increased activation and maturation synergistically, indicating they must work through different pathways. CpG-generated DCs were more effective at inducing T-cell proliferation in an MLR and induced more IFN-γ but less IL-5 than DCs matured in the absence of CpG, supplying further evidence that CpG enhances a Th1 response. Decreased IL-5 synthesis in the presence of CpG-DNA was also described by others (35). The effects of CpG oligonucleotides on primary blood DCs were CpG specific, since control oligonucleotides with methylated CpGs and oligonucleotides with GpC instead of CpGs were inactive. Both phosphorothioate and phosphodiester oligonucleotides containing CpG motifs were active. However, as described earlier (36), high concentrations of phosphorothioate oligonucleotides showed a CpG-independent background activity.
Peripheral blood DCs but not monocyte-derived DCs responded to CpG DNA. In contrast, primary blood DCs showed only little response to LPS, whereas monocyte-derived DCs were highly sensitive to LPS, suggesting an inverse relation of LPS and CpG sensitivity. Recently we found that the response of human monocytes to CpG is low (28). One might speculate that the low CpG sensitivity of human monocytes is maintained during generation of DCs from human monocytes with GMCSF and IL-4.
Ultrastructural examination of DCs generated in the presence of CpG DNA revealed electron-dense multilaminar intracytoplasmic bodies and multivesicular structures, which were not present in DCs generated with GMCSF alone. Multilaminar membrane structures have previously been described as MHC class II compartments with a highly specialized role in antigen processing and presentation. High numbers of these multilaminar structures were found in DCs derived from CD34-positive progenitor cells, whereas they were less frequent in monocyte-derived DCs (37).
It has been shown in mice that CpG up-regulates MHC II and costimulatory molecules on murine Langerhans cells (38). In another study, similar changes were described for murine bone marrow-derived DCs (39). In both studies DCs were defined by their expression of MHC II, which may include other myelomonocytic cells in the analysis as well. In contrast to human monocytes, murine monocytes/macrophages are known to secrete high amounts of inflammatory cytokines in response to CpG. Thus, a secondary indirect effect of CpG on DCs in these cell preparations might have contributed to the activation of DCs. In the present study we show that purified human blood DCs are highly sensitive to CpG, whereas their response to LPS is barely detectable.
Recently the central role of CD40 ligation for the so-called superactivation of DCs has been identified (40–43). CD40 ligation on DCs is required for the DC-dependent induction of cytotoxic T cells from naive T cells. CD40 ligand present on the surface of activated T helper cells provides this signal under physiologic circumstances. CpG and CD40 both activate c-Jun NH2-terminal kinase and p38, but do not activate the extracellular receptor kinase (Erk) in B cells (G.H. and A.M.K., unpublished results) (44–46).
There are a number of potential advantages of CpG-derived blood DCs for ex vivo immunotherapeutic strategies compared with monocyte-derived or stem cell-derived DCs. The use of CpG allows for rapid generation of functionally active DCs within 2 days. CpG DNA is a single, easily manufactured, chemically defined reagent. CpG-derived DCs enhance Th1 activity, whereas IL-4 used in the other approaches is a Th2 cytokine, which theoretically may be less effective for the generation of optimal cytotoxic T cell responses. CpG treatment results in DCs with physiologic characteristics, as compared with monocyte-derived DCs with a tendency to dedifferentiate toward macrophages. Further studies will be required to determine whether these potential benefits are of practical significance.
Our data also suggest that systemic administration of CpG could enhance the availability of immature and mature DCs in the blood and in tissues, and so increase the efficacy of immunization. On the basis of their synergistic activity in vitro, the combination of CpG and GMCSF may be useful in vivo in humans, as we have demonstrated in mice (47). Obviously, combination of CpG with other reagents that increase the number of dendritic precursor cells in peripheral blood such as Flt3 ligand (48) might further improve the method for clinical application. In conclusion, the present study provides the functional rationale and the methods for the use of CpG for DC-based immunotherapy against cancer and infectious disease.
Acknowledgments
We thank Ae-Kyung Yi and Bernhard Noll for helpful discussions. G.H. is supported by Grant Ha 2780/1-1 of the Deutsche Forschungsgemeinschaft. A.M.K. was supported by grants from the Department of Veterans Affairs and by National Institutes of Health Grant PO1CA66570. Services were provided by the University of Iowa Diabetes and Endocrinology Center (National Institutes of Health Grant DK25295). Support was also received from CpG ImmunoPharmaceuticals, Hilden, Germany, and Wellesley, MA.
ABBREVIATIONS
- Th1 and Th2
T helper 1 and 2
- DCs
dendritic cells
- GMCSF
granulocyte–macrophage colony-stimulating factor
- LPS
lipopolysaccharide
- PBMC
peripheral blood mononuclear cells
- CFSE
5-(and 6-)carboxyfluorescein diacetate succinimidyl ester
- MLR
mixed lymphocyte reaction
- ICAM-1
intercellular adhesion molecule 1
- MFI
mean fluorescence intensity
References
- 1.Krieg A M, Yi A-K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. Nature (London) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 2.Weiner G J, Liu H M, Wooldridge J E, Dahle C E, Krieg A M. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu R S, Targoni O S, Krieg A M, Lehmann P V, Harding C V. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazolot-Millan C L, Weeratna R, Krieg A M, Siegrist C A, Davis H L. Proc Natl Acad Sci USA. 1998;95:15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis H L, Weeranta R, Waldschmidt T J, Tygrett L, Schorr J, Krieg A M. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 6.Lipford G B, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 7.Wooldridge J E, Ballas Z, Krieg A M, Weiner G J. Blood. 1997;89:2994–2998. [PubMed] [Google Scholar]
- 8.Zimmermann S, Egeter O, Hausmann S, Lipford G B, Rocken M, Wagner H, Heeg K. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]
- 9.Kline J N, Waldschmidt T J, Businga T R, Lemish J E, Weinstock J V, Thorne P S, Krieg A M. J Immunol. 1998;160:2555–2559. [PubMed] [Google Scholar]
- 10.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 11.Fields R C, Shimizu K, Mule J J. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada H, Tahara H, Shurin M R, Attanucci J, Giezeman-Smits K M, Fellows W K, Lotze M T, Chambers W H, Bozik M E. Int J Cancer. 1998;78:196–201. doi: 10.1002/(sici)1097-0215(19981005)78:2<196::aid-ijc13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Su H, Messer R, Whitmire W, Fischer E, Portis J C, Caldwell H D. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMatos P, Abdel-Wahab Z, Vervaert C, Hester D, Seigler H. J Surg Oncol. 1998;68:79–91. doi: 10.1002/(sici)1096-9098(199806)68:2<79::aid-jso3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Yang S, Darrow T L, Vervaert C E, Seigler H F. Cell Immunol. 1997;179:84–95. doi: 10.1006/cimm.1997.1151. [DOI] [PubMed] [Google Scholar]
- 16.Nair S K, Snyder D, Rouse B T, Gilboa E. Int J Cancer. 1997;70:706–715. doi: 10.1002/(sici)1097-0215(19970317)70:6<706::aid-ijc13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Nestle F O, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg S A, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Dudley M E, Schwarz S L, Spiess P J, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu F J, Benike C, Fagnoni F, Liles T M, Czerwinski D, Taidi B, Engleman E G, Levy R. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 20.Tjoa B A, Simmons S J, Bowes V A, Ragde H, Rogers M, Elgamal A, Kenny G M, Cobb O E, Ireton R C, Troychak M J, et al. Prostate. 1998;36:39–44. doi: 10.1002/(sici)1097-0045(19980615)36:1<39::aid-pros6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 22.Mortarini R, Anichini A, Di Nicola M, Siena S, Bregni M, Belli F, Molla A, Gianni A M, Parmiani G. Cancer Res. 1997;57:5534–5541. [PubMed] [Google Scholar]
- 23.O’Doherty U, Steinman R M, Peng M, Cameron P U, Gezelter S, Kopeloff I, Swiggard W J, Pope M, Bhardwaj N. J Exp Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hausser G, Ludewig B, Gelderblom H R, Tsunetsugu-Yokota Y, Akagawa K, Meyerhans A. Immunobiology. 1997;197:534–542. doi: 10.1016/S0171-2985(97)80085-X. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann G, Krug A, Eigler A, Moeller J, Murphy J, Albrecht R, Endres S. Antisense Nucleic Acid Drug Dev. 1996;6:291–299. doi: 10.1089/oli.1.1996.6.291. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann G, Krug A, Bidlingmaier M, Hacker U, Eigler A, Albrecht R, Strasburger C J, Endres S. J Pharmacol Exp Ther. 1998;285:920–928. [PubMed] [Google Scholar]
- 27.Lyons A B, Parish C R. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 28.Hartmann G, Krieg A M. Gene Ther. 1999;6:893–903. doi: 10.1038/sj.gt.3300880. [DOI] [PubMed] [Google Scholar]
- 29.Moldoveanu Z, Love-Homan L, Huang W Q, Krieg A M. Vaccine. 1998;16:1216–1224. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- 30.Sun S, Zhang X, Tough D F, Sprent J. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krieg A M, Love H L, Yi A K, Harty J T. J Immunol. 1998;161:2428–2434. [PubMed] [Google Scholar]
- 32.Anitescu M, Chace J H, Tuetken R, Yi A K, Berg D J, Krieg A M, Cowdery J S. J Interferon Cytokine Res. 1997;17:781–788. doi: 10.1089/jir.1997.17.781. [DOI] [PubMed] [Google Scholar]
- 33.Macfarlane D E, Manzel L, Krieg A M. Immunology. 1997;91:586–593. doi: 10.1046/j.1365-2567.1997.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L J, Tedder T F. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]
- 35.Broide D, Schwarze J, Tighe H, Gifford T, Nguyen M D, Malek S, Van U J, Martin O E, Gelfand E W, Raz E. J Immunol. 1998;161:7054–7062. [PubMed] [Google Scholar]
- 36.Hartmann G, Krug A, Waller-Fontaine K, Endres S. Mol Med. 1996;2:429–438. [PMC free article] [PubMed] [Google Scholar]
- 37.Herbst B, Kohler G, Mackensen A, Veelken H, Mertelsmann R, Lindemann A. Br J Haematol. 1997;99:490–499. doi: 10.1046/j.1365-2141.1997.4283238.x. [DOI] [PubMed] [Google Scholar]
- 38.Jakob T, Walker P S, Krieg A M, Udey M C, Vogel J C. J Immunol. 1998;161:3042–3049. [PubMed] [Google Scholar]
- 39.Sparwasser T, Koch E S, Vabulas R M, Heeg K, Lipford G B, Ellwart J W, Wagner H. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Lanzavecchia A. Nature (London) 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 41.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. Nature (London) 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 42.Ridge J P, Di Rosa F, Matzinger P. Nature (London) 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 43.Bennett S R, Carbone F R, Karamalis F, Flavell R A, Miller J F, Heath W R. Nature (London) 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 44.Yi A K, Krieg A M. J Immunol. 1998;161:4493–4497. [PubMed] [Google Scholar]
- 45.Sakata N, Patel H R, Terada N, Aruffo A, Johnson G L, Gelfand E W. J Biol Chem. 1995;270:30823–30828. doi: 10.1074/jbc.270.51.30823. [DOI] [PubMed] [Google Scholar]
- 46.Craxton A, Shu G, Graves J D, Saklatvala J, Krebs E G, Clark E A. J Immunol. 1998;161:3225–3236. [PubMed] [Google Scholar]
- 47.Liu H M, Newbrough S E, Bhatia S K, Dahle C E, Krieg A M, Weiner G J. Blood. 1998;92:3730–3736. [PubMed] [Google Scholar]
- 48.Hubert P, Greimers R, Franzen-Detrooz E, Doyen J, Delanaye P, Boniver J, Delvenne P. Cancer Immunol Immunother. 1998;47:81–89. doi: 10.1007/s002620050507. [DOI] [PMC free article] [PubMed] [Google Scholar]