Short abstract
Destruction of cartilage and bone are hallmarks of human rheumatoid arthritis (RA), and controlling these erosive processes is the most challenging objective in the treatment of RA. Systemic interleukin-4 treatment of established murine collagen-induced arthritis suppressed disease activity and protected against cartilage and bone destruction. Reduced cartilage pathology was confirmed by both decreased serum cartilage oligomeric matrix protein (COMP) and histological examination. In addition, radiological analysis revealed that bone destruction was also partially prevented. Improved suppression of joint swelling was achieved when interleukin-4 treatment was combined with low-dose prednisolone treatment. Interestingly, synergistic reduction of both serum COMP and inflammatory parameters was noted when low-dose interleukin-4 was combined with prednisolone. Systemic treatment with interleukin-4 appeared to be a protective therapy for cartilage and bone in arthritis, and in combination with prednisolone at low dosages may offer an alternative therapy in RA.
Keywords: bone destruction, cartilage oligomeric matrix protein levels, collagen-induced arthritis, interleukin-4, prednisolone
Abstract
Introduction:
Rheumatoid arthritis (RA) is associated with an increased production of a range of cytokines including tumour necrosis factor (TNF)-α and interleukin (IL)-1, which display potent proinflammatory actions that are thought to contribute to the pathogenesis of the disease. Although TNF-α seems to be the major cytokine in the inflammatory process, IL-1 is the key mediator with regard to cartilage and bone destruction. Apart from direct blockade of IL-1/TNF, regulation can be exerted at the level of modulatory cytokines such as IL-4 and IL-10. IL-4 is a pleiotropic T-cell derived cytokine that can exert either suppressive or stimulatory effects on different cell types, and was originally identified as a B-cell growth factor and regulator of humoral immune pathways. IL-4 is produced by activated CD4+ T cells and it promotes the maturation of Th2 cells. IL-4 stimulates proliferation, differentiation and activation of several cell types, including fibroblasts, endothelial cells and epithelial cells. IL-4 is also known to be a potent anti-inflammatory cytokine that acts by inhibiting the synthesis of proinflammatory cytokines such as IL-1, TNF-α, IL-6, IL-8 and IL-12 by macrophages and monocytes. Moreover, IL-4 stimulates the synthesis of several cytokine inhibitors such as interleukin-1 receptor antagonist (IL-1Ra), soluble IL-1-receptor type II and TNF receptors IL-4 suppresses metalloproteinase production and stimulates tissue inhibitor of metalloproteinase-1 production in human mononuclear phagocytes and cartilage explants, indicating a protective effect of IL-4 towards extracellular matrix degradation. Furthermore, IL-4 inhibits both osteoclast activity and survival, and thereby blocks bone resorption in vitro. Of great importance is that IL-4 could not be detected in synovial fluid or in tissues. This absence of IL-4 in the joint probably contributes to the disturbance in the Th1/Th2 balance in chronic RA.
Collagen-induced arthritis (CIA) is a widely used model of arthritis that displays several features of human RA. Recently it was demonstrated that the onset of CIA is under stringent control of IL-4 and IL-10. Furthermore, it was demonstrated that exposure to IL-4 during the immunization stage reduced onset and severity of CIA. However, after cessation of IL-4 treatment disease expression increased to control values.
Aims:
Because it was reported that IL-4 suppresses several proinflammatory cytokines and matrix degrading enzymes and upregulates inhibitors of both cytokines and catabolic enzymes, we investigated the tissue protective effect of systemic IL-4 treatment using established murine CIA as a model. Potential synergy of low dosages of anti-inflammatory glucocorticosteroids and IL-4 was also evaluated.
Methods:
DBA-1J/Bom mice were immunized with bovine type II collagen and boosted at day 21. Mice with established CIA were selected at day 28 after immunization and treated for days with IL-4, prednisolone, or combinations of prednisolone and IL-4. Arthritis score was monitored visually. Joint pathology was evaluated by histology, radiology and serum cartilage oligomeric matrix protein (COMP). In addition, serum levels of IL-1Ra and anticollagen antibodies were determined.
Results:
Treatment of established CIA with IL-4 (1 μg/day) resulted in suppression of disease activity as depicted in Figure 1. Of great interest is that, although 1 μg/day IL-4 had only a moderate effect on the inflammatory component of the disease activity, it strongly reduced cartilage pathology, as determined by histological examination (Fig. 1). Moreover, serum COMP levels were significantly reduced, confirming decreased cartilage involvement. In addition, both histological and radiological analysis showed that bone destruction was prevented (Fig. 1). Systemic IL-4 administration increased serum IL-1Ra levels and reduced anticollagen type II antibody levels. Treatment with low-dose IL-4 (0.1 μg/day) was ineffective in suppressing disease score, serum COMP or joint destruction. Synergistic suppression of both arthritis severity and COMP levels was noted when low-dose IL-4 was combined with prednisolone (0.05 mg/kg/day), however, which in itself was not effective.
Discussion:
In the present study, we demonstrate that systemic IL-4 treatment ameliorates disease progression of established CIA. Although clinical disease progression was only arrested and not reversed, clear protection against cartilage and bone destruction was noted. This is in accord with findings in both human RA and animal models of RA that show that inflammation and tissue destruction sometimes are uncoupled processes. Of great importance is that, although inflammation was still present, strong reduction in serum COMP was found after exposure to IL-4. This indicated that serum COMP levels reflected cartilage damage, although a limited contribution of the inflamed synovium cannot be excluded.
Increased serum IL-1Ra level (twofold) was found after systemic treatment with IL-4, but it is not likely that this could explain the suppression of CIA. We and others have reported that high dosages of IL-1Ra are needed for marked suppression of CIA. As reported previously, lower dosages of IL-4 did not reduce clinical disease severity of established CIA. Of importance is that combined treatment of low dosages of IL-4 and IL-10 appeared to have more potent anti-inflammatory effects, and markedly protected against cartilage destruction. Improved anti-inflammatory effect was achieved with IL-4/prednisolone treatment. In addition, synergistic effects were found for the reduction of cartilage and bone destruction. This indicates that systemic IL-4/prednisolone treatment may provide a cartilage and bone protective therapy for human RA.
Figure 1.
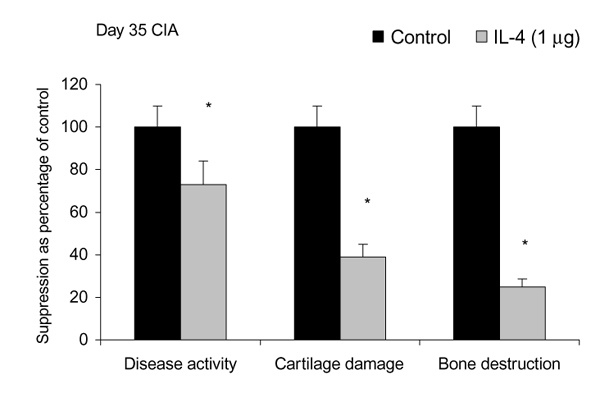
Effects in mice of treatment with interleukin-4 or control on disease activity, cartilage damage and bone destruction. Mice were treated intraperitoneally for 7 days with either vehicle (control) or 1 μg/day interleukin-4 (IL-4). CIA, collagen-induced arthritis. *P < 0.05, versus control, by Mann-Whitney U test.
Introduction
Interleukin (IL)-4 is a pleiotropic T-cell-derived cytokine that can exert either suppressive or stimulatory effects on different cell types. It was originally identified as a B-cell growth factor and regulator of humoral immune pathways [1,2]. IL-4 is produced by activated CD4+ T cells and it promotes the maturation of Th2 cells. IL-4 inhibits the differentiation of naïve T cells to Th1 and cytokine (ie IL-2 and interferon-γ) production by Th1 cells [3]. IL-4 stimulates proliferation, differentiation or activation of several cell types, including fibroblasts, endothelium cells and epithelium cells [4]. IL-4 is also known to be a potent anti-inflammatory cytokine that acts by inhibiting the synthesis of proinflammatory cytokines such as IL-1, tumour necrosis factor (TNF)-α, IL-6, IL-8 and IL-12 by macrophages and monocytes [5,6,7]. Moreover, IL-4 stimulates the synthesis of several cytokine inhibitors such as interleukin-1 receptor antagonist (IL-1Ra), IL-1-receptor type II and TNF receptors [8,9,10]. IL-4 suppresses metalloproteinase production and stimulates tissue inhibitor of metalloproteinase-1 production in human mononuclear phagocytes and cartilage explants, indicating a protective effect of IL-4 towards extracellular matrix degradation [11,12]. Furthermore, IL-4 inhibits both osteoclast activity and survival, and thereby blocks bone resorption in vitro [13,14].
RA is associated with an increased production of a range of cytokines including TNFα and IL-1, which display potent proinflammatory actions that are thought to contribute to the pathogenesis of rheumatoid arthritis (RA) [15,16]. Although TNF-α seems to be the major cytokine involved in the inflammatory process, IL-1 is the key mediator with regard to cartilage and bone destruction [17,18]. Apart from direct blockade of IL-1/TNF, regulation can be exerted at the level of modulatory cytokines such as IL-4 and IL-10. Of great importance is that IL-4 could not be detected in synovial fluid and tissues [19,20], and this lack of IL-4 is likely to contribute to the uneven Th1/Th2 balance in chronic RA.
Although having a number of side effects, including osteoporosis and reduced adrenal function, glucocorticoids are potent and commonly used anti-inflammatory agents in human RA. Glucocorticoids downregulate proinflammatory cytokine production, such as IL-1 and TNF-α, by macrophages and monocytes via several mechanisms. One mechanism is through enhanced IκBα protein synthesis. IκBα forms inactive cytoplasmic complexes with nuclear factor-κB, which itself activates many immunoregulatory genes in response to proinflammatory cytokines [21,22]. Other mechanisms of action that have been reported recently [23] are downmodulation of histone acetyltransferase and upregulation of histone deacetyltransferase, which both affected messenger RNA transcription negatively.
Murine collagen-induced arthritis (CIA) is a widely used experimental model of arthritis. Neutralization of the monokines IL-1 and TNF-α before or during onset of arthritis arrested the development of CIA [24,25]. Expression of CIA is also under particularly stringent control by IL-4 and IL-10. Treatment with anti-IL-4/anti-IL-10 shortly before onset accelerated the disease expression [26]. Furthermore, it was demonstrated that IL-12 plays a crucial role in the development of CIA, because blockade of endogenous IL-12 completely prevented onset of the disease [27]. In accord with these findings, during onset of CIA predominantly Th1 responses towards collagen type II were found [28,29]. It has been claimed [30,31] that IL-4 exposure could induce immune deviation by enhanced development of Th2-like primary CD4 effector cells. Several animal studies indicated that IL-4 administration, starting just after immunization with the disease-inducing agent, ameliorated Th1-mediated models of autoimmune diseases such as diabetes in nonobese diabetic mice and experimental arthritis [32,33,34].
In the present study the effects of systemic high dose IL-4 therapy in established CIA were investigated. Furthermore, the potential synergy of combined prednisolone and IL-4 treatment were examined. We investigated the protective effect of IL-4 alone or in combination with prednisolone on disease activity as well as cartilage and bone destruction as determined histologically, radiologically and by serum measurements of cartilage oligomeric matrix protein (COMP). Anticollagen type II specific antibodies and serum IL-1Ra levels were assessed, in order to obtain an insight into the mechanism of action. The findings suggest that IL-4 treatment protects against cartilage and bone destruction, and that combined IL-4/steroid treatment may provide a safe, anti-inflammatory and anti-destructive therapy in human RA.
Materials and methods
Animals
Male DBA-1/Bom mice were purchased from Bomholdgård (Ry, Denmark). The mice were housed in filter top cages, and were given free access to water and food. The mice were immunized at the age of 10–12 weeks.
Materials
Complete Freund's adjuvant and Mycobacterium tuberculosis (strain H37Ra) were obtained from Difco Laboratories (Detroit, MI, USA). Bovine serum albumin and prednisolone 21-sodium succinate (P-4153) were purchased from Sigma Chemicals (St Louis, MO, USA). Antimurine IL-1Ra antibodies (capture MAP-480, detection BAF-480) were obtained from R&D Systems (Minneapolis, MN, USA). PolyHRP-streptavidine (M2032) and Caseine colloid buffer (M2052) was from CLB (Amsterdam, The Netherlands). Recombinant murine IL-1Ra was purchased from R&D systems. Recombinant murine IL-4 (6.5 × 107 U/mg) was kindly provided by Dr S Smith (Schering-Plough, Kenilworth, NJ, USA).
Collagen preparation
Articular cartilage was obtained from metacarpophalangeal joints of 1–2 year old cows. Bovine type II collagen was prepared according to the method of Miller and Rhodes [35]. It was dissolved in 0.05 mol/l acetic acid (5 mg/ml) and stored at -70ºC.
Immunization
Bovine type II collagen was diluted with 0.05 mol/l acetic acid to a concentration of 2 mg/ml and was emulsified in an equal volume of complete Freund's adjuvant (2 mg/ml MT H37Ra). The mice were immunized intradermally at the base of the tail with 100 μl emulsion (100 μg collagen). At day 21 the animals were boosted with an intra-peritoneal injection of 100 μg collagen type II, diluted in phosphate-buffered saline (pH 7.4).
Assessment of arthritis
Mice were examined for visual appearance of arthritis in peripheral joints, and scores for severity were given (arthritis score) as previously described [17,18,25,26,27]. Mice were considered arthritic when significant changes in redness and/or swelling were noted in digits or in other parts of the paws. At later time points ankylosis was also included in the arthritis score. Clinical severity of arthritis was graded on a scale of 0–2 for each paw, according to changes in redness and swelling: 0, no changes; 0.5, significant; 1.0, moderate; 1.5, marked; and 2.0, maximal swelling and redness, and later on ankylosis. Arthritis score (mean± stan-dard deviation) was expressed as cumulative value for all paws, with a maximum of eight and expressed as percentage of the initial score at the beginning of treatment.
Treatment of collagen-induced arthritis with interleukin-4, prednisolone or interleukin-4/prednisolone
To evaluate the effect of IL-4, prednisolone or the combination IL-4/prednisolone on established CIA, mice with CIA were selected at day 28 and divided into groups of at least 10 mice with similar arthritis scores. Thereafter, mice were treated twice a day intraperitoneally with IL-4 (0.1 or 1μg/day), prednisolone (0.05 mg/kg/day), or with IL-4 and prednisolone (at the same doses for the noncombined regimens) for each of several days as indicated in the results.
Determination of interleukin-1 receptor antagonist levels
IL-1Ra was measured using enzyme-linked immunosorbent assay (ELISA). Briefly, Nunc Maxisorb ELISA plates (Nunc, Rostilde, Denmark) were coated with capture antibodies (5 μg/ml, carbonate buffer, pH 9.6, 24 h at 4°C), and thereafter nonspecific binding sites were blocked with 1% bovine serum albumin/phosphate-buffered saline-Tween. Standards and unknown samples were diluted in normal DBA-1 serum and incubated for 3 h at room temperature. Biotinylated detection antibodies were added at concentrations of 0.2–0.4 μg/ml in 0.5% bovine serum albumin in phosphate-buffered slaine-Tween for 1.5 h at room temperature. Thereafter plates were incubated with PolyHRP (0.1 μg/ml in 1% caseine colloid buffer) for 45 min and orthophenylenediamine (0.8 mg/ml) was used as substrate. Plates were read at 495 nm.
Measurement of cartilage oligomeric matrix protein
At the end of the experiments, serum samples were taken and murine cartilage oligomeric matrix protein (COMP) levels were determinated using ELISA under similar conditions as those described for the assay for human COMP [36]. The assay was modified by using rat COMP for coating the microtitre plates, the standard curve included in each plate and by using the polyclonal antiserum raised against rat COMP to detect the antibody [37,38]. A high cross-reactivity was found to murine COMP [39]. This was shown by parallel dilution curves of murine sera to the standard curve prepared with rat COMP, as well as in experiments in which a dilution of murine serum was added to the standard curve.
Determination of anticollagen antibodies
Antibodies against bovine type II collagen were examined by using an ELISA. Titres of total IgG, IgG1 and IgG2a were measured. Briefly, plates were coated with 10 μg bovine type II, and thereafter nonspecific bindings sites were blocked with 0.1 mol/l ethanolamin (Sigma Chemicals). Serial 1 : 2 dilutions of the sera were added, followed by incubation with isotype-specific goat antimouse peroxidase (Southern Biotechnology Associates, Birmingham, AL, USA) and substrate (5-aminosalicyclic acid; Sigma Chemicals). Plates were read at 492 nm. Titres were expressed as means ± standard deviation dilution, which gives the half maximal value.
Radiological analysis of bone destruction
At the end of the experiments, knee joints were removed and used for radiological analysis as a measure of bone destruction. Radiographs were carefully examined using a stereo microscope, and joint destruction was graded on a scale from 0 to 5, ranging from no damage, minor bone destruction observed as one enlightened spot, moderate changes, two to four spots observed in one area, marked changes, two to four spots observed in more areas, severe erosions afflicting the joint, complete destruction of joint and/or new bone formations. Bone destruction was scored on the femoral head, tibia and patella as described previously [17].
Histology
Mice were killed by ether anaesthesia. Knee joints were removed and fixed for 4 days in 4% formaldehyde. After decalcification in 5% formic acid, the specimens were processed for paraffin embedding [17,18,25,26,27]. Tissue sections (7 μm thick) were stained with haematoxylin and eosin, or safranin O. Histopathological changes were scored using the following parameters.
Infiltration of cells was scored on a scale from 0 to 3, depending on the amount of inflammatory cells in the synovial tissues. Inflammatory cells in the joint cavity were graded on a scale from 0 to 3 and expressed as exudate. Cartilage proteoglycan depletion was determined using safranin O staining. The loss of proteoglycans was scored on a scale from 0 to 3, ranging from fully stained cartilage to destained cartilage or complete loss of articular cartilage. A characteristic parameter in CIA is the progressive loss of articular cartilage. This destruction was separately graded on a scale from 0 to 3, ranging from the appearance of dead chondrocytes (empty lacunae) to complete loss of the articular cartilage. Bone erosion was scored on a scale ranging from 0 to 3, ranging from no abnormalities to complete loss of cortical and trabecular bone of the femoral head and patella. Histopathological changes in the knee joints were scored in the patella/femur region on 5 semiserial sections of the joint, spaced 70 μm apart. Scoring was performed on decoded slides by two observers, as described earlier [17,18,25,26,27].
Statistical analysis
Differences between experimental groups were tested using the Mann-Whitney U test, unless otherwise stated.
Results
Amelioration of arthritis score in collagen-induced arthritis by in vivo treatment of interleukin-4
To investigate effects of in vivo treatment of established CIA with IL-4, mice that expressed CIA at day 28 after immunization were injected intraperitoneally with vehicle, 0.1 or 1 μg IL-4 per day. Figure 2 shows that administration of 1 μg/day IL-4 results in significant amelioration of the arthritis score, but a lower dosage of 0.1 μg/day IL-4 was without effect. The anti-inflammatory effect of 1 μg/day IL-4 was further illustrated in Figure 3, in which disease progression is expressed as change in (Δ) disease activity of all individual mice. Increased severity of CIA score can be seen in animals treated either with vehicle or 0.1 μg/day IL-4, whereas significantly decreased disease activity was noted after treatment with 1 μg/day IL-4. Histology revealed that no effect was found on the influx of inflammatory cells in joint tissues of IL-4-treated animals when compared with the vehicle-treated animals (Table 1).
Figure 2.
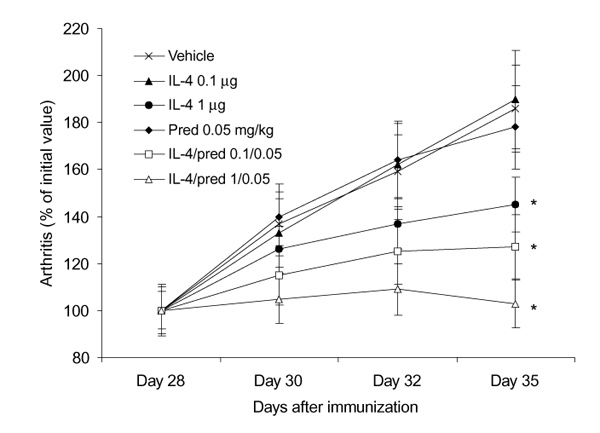
Dose dependent suppression of disease activity of collagen-induced arthritis (CIA) by interleukin (IL)-4 and the combination of IL-4/prednisolone (Pred). Mice with established CIA were divided into separate groups of at least 10 mice. Groups were treated intraperitoneally twice a day with vehicle, IL-4, prednisolone, or combined IL-4/prednisolone for 8 consecutive days. The data represent the mean arthritis score, expressed as percentage of initial value at day 28. Experiments were repeated once with approximately the same outcome. *P < 0.05, versus vehicle, by Mann-Whitney U test.
Figure 3.
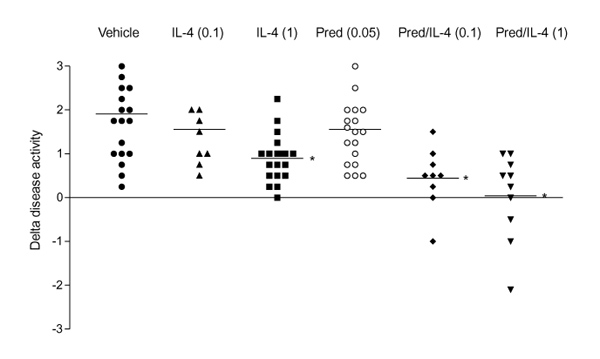
Dose-dependent arrest of disease activity by treatment with interleukin (IL)-4 and IL-4/prednisolone (Pred). The enhanced disease activity between days 28 and 35 of each individual mouse is expressed as change in (Δ) disease activity. For treatment protocol, see Fig. 2. P < 0.05, versus vehicle, by Mann-Whitney U test.
Table 1.
Effect of prednisolone, interleukin (IL)-4 or IL-4/prednisolone treatment on the joint pathology of collagen-induced arthritis in Mice
| Cartilage | Proteoglycan | Bone | ||||
| Treatment | Dose | Infiltrate | destruction | loss | erosin | n |
| Vehicle | - | 2.3 ± 0.9 | 2.2 ± 0.9 | 2.7 ± 1.0 | 1.9 ± 0.9 | 20 |
| Prednisolone | 0.05 | 2.1 ± 0.8 | 2.1 ± 1.2 | 2.6 ± 0.6 | 1.7 ± 1.1 | 20 |
| IL-4 | 0.1 | 2.5 ± 0.7 | 2.5 ± 0.8 | 2.9 ± 0.3 | 2.0 ± 0.8 | 10 |
| IL-4 | 1 | 2.0 ± 1.0 | 1.2 ± 0.8* | 2.0 ± 0.7 | 0.6 ± 0.6* | 20 |
| IL-4/prednisolone | 0.1/0.05 | 2.1 ± 0.4 | 1.6 ± 0.7 | 2.5 ± 0.8 | 2.1 ± 0.6 | 10 |
| IL-4/prednisolone | 1/0.05 | 1.2 ± 0.5* | 1.1 ± 0.9* | 1.4 ± 0.7* | 0.4 ± 0.5* | 10 |
Histopathology scores of arthritic knee joints after treatment with vehicle, IL-4, prednisolone or the combination of IL-4/prednisolone. Mice were sacrified and knee joints were used for histology. Histology was scored as indicated in the Materials and methods section. Mice were treated twice a day intraperitoneally with either prednisolone (0.05 mg/kg), or IL-4 (0.1 or 1 μg/day], or IL-4 (at both dosages) combined with prednisolone (0.05 mg/kg). *P <0.05, versus vehicle, by Mann-Whitney U test.
Interleukin-4 protects against cartilage destruction
Systemic treatment with high-dose IL-4 (1μg/day) significantly decreased cartilage destruction, determined as chondrocyte death and cartilage erosions (Fig. 4,Table 1). It did not result in a significantly reduced loss of matrix proteoglycans, as determined by safranin O staining (Fig. 5, Table 1). It has been demonstrated (data not shown) that there is a strong correlation between severe cartilage damage and increased serum COMP levels during murine CIA. In naïve DBA-1 mice, serum COMP levels are approximately 4.0 μg/ml and COMP levels increased up to 8–12 μg/ml in mice with fully established CIA. Serum COMP levels were determined in the various groups to identify the protection against severe cartilage destruction by IL-4. Figure 6 shows that elevated COMP in CIA were not reduced by treatment with low-dose IL-4. It is of particular interest, that treatment with high-dose IL-4 (1 μg/day) significantly reduced serum COMP levels to values found in nonarthritic control animals.
Figure 4.
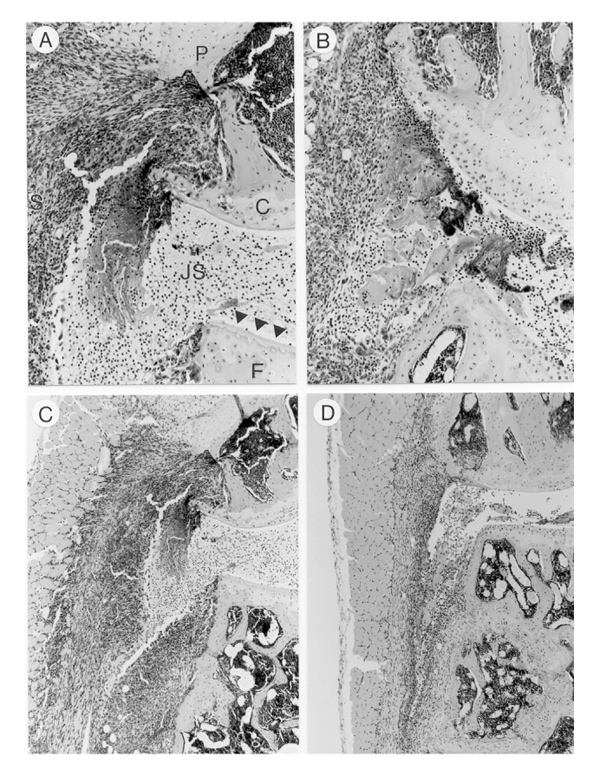
Interleukin (IL)-4 treatment reduced cartilage destruction, whereas IL-4/prednisolone treatment additionally decreased cell influx. (a) Knee joint from vehicle-treated mouse. Severe cartilage destruction can be seen. Empty lacunae reflects chondrocyte death as marker of cartilage destruction, indicated by arrows. (b) Knee joint of a mouse treated with IL-4 1 μg/kg/day for eight consecutive days. Note the reduced cartilage destruction and chondrocyte death. (c) Knee joint of vehicle-treated animal. Note the severe cell influx in synovial tissues and joint cavity. (d) Knee joint of a mouse treated with IL-4/prednisolone (1 μg per day/0.05 mg per kg). Note the marked reduction of cell influx. All specimens were sampled at day 35. P, patella; F, femur; JS, joint space; C, cartilage; S, synovium. Haematoxylin and eosin staining was used. Original magnifications: × 200 (a, b) and × 100 (c, d).
Figure 5.
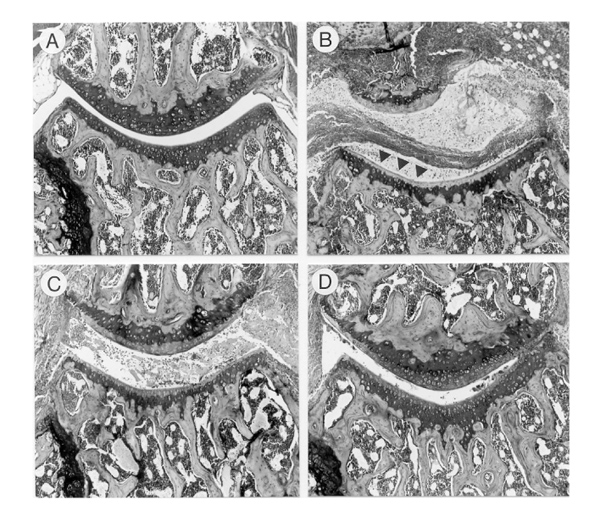
Effect of IL-4 or IL-4/prednisolone treatment on matrix proteoglycan loss. (a) Knee joint of a control naïve mouse. The fully stained cartilage layers indicate no loss of proteoglycans. (b) Knee joint of an arthritic mouse treated with vehicle. Note the severe joint inflammation and complete loss of safranin O staining of the cartilage layers (indicated by arrows). (c) Mouse treated with IL-4 (1 μg/day). Loss of matrix proteoglycan can still be seen. (d) Knee joint of a mouse treated with IL-4/prednisolone (1 μg per day/0.05 mg per kg). Marked reduction in matrix proteoglycan depletion after combined treatment. For details see Fig. 4. Safranin O staining, original magnification × 100.
Figure 6.
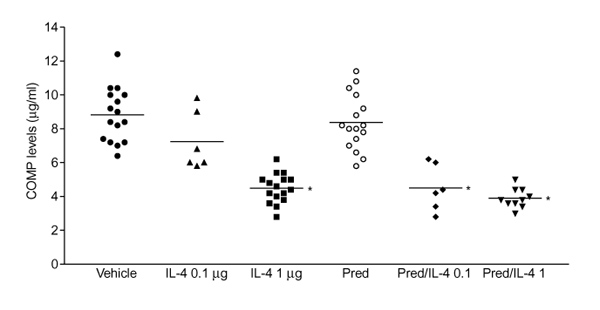
Serum cartilage oligomeric matrix protein (COMP) level as a marker of cartilage turnover. Suppression of serum COMP was found after treatment with interleukin (IL)-4 and IL-4/prednisolone (Pred). IL-4(1 μg/day) and both doses (0.1 μg per day/0.05 mg per kg per day; and 1 μg per day/0.05 mg per kg per day) of IL-4/prednisolone reduced serum COMP levels to basic levels as found in nonimmunized animals (4.2 ± 0.2 μg/ml). The data represent the mean± standard deviation COMP level of at least six sera per group. *P < 0.01, versus vehicle, by Mann-Whitney U test.
Interleukin-4 protects against bone destruction
Bone destruction, which is a common feature of murine collagen arthritis, was examined by radiological analysis. Radiographs of knee joints were taken at the end of the treatment period. Figure 7 showed that treatment with 1 μg/day IL-4 was sufficient to prevent bone destruction, determined as bone erosions on the head of the femur, the patella and the tibia. Little or no effect was noted after treatment with low-dose IL-4. Histological analysis of knee joints corroborated the protective effect of IL-4 (Table 1). Figure 8 (a, c) depicts degradation of patellar and femural cortical bone by osteoclasts in the vehicle-treated group, whereas almost no osteoclasts were seen in the IL-4-treated group (Fig. 8d).
Figure 7.
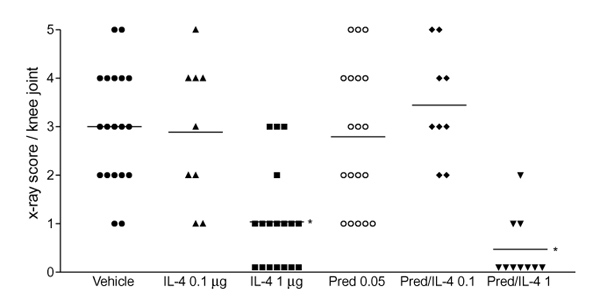
Protection of interleukin (IL)-4 and IL-4/prednisolone (Pred) treatment on bone destruction. Knee joints were isolated at day 35 and bone destruction was examined by radiographic analysis. For treatment scheme see Fig. 2. Erosions were scored on a scale ranging from 0 to 5 on the femur head, tibia and patella. Each group consists of at least nine knee joints per group. *P < 0.01, versus vehicle, by Mann-Whitney U test.
Figure 8.
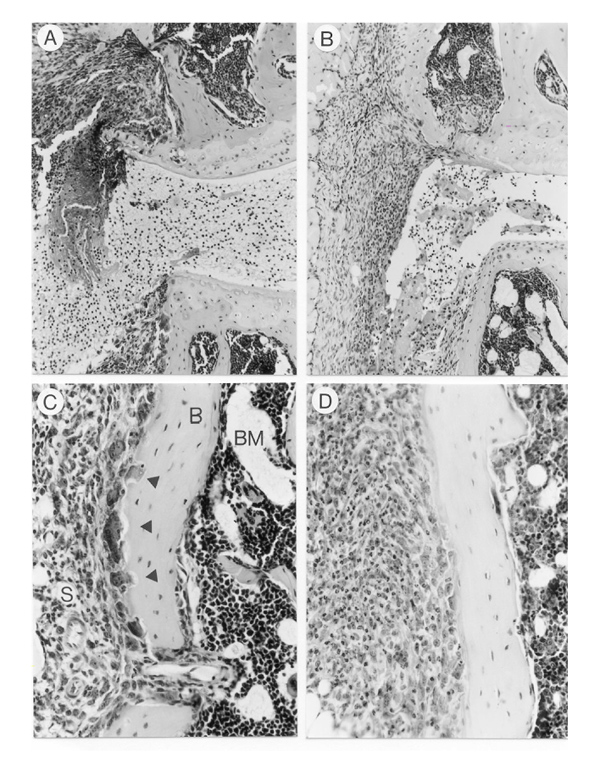
Bone destruction is prevented by interleukin (IL)-4 and IL-4/prednisolone treatment. (a) Severe bone destruction in patella and femur in knee joint of vehicle-treated animal. (b) Almost no bone degradation was noted after treatment with IL-4/prednisolone (1 μg per day/0.05 mg per kg). (c) Bone destruction in femur of a vehicle-treated animal at higher magnification. Osteoclasts, large multinuclear cells, located at the site of bone destruction (arrows). (d) No osteoclast-like cells were found in IL-4 (1 μg/day) treated animals. For treatment details see Fig. 4. S, synovium; B, bone; BM, bone marrow. Original magnifications × 200 (a, b), × 400 (c, d).
Combined interleukin-4/prednisolone treatment
We examined potential synergistic effects of IL-4 and prednisolone, using low-dose prednisolone (0.05 mg/kg/day) and 0.1 or 1μg/day IL-4. Treatment of CIA with IL-4/prednisolone completely arrested the development of inflammatory signs of CIA (Figs 2 and 3). Both combinations tested revealed full suppression of disease progression. In accord with previous observations, mice treated with 0.05 mg/kg/day prednisolone alone did not show significant suppression of arthritis. Histology taken after 7 days of treatment showed enhanced safranin O staining only in animals treated with IL-4/prednisolone (1 μg per kg/0.05 kg daily), indicating reduced depletion of matrix proteoglycans (Table 1, Fig. 5d). This was in accord with the marked reduction in joint inflammation, as can be seen in Figure 4d. Both combinations of IL-4 and prednisolone reduced serum COMP to values found in naïve DBA-1 mice. Interestingly, synergistic suppression of serum COMP was noted after exposure to low-dose IL-4 and prednisolone (Fig. 6). In contrast to serum COMP levels, combined IL-4/prednisolone treatment did not result in synergistic protection against bone destruction. High-dose IL-4 alone was already highly effective, and the combination of IL-4 with prednisolone did not improve the effect further, or was there an adverse effect of prednisolone (Table 1, Figs 7 and 8b). Treatment of CIA with 1 μg/day IL-4 alone and in combination with prednisolone (0.05 mg/kg/day) for 7 days caused similar reduction in osteoclast numbers (data not shown).
Effect of interleukin-4, or interleukin-4/prednisolone treatment on interleukin-1 receptor antagonist and anticollagen antibody levels
Serum IL-1Ra levels were determined at the end of the experiments and Table 2 shows a twofold increase after IL-4 treatment (1μg/day dose). Treatment with 0.1μg/day IL-4 showed no significant effects on serum IL-1Ra levels. Prednisolone reduced IL-1Ra levels when compared with vehicle-treated animals. In accord with these findings, combined IL-4/prednisolone (1 μg per day/ 0.05 mg per kg per day) treatment resulted in lower IL-1Ra levels than found with IL-4 alone.
Table 2.
Serum interleukin-1 receptor antagonist (IL-1Ra levels) after treatment with either interleukin (IL)-4, prednisolone, or IL-4/prednisolone
| Treatment | Dose | IL-1Ra (pg/ml) |
| Vehicle | - | 414 ± 155 |
| IL-4 | 0.1 | 386 ± 213 |
| IL-4 | 1 | 838 ± 187* |
| Prednisolone | 0.05 | 326 ± 165 |
| IL-4/prednisolone | 0.1/0.05 | 422 ± 129 |
| 1/0.05 | 499 ± 187 |
Serum IL-1Ra was determined using enzyme-linked immunosorbent assay at day 35 after immunization. Mice were treated as indicated in Table 1. The data represent the mean± standard deviation of at least eight mice per group. The sensitivity of the IL-1Ra assay was to within 160 pg/ml. *P < 0.05, versus vehicle, by Mann-Whitney U test.
Anticollagen antibodies were assayed at the end of treatment period at day 35. The antibody levels increased rapidly after clinical expression of CIA around day 28 after immunization. After IL-4 (1μg/day) treatment for 7 days, total IgGs levels as well as IgG1 and IgG2a anticollagen type II antibody levels were lower compared with vehicle treated animals (Fig. 9). Although all anticollagen type II antibodies were reduced, IgG2a levels showed the most prominent reduction, indicating an effect on the Th1 rather than on the Th2 immune response. No decreased anticollagen type II antibody levels were found after treatment with low-dose IL-4. The high-dose IL-4/prednisolone regimen reduced anticollagen type II antibodies to levels similar to those found after treatment with 1μg/day IL-4.
Figure 9.
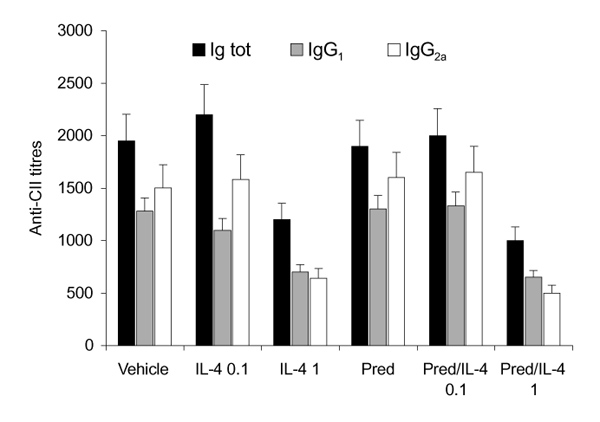
Interleukin (IL)-4 or IL-4/prednisolone (Pred) treatment is associated with reduced anticollagen type II (CII) antibody levels. Treatment with 1 μg/day IL-4 resulted in lower anticollagen type II antibodies. Total Immunoglobulins (Ig tot), IgG1 and IgG2a levels were reduced. Similar effects were found after treatment with IL-4/prednisolone (1 μg per day/0.05 mg per kg). Anticollagen type II levels were determined in at least six mice per group. Data are expressed as means ± standard deviation dilution, which gives the half maximal value.
Discussion
The present study demonstrates clear tissue-protective effects of IL-4, although IL-4 did not prove to be a very potent anti-inflammatory cytokine. Both cartilage and bone erosion were prevented by IL-4 treatment of established CIA. Combination with low-dose prednisolone enhanced the anti-inflammatory capacity of IL-4. This might offer an attractive alternative to the use of high-dose prednisolone, because it can circumvent the unwanted side effects of the drug, including steroid-induced osteoporosis.
In previous studies of murine collagen arthritis [17,18,25] it was shown that TNF-α is important at onset of the disease, whereas IL-1 is the dominant cytokine, not only at the onset, but also in the progression of the arthritis and the concomitant cartilage destruction. Further support for the critical role of IL-1 is provided by the absence of collagen arthritis in IL-1β-deficient mice, and the marked reduction of this arthritis in ICE-deficient mice as well as in normal mice treated with IL-1β-converting enzyme inhibitors [40,41]. Moreover, reduced onset of arthritis was noted in TNF-receptor-deficient mice, but once a joint was afflicted the arthritis progressed to full-blown expression and cartilage destruction, again emphasizing that TNF is important in onset, but is not the dominant cytokine in progression and tissue destruction [42].
In recent studies, it was clearly demonstrated that onset of CIA is under stringent control of IL-4 and IL-10, because blockade of both IL-4 and IL-10 by the use of antibodies accelerated disease onset [26]. Furthermore, treatment of established murine CIA with low-dose IL-4 showed no suppressive effect on disease activity and joint pathology. Interestingly, combination of low-dose IL-4 and IL-10 appeared to have more potent anti-inflammatory effects, and resulted in protection against cartilage pathology [26]. Systemic treatment of murine CIA with high-dose IL-4 (3 μg/day) during the immunization stage delays onset as well as reduces severity. When IL-4 administration was terminated, however, disease expression and activity rapidly accelerated and was indistinguishable from that in the vehicle-treated control group [34]. Systemic IL-4 treatment of streptococcal cell wall arthritis in rats resulted in suppression of disease activity, and ameliorated the chronic destructive process leading to decreased lesions [33]. This was associated with enhanced levels of IL-1Ra, the natural inhibitor of IL-1, which is in accord with observations in the present study and with studies in humans systemically treated with IL-4 [43]. However, it is not likely that the twofold increment in serum IL-1Ra levels, found after IL-4 exposure, is sufficient to suppress CIA. As previously mentioned, blockade of IL-1 by anti-IL-1 antibodies or very high-dose IL-1Ra completely suppressed CIA and lead to full protection against joint pathology [17,18,25]. Whether IL-4 acts locally or systemically is at present unknown. Further experiments on biodistribution of IL-4 are needed to resolve this issue.
IL-4 levels are virtually undetectable in arthritic tissue of RA patients, suggesting that the disease is either a selective Th1 process or is not driven at all by T cells. An alternative explanation could be the fact that IL-1α and IL-1β specifically inhibit IL-4 synthesis by T cells [44]. Other proinflammatory cytokines, such as TNF-α, IL-6 and IL-12 did not decrease IL-4 production, indicating the pivotal role of IL-1 in RA. It is known that IL-4 has a suppressive effect on Th1 activity and is a crucial factor in differentiation of naïve T cells into the Th2 phenotype. This suppression has been suggested to be due to the inhibitory effect of IL-4 on IL-12 generation by antigen-presenting cells and macrophages [7]. IL-12, on the other hand, is a potent stimulator of the generation of Th1 cells. Analysis of anticollagen type II antibodies revealed that systemic IL-4 treatment did not alter the balance of IgG2a/IgG1 antibodies, indicating no suppressive effect on the Th1 immune response. Total anticollagen type II antibody levels were lower in both IL-4 (1 μg/day) and IL-4/prednisolone treated animals when compared with the vehicle group. We have previously found that anticollagen type II antibody levels rapidly increased after onset of CIA and reached the highest levels after 7 days (Joosten LAB, unpublished data). IL-4 treatment arrested the development of high anticollagen type II antibody levels after onset and did not alter IgG2a/IgG1 balance.
Cartilage alterations were screened for by histology as well as COMP levels in sera of mice at the end of the experiments. COMP is a prominent component of articular cartilage. In a process affecting cartilage turnover, fragments are released and eventually reach the circulation. Thus, serum levels may be used as a marker of generalized cartilage turnover [44,45]. More recent studies [46,47] have demonstrated the production of COMP by activated synovial cells and synovial tissue of RA and osteoarthritis patients. Although the relative contribution to serum levels is not firmly established, important information has been obtained from studies of collagen arthritis in rats. Thus, increased serum COMP levels are seen at time points when erosive changes appear in cartilage, whereas in early stages with marked inflammation in the synovium no increased COMP levels are seen [37,38] (Larsson E, Saxne T, unpublished data). Furthermore, serum COMP levels are reduced to normal in murine CIA after treatment with IL-1-blocking antibodies, in correspondence with a marked suppression of the cartilage lesion as viewed histologically [17]. Thus, evidence so far indicates that changes in serum COMP relate to changes in the cartilage turnover. In accord with these findings, low-dose IL-4/prednisolone treatment did not suppress disease activity, largely reflecting synovitis, but clearly reduced serum COMP levels. Histology interestingly revealed that serum COMP levels correlated more with cartilage erosions than with loss of matrix proteoglycans, which is a reversible process.
Recently, it was shown that expression of neo-epitope VDIPEN correlated with marked cartilage erosions during experimental arthritis. This neoepitope is formed by proteolytic cleavage of aggrecan by matrix metalloproteinases (MMPs). VDIPEN expression reflects MMP-3 (eg stromelysin) activity and it colocalized with collagen breakdown epitopes, indicating severe cartilage damage by MMPs [48,49]. It was demonstrated that IL-4 down-regulates both stromelysin and collagenase synthesis and thereby contributed to inhibition of cartilage destruction [11,12,50]. Thus, reduction of cartilage destruction found after IL-4 treatment may well be due to a lower production of MMPs and/or inhibition of their activity. The fact that IL-4 treatment did not protect against proteoglycan loss does suggest that IL-4 has no major suppressive effect on aggrecanase. In a previous study [51] we showed that early proteoglycan loss is mediated by aggrecanase, whereas erosive, late destruction is linked to stromelysin.
Control of bone destruction is a most challenging objective in treatment of RA. In areas of tumour-like synovial tissue, erosion of subchondral and cortical bone is common, leading to the characteristic erosions seen on radiography. Osteoclasts can be seen in the areas of bone destruction during CIA. It has been reported that IL-4 inhibits bone resorption by inhibition of osteoclast development and activity in vitro [13,14]. Here, we report for the first time that systemic IL-4 treatment of established CIA markedly reduced bone erosions, examined by radiographic analysis and histopathology. Neither bone destruction nor osteoclasts were noted in arthritic knee joints of animals treated with high-dose IL-4, indicating decreased formation of these cells. IL-4 furthermore downregulates IL-1, IL-6, TNF-α and prostaglandin E2 production in several cell types that play a role in the resorption process of the bone. Interestingly, blocking studies with neutralizing antibodies directed against IL-4 in CIA indicated that the endogenous cytokine inhibited bone destruction. In animals treated with anti-IL-4, bone destruction determined by radiographic analysis was aggravated compared with that in vehicle-treated animals (data not shown).
Glucocorticoids are potent and commonly accepted anti-inflammatory agents, but the major drawback on continued usage in arthritis is the severe negative effect on the bone. More recent studies on the mechanism of action revealed strong downregulation of macrophage production of the proinflammatory cytokines TNF-α and IL-1, related to enhanced IκBα synthesis. Intriguingly, over a large dose range steroids not only inhibit TNF and IL-1, but also reduce the production of IL-1Ra and regulatory cytokines such as IL-4 and IL-10 [52]. This suggests that the net effect in joint inflammation is impaired by the lack of the protective cytokines, which inhibit TNF/IL-1 production as well as induce potent upregulators of scavengers such as soluble receptors for TNF and IL-1, and IL-1Ra [8,9,53]. Moreover, IL-4 powerfully reduces inducible nitric oxide synthase expression, thereby counteracting the suppressive effect of IL-1 on chondrocyte proteoglycan synthesis, which is mainly nitric oxide mediated. Evidence for the latter was provided in in vitro studies with nitric oxide inhibitors. In further support of a role in vivo, we recently demonstrated that IL-1 failed to inhibit chondrocyte proteoglycan synthesis in inducible nitric oxide synthase deficient mice [54].
The present data clearly demonstrates the synergistic effect of combination therapy of low-dose prednisolone and IL-4. Low-dose IL-4 was without suppressive effect on clinical disease activity, which is in accord with previous studies [39]. However, when combined with prednisolone the progression of CIA was completely arrested. Furthermore, synergistic suppression of cartilage destruction was demonstrated by lowered serum COMP levels, which was also reflected by histology. Only combined therapy with high-dose IL-4 and prednisolone was able to suppress the influx of inflammatory cells in joint tissues and reduce the loss of matrix proteoglycans.
In conclusion, IL-4 might offer an alternative cartilage-and bone-protective therapy that is complementary to TNF/IL-1 inhibitors. Its limited effect on the inflammatory process warrants combination with other therapeutic modalities. The present data suggest that combination with prednisolone at low dosages provides an intriguing option. In accord with earlier observations of both IL-10/prednisolone and IL-4/IL-10 synergy [26,39], it must be considered that a cocktail of IL-4, IL-10 and low-dose glucocorticosteroids or glucocorticoids might be an even more efficacious therapy for human RA.
References
- Mosmann TR, Coffman RL. Th1 and Th2 cells: different pattern of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Paul WE, Ohara J. B-cell stimulatory factor-1/interleukin-4. . Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomarat P, Banchereau J. An update on interleukin-4 and its receptor. Eur Cytokine Net. 1997;8:333–344. [PubMed] [Google Scholar]
- Hart PA, Vitti GF, Burgess DR, et al. Potential anti-inflammatory effects of interleukin-4: suppression of human monocyte tumor necrosis factor α, interleukin-1 and prostaglandin E2. Proc NatlAcad Sci USA. 1989;86:3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TeVelde AA, Huijbens RJF, Heije K, De Vries JE, Figdor CD. Interleukin-4 (IL-4) inhibits secretion of IL-1 β, tumor necrosis factor α, and IL-6 by human monocytes. . Blood. 1990;76:1392–1397. [PubMed] [Google Scholar]
- DeKruyff RH, Fang Y, Wolf SF, Umetsu DT. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T-cells through an effect on antigen-presenting cells. J Immunol. 1995;154:2578–2585. [PubMed] [Google Scholar]
- Vannier E, Miller LC, Dinarello CA. Coordinated anti-inflammatory effects of interleukin-4: IL-4 suppresses IL-1 production but upregulates gene expression and synthesis of interleukin-1 receptor antagonist. . Proc Natl Acad Sci USA. 1992;89:4076–4080. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Cope AP, Gibbons DL, Aderka D. Differential regulation of tumor necrosis factor receptors (TNF-R) by IL-4: upregulation of p55 and p75 TNF-R on synovial joint mononuclear cells. Cytokine. 1993;3:205–208. doi: 10.1016/1043-4666(93)90006-q. [DOI] [PubMed] [Google Scholar]
- Lacraz S, Nicod LP, Rochemonteix BG, et al. Suppression of metalloproteinase biosynthesis human alveolar macrophages by interleukin-4. J Clin Invest. 1992;90:382–388. doi: 10.1172/JCI115872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston TE, Ellis AJ, Bigg H, et al. Interleukin-4 blocks the release of collagen fragments from bovine nasal cartilage treated with cytokines. Biochim Biophys Acta. 1996;1314:226–232. doi: 10.1016/s0167-4889(96)00107-3. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Watanabe K, Morimoto I, et al. Interleukin-4 inhibits spontaneous and parathyroid hormone-related protein-stimulated osteoclast formation in mice. J Bone Miner Res. 1994;9:1533–1539. doi: 10.1002/jbmr.5650091005. [DOI] [PubMed] [Google Scholar]
- Miossec P, Chomarat P, Dechanet J, et al. Interleukin-4 inhibits bone resorption through and effect on osteoclasts and proinflam-matory cytokines in an ex vivo model of bone resorption in rheumatoid arthritis. Arthritis Rheum. 1994;12:1715–1722. doi: 10.1002/art.1780371202. [DOI] [PubMed] [Google Scholar]
- Arend WP, Dayer JM. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- Bucala R, Ritchlin C, Winchester R, Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991;173:569–574. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LAB, Helsen MMA, Saxne T, et al. IL-1 α,β blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF α blockade only ameliorates joint inflammation. . J Immunol. 1999 in press [PubMed] [Google Scholar]
- Van de Berg WB, Joosten LAB, Helsen MMA, van de Loo FAJ. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol. 1994;95:237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Chomarat P, Dechanet J. Bypassing the antigen to control rheumatoid arthritis. Immunology Today. 1996;17:170–173. doi: 10.1016/0167-5699(96)80615-3. [DOI] [PubMed] [Google Scholar]
- Miossec P, Van den Berg WB. Th1/Th2 cytokine balances in arthritis. Arthritis Rheum. 1997;40:2105–2115. doi: 10.1002/art.1780401203. [DOI] [PubMed] [Google Scholar]
- Scheiman RI, Cogswell PC, Lofquist AK, Baldwin AS. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- Auphan N, Didonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: Inhibition of NF-κ B activity through induction of IκB synthesis. . Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci Colch. 1998;94:557–578. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN. Antitumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Prod Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LAB, Helsen MMA, Van de Loo FAJ, van den Berg WB. Anticytokine treatment of established collagen type II arthritis in DBA/1 mice: a comparative study using anti-TNF alpha, anti-IL-1 alpha, beta and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- Joosten LAB, Lubberts E, Durez P, et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis: protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- Joosten LAB, Lubberts E, Helsen MMA, van den Berg WB. Dual role of IL-12 in early and late stages of murine collagen type II arthritis. J Immunol. 1997;159:4094–4102. [PubMed] [Google Scholar]
- Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine pattern and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- Doncardi A, Stasiuk LM, Fournier C, Abehsira-Amar O. Conversion in vivo from an early dominant Th0/Th1 response to a Th2 phenotype during the development of collagen-induced arthritis. Eur JImmunol. 1997;27:1451–1458. doi: 10.1002/eji.1830270623. [DOI] [PubMed] [Google Scholar]
- Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. . Immunology Today. 1996;17:225–231. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Swain SL, Bradly LM. Enhanced development of Th2-like primary effectors in response to sustained exposure to limited rIL-4 in vivo. . J Immunol. 1996;156:3267–3274. [PubMed] [Google Scholar]
- Rapoport MJ, Jaramillo A, Zipris D, et al. Interleukin-4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med . 1993;178:87–95. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JB, Wong HL, Costa GL, Bienkowski MJ, Wahl SM. Suppression of monocyte function and differential regulation of IL-1 and IL-1Ra by IL-4 contribute to resolution of experimental arthritis. JImmunol. 1993;151:4344–4351. [PubMed] [Google Scholar]
- Horsfall AC, Butler DM, Marinove L, et al. Suppression of collagen-induced arthritis by continuous administration of IL-4. . J Immunol. 1997;159:5687–5696. [PubMed] [Google Scholar]
- Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;2:33–65. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Saxne T, Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31:583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- Larsson E, Mussener Å, Heinegård D, Klareskog L, Saxne T. Increased serum levels of cartilage oligomeric matrix protein and bone sialoprotein in rats with collagen arthritis. Br J Rheumatol. 1997;36:1258–1261. doi: 10.1093/rheumatology/36.12.1258. [DOI] [PubMed] [Google Scholar]
- Vingsbo-Lundberg C, Saxne T, Olsson H, Holmdahl R. Increased serum levels of cartilage oligomeric matrix protein in chronic erosive arthritis in rats. Arthritis Rheum. 1998;41:544–550. doi: 10.1002/1529-0131(199803)41:3<544::AID-ART21>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LAB, Helsen MMA, Saxne T, et al. Synergistic protection against cartilage destruction by low dose prenisolone and interleukin-10 in established murine collagen arthritis. Inflamm Res. 1999;48:48–55. doi: 10.1007/s000110050396. [DOI] [PubMed] [Google Scholar]
- Christen A, Mudgett JS, Orevillo CJ, et al. Collagen-induced arthritis in the interleukin-1β knock-out mouse [abstract]. Trans Orthoped Res Soc. 1996;21:169. [Google Scholar]
- Ku G, Faust T, Lauffer LL, Livingston DJ, Harding MW. IL-1β converting enzyme inhibition blocks progression of type II collagen-induced arthritis in mice. Cytokine . 1996;8:377–386. doi: 10.1006/cyto.1996.0052. [DOI] [PubMed] [Google Scholar]
- Mori L, Iselin S, De Libero G, Lesslauer W. Attenuation of collagen-induced arthritis in 55 kD TNF receptor type I (TNFRI)-IgG1 treated and TNFRI deficient mice. J Immunol. 1996;157:3178–3185. [PubMed] [Google Scholar]
- Wong HL, Costa MT, Lotze MT, Wahl SM. Interleukin (IL) 4 differently regulates monocyte IL-1 family gene expression and synthesis in vitro and in vivo. J Exp Med. 1993;177:775–781. doi: 10.1084/jem.177.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborg C, Imfeld KL, Zaldivar F, Wang Z, Buckingham BA, Berman MA. IL-4 expression in human T cells is selectively inhibited by IL-1α and IL-1β. J Immunol . 1995;155:5206–5212. [PubMed] [Google Scholar]
- Månsson B, Carey D, Alini M, et al. Cartilage and bone metabolism in rheumatoid arthritis. J Clin Invest. 1995;95:1071–1077. doi: 10.1172/JCI117753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare PE, Carlson CS, Stollerman ES, et al. Expression of cartilage oligomeric matrix protein by human synovium. . FEBS Lett. 1997;412:249–252. doi: 10.1016/s0014-5793(97)00789-8. [DOI] [PubMed] [Google Scholar]
- Di Cesare PE, Fang C, Leslie MP, et al. Localization and expression of cartilage oligomeric matrix protein by human rheumatoid and osteoarthritic synovium and cartilage. J Orthop Res. 1999;19:437–445. doi: 10.1002/jor.1100170321. [DOI] [PubMed] [Google Scholar]
- Van Meurs JBJ, van Lent PLEM, Singer II, et al. Interleukin-1 receptor antagonist prevents expression of the metalloproteinase-generated neoepitope VDIPEN in antigen-induced arthritis. . Arthritis Rheum. 1998;41:647–656. doi: 10.1002/1529-0131(199804)41:4<647::AID-ART11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Van Meurs JBJ, van Lent PLEM, Holthuysen AEM, et al. Cleavage of aggrecan at Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: a pivotal role for stromolysin-1 in MMP activity. Arthritis Rheum. 1999 in press doi: 10.1002/1529-0131(199910)42:10<2074::AID-ANR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Nemoto O, Yamada H, Kikuchi T, et al. Suppression of matrix metalloproteinase-3 synthesis by interleukin-4 in human articular chon-drocytes. J Rheumatol. 1997;24:1774–1779. [PubMed] [Google Scholar]
- Van Meurs JBJ, van Lent PLEM, Holthuysen AEM, et al. Kinetics of aggrecanase and metalloproteinase induced neoepitopes in various stages of cartilage destruction in murine arthritis. . Arthritis Rheum. 1999;42:1128–1139. doi: 10.1002/1529-0131(199906)42:6<1128::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kunicka JE, Talle MA, Denhardt GH, et al. Immunosuppression by glucocorticoids: inhibition of production of multiple lymphokines by in vivo administration of dexamethasone. Cell Immunol. 1993;149:39–49. doi: 10.1006/cimm.1993.1134. [DOI] [PubMed] [Google Scholar]
- Joyce DA, Steer JH, Kloda A. Dexamethasone antagonizes IL-4 and IL-10-induced release of IL-1RA by monocytes but augments IL-4, IL-10 and TGF β induced suppression of TNFα release. J Interferon Cytokine Res. 1996;16:511–517. doi: 10.1089/jir.1996.16.511. [DOI] [PubMed] [Google Scholar]
- Van de Loo FAJ, Arntz OJ, Enckevort FHJ, van Lent PLEM, van den Berg WB. Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS-2 deficient mice and in anti-interleukin-1 treated wild type mice with unabated joint inflammation. Arthritis Rheum. 1998;41:634–646. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]


