Abstract
In the CNS, there are widespread and diverse interactions between growth factors and estrogen. Here we examine the interactions of estrogen and brain-derived neurotrophic factor (BDNF), two molecules that have historically been studied separately, despite the fact that they seem to share common targets, effects, and mechanisms of action. The demonstration of an estrogen-sensitive response element on the BDNF gene provided an impetus to explore a direct relationship between estrogen and BDNF, and predicted that the effects of estrogen, at least in part, might be due to the induction of BDNF. This hypothesis is discussed with respect to the hippocampus, where substantial evidence has accumulated in favor of it, but alternate hypotheses are also raised. It is suggested that some of the interactions between estrogen and BDNF, as well as the controversies and implications associated with their respective actions, may be best appreciated in light of the ability of BDNF to induce neuropeptide Y (NPY) synthesis in hippocampal neurons. Taken together, this tri-molecular cascade, estrogen-BDNF-NPY, may be important in understanding the hormonal regulation of hippocampal function. It may also be relevant to other regions of the CNS where estrogen is known to exert profound effects, such as amygdala and hypothalamus; and may provide greater insight into neurological disorders and psychiatric illness, including Alzheimer’s disease, depression and epilepsy.
Keywords: Alzheimer’s disease, dentate gyrus, depression, epilepsy, estradiol, granule cell, memory, mossy fibers, neurogenesis, neuroprotection, neuropeptide Y, neurotrophin, estrogen receptor α, estrogen receptor β
I. Introduction
There is a long history of studies that have examined steroid hormone and growth factor interactions. These studies initially focused on the periphery, and then expanded into the central nervous system (CNS). In the CNS, one of the best examples of a steroid hormone that has widespread and diverse interactions with growth factors is estrogen, one of the two principal steroid hormones produced by the ovaries. Thus, there is a substantial interest in the relationship between estradiol and growth factors, and a large literature has developed that provides evidence for how these interactions occur. The majority of interactions can be conceptualized in three ways: convergence of estrogen and growth factor signal transduction, induction by estrogen of specific growth factors (or vice-versa), and dependence of either growth factor effects on estrogen, or the converse.
Convergence involves the ability of estrogen and growth factors to activate the same effectors: an example of this is the activation of the same signaling cascades. The discussion below highlights the interaction between estrogen and the neurotrophin BDNF, and this is a good example of a steroid and growth factor that indeed activate a number of common signaling cascades (Figure 1, Table 1).
Figure 1.
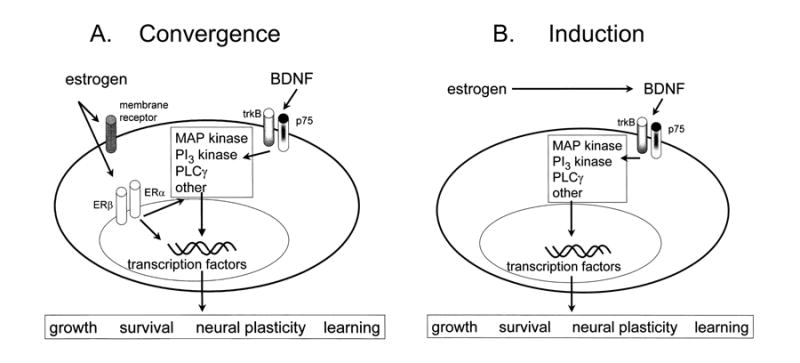
Estrogen-BDNF interactions: convergence vs. induction.
A. A schematic illustrates the potential convergence of estrogen- and BDNF-induced signal transduction. Estrogen may act either on membrane or nuclear receptors, and possibly both. These receptors are not precisely defined, so their similarity in the diagram is for schematic purposes only. BDNF acts on either trkB or p75 receptors to activate similar signaling pathways as estrogen, and major targets include transcription factors that influence growth, survival, neural plasticity, and learning in hippocampus, as well as other effects. Estrogen and BDNF may not necessarily act on the same cell, as shown, but cells that are distinct, such as pyramidal cells and GABAergic interneurons, as well as glia.
B. An alternative to the convergence shown in part A is that estrogen and BDNF interact directly, because estrogen induces BDNF gene expression, which in turn acts on trkB and p75 to exert its effects. Induction of this kind may occur by an estrogen-sensitive response element (ERE) on the BDNF gene or by estrogen-induced increase in neural activity that upregulates BDNF, because BDNF expression is regulated by activity (for further discussion, see text).
Table 1.
Convergence of Estradiol and BDNF on similar signaling cascades in hippocampal neurons.
| Pathway/target activates | Evidence that estradiol activates the target in hippocampaus | Evidence that BDNF the target in hippocampus |
|---|---|---|
| MAP kinase | [48, 151–153] | [154] |
| ERK | [48, 152] | [155, 156] |
| PI3 kinase/Akt | [157, 158] | [154] |
| CaMKII | [159] | [55, 160, 161] |
| CREB | [41, 85, 162] | [155, 156, 160, 163] |
| Src/Fyn | [153, 164] | [165] |
Examples of signal transduction pathways and mediators that have been shown to be activated by either estrogen or BDNF. For simplicity of presentation, distinct members of the same pathway are separated to show evidence for multiple steps in each pathway (e.g, MAP kinase, ERK).
Induction would be illustrated by the facilitation of growth factors or their actions by steroid hormones, or the converse. Again, estrogen and BDNF provide an example, in light of the fact that an estrogen response element (ERE) is present on the BDNF gene, and estrogen appears to induce BDNF expression.
Dependence, the third major category of potential interactions, to some extent overlaps with induction – for example, induction of growth factor synthesis may be required for full expression of steroid effects on target tissues [1, 2]. However, it also includes examples of situations in which components of steroid response pathways may be required for full expression of growth factor effects. For example, in the case of insulin-like growth factor 1 (IGF-1), estrogen receptor α (ERα) is an obligate partner in the activation of IGF-1 receptor activated signaling. This appears to due to the fact that ERα is an integral part of the IGF-1 receptor complex. Without both estrogen and IGF-1, IGF1 receptors do not activate the normal IGF-1 signaling pathways [3].
II. Estrogen and BDNF in hippocampus
A. Rationale
As implied above, there are many reasons at the present time to be interested in how estrogen and BDNF may interact. It is important to note that this began with studies that identified a complementary expression of estrogen, estrogen receptors, and different members of the neurotrophin family, including NGF (nerve growth factor) and BDNF. This pattern of expression strongly suggested functional interactions, and these were predicted from the initial expression studies [4].
Why focus on estrogen and BDNF in hippocampus, specifically? The evidence for an interaction between estrogen and BDNF is perhaps best developed for this structure. Not only is there complementary expression of estrogen receptors and BDNF in the hippocampus, there is also a long history of studies showing similar effects of estrogen and BDNF in this region of the brain (Tables 1, 2). Finally, for both estrogen and BDNF, the effects that have been observed exhibit unique and characteristic response patterns, that are not shared by any other humoral agent. Thus, estrogen has striking effects that range from alterations in behavior [5–13], structure (spine morphology, density, and spine synapses; Table 2), and physiology (potentiation of glutamatergic inputs in area CA1; Table 2) including specific receptor-mediated actions, such as actions at the NMDA receptor (although not exclusively; [14]). BDNF is the one other molecule that has been shown to exert many, if not all, of these same specific effects (Table 2). This is perhaps not surprising, because both estrogen and BDNF have been shown to exert effects via the same cellular mechanisms, including extracellular signal-regulated kinase (ERK) phosphorylation, cyclic AMP response element binding protein (CREB)-dependence, and phosphorylation of the NR2B subunit of the NMDA receptor (Tables 1, 2).
Table 2.
Similarities in hippocampal actions of estradiol and BDNF.
| ↑Estradiol (males) | ↑Estradiol (Ovx females) | ↑Estradiol (Intact females) | ↑BDNF (males) | ↑BDNF transgenics (males) | ↑TrkB transgenics (males) | |
|---|---|---|---|---|---|---|
| Physiology in hippocampal slices (area CA1) | ||||||
| Potentiation | ||||||
| Potentiation | [47, 151] | [14, 50, 166–168] | [169, 170] | [34, 171, 172] | [173–175] | [176] |
| Lack of potentiation | [177] | [45] | ||||
| Inhibition | ||||||
| Increased | [49] | [178] | ||||
| Decreased or no effect | [14, 49, 167] | [177–179] | ||||
| NMDA receptor dependence | [47] | [14, 48, 49] | [52–55] | |||
| Decreased slow AHP | [56, 58] | [57] | ||||
| Memory | ||||||
| Facilitation | [11, 180–182] | [5–7, 9–13] | [34, 165, 183, 184] | [34, 165, 184, 185] | [186] | |
| No effect or facil. if ↓ estradiol | [46] | [44] | ||||
| Decrease | [187] | |||||
| Plasticity | ||||||
| Dendritic spines | ||||||
| Changes | [41, 59] | [59–62, 65, 188, 189] | [64] | [41, 66–70, 184] | ||
| No effect | [190] | |||||
| Neurogenesis | ||||||
| Increased adult neurogenesis | [74] | [73, 75] | [73] | [72, 191, 192] | ||
| Decrease | [74] | [75] | [193, 194] | |||
| Pathophysiology | ||||||
| Neuroprotection | ||||||
| Ischemia | ||||||
| Neuroprotection | [195, 196] | [31, 197–200] | [201] | [202–206] | [207] | |
| Exacerbation or no effect | [208] | [209] | [210, 211] | [212] | ||
| Kainic acid | ||||||
| Neuroprotection | [213–215] | [216–219] | [220] | |||
| Exacerbation of damage | [218] | |||||
| Seizure susceptibility | ||||||
| Status epilepticus or seizure threshold | ||||||
| Decreased | [218, 221] | [189, 216, 218, 222, 223] | [222, 224] | [115] | [45] | [212] |
| Increased or no effect | [223] | |||||
| Kindling | ||||||
| Facilitation | [225, 226] | [227] | [228] | [229–231] | ||
| Inhibition or no change | [225, 226] | [232] | [233–235] | |||
For each response category listed on the left, evidence that the response is influenced by estradiol and BDNF are shown. Increased estradiol in males refers to studies which used male rats and either administered estradiol or experimentally increased estradiol. Increased estradiol in ovariectomized (Ovx) rats refers to the analogous experiments in Ovx female rats. Very different doses and durations of estradiol treatment were used, and these factors, as well as others, could be responsible for the distinct effects that are listed. For proestrous females, effects were relative to animals examined during other endocrine conditions, such as a distinct time of the estrous cycle or after ovariectomy. ‘Increased BDNF in males’ is analogous to ‘Increased estradiol in males,’ except that BDNF was used, not estradiol. BDNF transgenics refer to animals that overexpressed BDNF and were male mice. TrkB transgenics refer to male mice that either had a mutation or change in expression of TrkB. The references listed under this heading provided evidence from animals with deficits in trkB function that ligands of trkB, like BDNF, would lead to the responses shown on the left (i.e. potentiation, changes in inhibition, etc.).
Responses include physiological studies in area CA1, and primarily reflect information based on hippocampal slices from rats. ‘Memory’ refers to behavioral tests in vivo for different types of learned tasks, and focuses on spatial learning and memory. However, many distinct behavioral tests have been used, and the references reflect a variety of tests that do not only examine spatial tasks. Neuroprotection refers to a decrease in cellular damage or neuronal loss after either an ischemic insult or seizures induced by the convulsant kainic acid. Seizures susceptibility or seizure threshold refers to an increased incidence, or severity of experimentally-induced status epilepticus or a decrease in seizure threshold in response to a convulsant stimulus. Regarding kindling, this refers to the facilitation of electrical kindling. Dendritic spine changes include increased density or morphological plasticity, as well as increased spine synapses. Increased neurogenesis refers to an increase in the incorporation of the mitotic marker bromodeoxyuridine in the adult dentate gyrus granule cell layer and subgranular zone, or other evidence of increased proliferation of dentate gyrus progenitors.
Another similarity is that both estradiol and BDNF have been shown to exert highly variable effects, sometimes facilitating certain hippocampal functions, while in other experimental situations the opposite has been documented. Importantly, the effects of estrogen and BDNF appear to vary in parallel. For example, both estradiol and BDNF are known to decrease excitotoxic neuronal loss, and hence have been touted as neuroprotective (Table 2). However, in other conditions they appear to make neuronal damage worse, the exact opposite effect. A similar situation exists with respect to the ability of estrogen or BDNF to promote or inhibit seizures (Table 2).
In summary, these intriguing observations suggest that it would be important to understand whether estrogen and BDNF act in concert, when this might occur, and what the mechanisms might be. Addressing these issues may provide insight into some of the fundamental aspects of hippocampal structure and function, and also have implications for our understanding of learning disorders, neuroprotection, and epilepsy.
B. Background
To begin to consider the potential ways estrogen and BDNF interact in hippocampus, it is helpful to review current concepts of the receptors and actions of each, which have been studied almost completely independently.
1. Estrogen, estrogen receptors, and their distribution in hippocampus
Estrogen actions in the CNS occur through receptor systems that are similar to those found in the reproductive target organs of estrogen. These receptor systems are not yet fully characterized. A major component of estrogen action is mediated via nuclear receptors, which act as hormone regulated transcription factors controlling the expression of estrogen sensitive genes. In addition, estrogen receptors are also found in association with both the plasma membrane and the mitochondria, where they may mediate rapid actions of the hormone that occur too quickly to be explained by genomic effects. ERα and estrogen receptor β (ERβ) are the best characterized estrogen receptors. Both of these receptors have the potential to mediate membrane as well as nuclear transcriptional effects of estrogen [15]. However, there may also be other receptor systems that contribute to estrogen action on the brain, most of which remain poorly characterized at the present time [16].
A primary source of estrogen is the periphery.. In females, peripheral estrogen synthesis occurs primarily in the ovaries, where testosterone is produced in the interstitial thecal cells and aromatized to estradiol within the granulosa cells. In males, gonadal synthesis of testosterone is converted to estrogen also, but to a much lesser extent.
Brain synthesis of estrogen is also possible, because enzymes for steroid synthesis are present, primarily in glial cells. Receent experiments have provided evidence that hippocampal synthesis does occur, which suggests that hippocampal synthesis of estrogen regulates estrogen-sensitive hippocampal responses [17, 18].
Estrogen receptor localization in hippocampus is difficult to define because of disparate findings in past studies, most likely due to the differences in antibodies used to judge receptor distribution. It also is likely that a consensus has been difficult because estrogen receptor (ER) localization changes with endocrine state. Thus, at proestrus, a different distribution of ERα was reported relative to other cycle stages [19, 20]. Changes in ERs may also occur after estradiol treatment [21, 22].
Past studies of ER distribution in hippocampus have provided a reasonable consensus on the following points.. If one considers just one species, the rat, and more specifically, just the adult hippocampus, both mRNA and expression of ERα and ERβ are found in numerous cell types. Thus, it has been reported that ERα is expressed in principal cells and in the neurons that use γ-aminobutyric acid (GABA) as a neurotransmitter (“GABAergic” neurons). Electron microscopic studies have shown that ERα is compartmentalized to many different sites, ranging from cytoplasmic to nuclear [19]. ERβ has also been shown to be expressed in these cell types [23, 24]. Most of these studies suggest that ERs are primarily neuronal.
What remains controversial is the preferential localization of the receptors in principal cells vs. GABAergic neurons, and relative concentrations in different subcellular compartments. Are ER primarily expressed on GABAergic neurons, and therefore primarily exert effects by modulation of inhibition? Initial studies suggested that this was the case [25] and indeed evidence from more recent work continues to support this hypothesis [26, 27]. However, even if GABAergic neurons are the primary cell type that express ER in hippocampus, exactly which GABAergic neurons express ER is not entirely clear. One study suggests that ERα is primarily located on neurons which co-express GABA and neuropeptide Y (NPY; [26]), which is notable in light of the potential for estrogen to induce NPY synthesis via BDNF gene expression, whereas other studies suggest ERα is specific to the subset of interneurons that are immmunoreactive for the calcium-binding protein parvalbumin[27]. These neurons are not identical, although there is some overlap [28, 29]. In the dentate gyrus, parvalbumin-immunoreactive neurons are the GABAergic neurons that form a basket-like plexus around granule cells, the so-called “basket” cells; NPY-expressing GABAergic neurons form a population that innervates the outer molecular layer primarily. However, some of the neurons that form part of the basket cell population do express NPY.
Another important issue is whether receptors are nuclear or non-nuclear, because of the obvious functional implications. Based not only on light but also electron microscopy, it appears that ERα and ERβ can be localized to the cytoplasm as well as the nucleus. Light level studies have shown, for example, that fixation, and other factors, can change the appearance of ERα and ERβ from somatic to dendritic compartments [22, 30]. Electron microscopy has detected ERα and ERβ in numerous subcellular locations [23, 31].
There are also differences in reports of the differential distribution of receptors on principal cell types, i.e, CA1 -CA3 pyramidal cells and granule cells. For example, some studies indicate that CA3 pyramidal cells express substantial ERα receptors [32] or demonstrate I125 - binding [33] in contrast to other reports that do not suggest this [19, 24, 27]. Likewise, some studies demonstrate ERβ in granule cells [23], but others suggest ERs are neglible in granule cells [27].
In summary, the adult rat hippocampus contains a robust population of ER, not only ERα but ERβ. They appear to be localized to many cell types, but a comprehensive understanding of ER distribution across different endocrine conditions, other conditions, and how this may change with stress, neuronal activity, or pathology has yet to emerge, even for the adult female rat, the most widely studied system. Many of the reasons are due to the inherent complexities of expression studies, which have shown that factors such as age, fixation and endocrine condition can lead to very different results. In addition, current antibodies are unlikely to provide the ability to completely recognize all types of ER, including not only ERα and ERβ in their classic forms, but also their potential variants.
2. BDNF, its receptors, and their distribution in hippocampus
The neurotrophin family is composed of several members, nerve growth factor (NGF), BDNF, neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4). The family and their receptors are schematically illustrated in Figure 2A.
Figure 2.
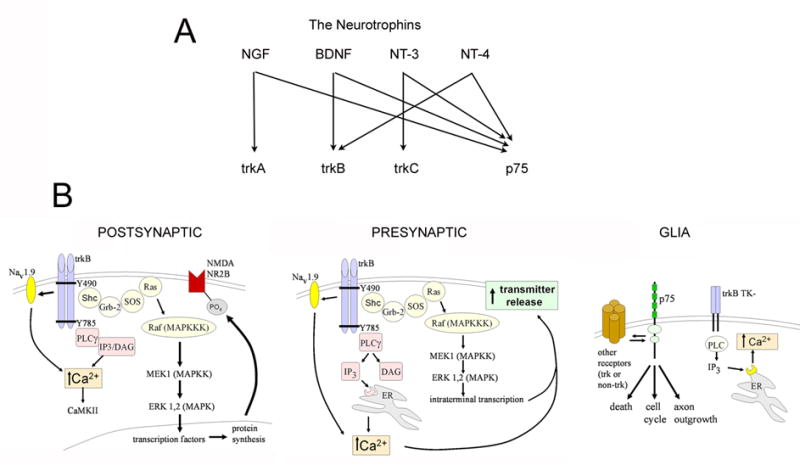
Neurotrophin receptors and targets of BDNF.
A. The Neurotrophin family includes nerve growth factor (NGF), BDNF, and also neurotrophin -3 (NT-3) and neurotrophin-4 (NT-4). Receptor specificity exists for trkA, for which NGF is the specific ligand, and trkC (NT-3 is the ligand), whereas trkB has two potential ligands, BDNF and NT-4 act. All neurotrophins bind to p75. BDNF is considered the main ligand for trkB in hippocampus, but the effects of NT-4 may be underestimated because in forebrain BDNF knockout mice, trkB can still be phosphorylated [236].
B. A schematic illustration of target pathways and molecules of trkB for postsynaptic, presynaptic, and glial processes. From [237].
There are two types of receptors for neurotrophins, either tropomyosin-kinases (trk) or the p75 receptor. BDNF and NT-4 bind to trkB, whereas all neurotrophins bind to p75. TrkB exists as a full-length and truncated receptor (the latter having two isoforms); the full-length form has received the greatest attention, but the truncated receptor is likely to have significant effects despite the fact that it lacks the internal kinase domain. Full-length trkB receptors autophosphorylate in response to ligand activation, and this, in turn, activates numerous signal cascades, as shown in Figure 2B. p75 also activates a number of potential intracellular mediators (Figure 2B).
Recent studies have shown that in addition to BDNF, a precursor to BDNF (proBDNF) also exerts biological effects. ProBDNF, appears to be released from the soma, where it is able to act preferentially at p75. Normally it is cleaved extracellularly by proteases, but it may exert important effects before cleavage in light of recent studies showing its ability to modulate hippocampal synaptic plasticity [34]. Figure 3 illustrates many of the potential locations of ligands and receptors for BDNF action in the rat dentate gyrus.
Figure 3.
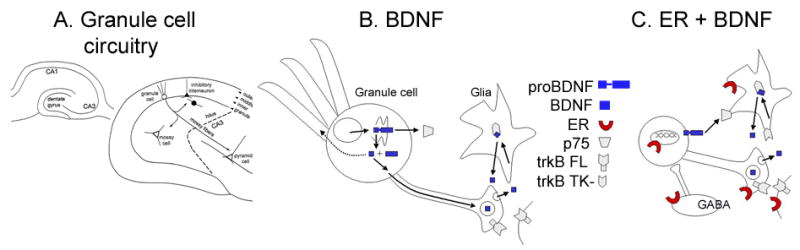
The expression of BDNF, its receptors, and estrogen receptors in the adult rat dentate gyrus.
A. A schematic illustrates the fundamental circuitry of the dentate gyrus. From [238].
B. The expression of BDNF and its receptors is shown schematically for the granule cell of the dentate gyrus and neighboring glia. BDNF is thought to be cleaved from its precursor, proBDNF and then anterogradely transported [36] to dense core vesicles. It may also be located or trafficked to dendrites under some conditions [37]. ProBDNF may be released and act on p75 receptors [34]. For further discussion, see text. TrkB FL refers to the full-length receptor; there also is a truncated receptor (TrkB TK-) that is likely to be associated with glia and potentially scavenge BDNF. C. The expression of BDNF, its receptors, and the potential locations of estrogen receptors in a simplified circuit of granule cell and GABAergic interneurons are illustrated. The GABAergic neurons, which express estrogen receptors may innervate granule cells. This, and evidence from CA1 studies that estradiol can disinhibit pyramidal cells [49], has led to the suggestion that estrogen may disinhibit granule cells indirectly [27]. However, estrogen receptors may be on a subset of interneurons that do not control granule cell inhibition [26] and even if so, estrogen actions on those GABAergic neurons may not lead to granule cell disinhibition, because such studies have not been conducted specifically (see text for further discussion).
BDNF is expressed in high concentrations in many of the areas of the CNS where estrogen is known to have robust effects, and the hippocampus is no exception. In the hippocampus of the adult rat, BDNF mRNA is expressed in all principal neurons, i.e, the dentate gyrus granule cells and hippocampal pyramidal cells. Interestingly, BDNF protein does not appear to be uniformly translated, because the highest concentration of mature BDNF appears to be in the granule cells, with relatively weak expression in pyramidal cells [35]. Within granule cells, BDNF is anterogradely transported to the axons, the mossy fibers [36].
Importantly, there are some reports that BDNF may be expressed to a greater extent in pyramidal cells than initial studies suggested. The evidence is based on the use of the antibody to BDNF that is commercially-available from Santa Cruz Biotechnology Inc., (Santa Cruz, CA) which shows a distinct pattern of expression relative to antibodies from other sources, such as Amgen-Regeneron Partners (Tarrytown, NY) [37, 38]. The reason for the different pattern of expression is currently unclear, but could relate to the fact that the different antibodies bind to distinct conformation of BDNF: vesicular BDNF located in axons may be more accessible or in a conformation more recognizable by one antibody. In contrast, non-vesicular, somatic/dendritic BDNF may be detected preferentially by the Santa Cruz antibody. Importantly, both antibodies withstand the classic tests of specificity (loss of reactivity upon incubation with BDNF, or loss of reactivity in BDNF knockout animals).
The hippocampal distribution of trkB has been characterized [39]. TrkB is present in a number of compartments within many hippocampal cell types, as well as glia [39]. p75 is also diverse, and in addition is present on the afferents of septal neurons that innervate the hippocampus [40]. Like ER, it is unclear how much BDNF may preferentially activate GABAergic interneurons relative to principal cells.
3. Comparison of estrogen and BDNF actions in hippocampus
The previous section outlined ligand and receptor distribution in hippocampus. Below is a comparison of some of the major effects of estrogen and BDNF in hippocampus. It is remarkable that they have almost always been studied independently (although there are exceptions [41]), because their effects are so similar.
As shown in Table 1, the effects of estrogen at the level of signaling parallel the effects of BDNF. However, estrogen and BDNF have more in common than shared target pathways, because there are many structural, electrophysiological, and behavioral changes in hippocampal neurons which are influenced by both estrogen and BDNF (Table 2).
For example, there are numerous reports that both estradiol and BDNF enhance hippocampal-dependent learning. This has been shown for male rats after manipulations that increase estradiol, and the reverse, that learning is worse when estradiol levels are low, for example in aromatase-knockout mice [42]. This has also been studied in female rats after ovariectomy and estradiol replacement, although it is difficult to compare some of these studies because doses of estradiol vary, as well as other potential factors such as age, time after ovariectomy, and housing [5–9, 11–13, 43]. The variability occurs not only in whether behavior is enhanced, but also the type of task. Thus, many studies of spatial learning (a classic hippocampal-dependent behavior) show an improvement after estradiol administration, but other types of memory can sometimes also improve, suggesting not only hippocampal effects but also non-hippocampal effects [5–9, 11–13, 43]. Learning appears to be enhanced on the morning of proestrus relative to other cycle stages or relative to ovariectomy [44]. Taken together, the studies of distinct endocrine conditions make a strong case for the positive effects of estradiol on hippocampal learning, whether it be due to an endogenous elevation in estradiol or exogenous administration.
Like the effects of estradiol, BDNF enhances learning (Table 2). Conversely, mutations in trkB or transgenics with altered trkB function show impaired learning (Table 2). However, too much BDNF, analogous to the effects of high doses of estrogen, appear to impair or have no effects on learning [45, 46]. Thus, for both estradiol and BDNF, the “dose-response” relationship appears to be non-linear (i.e, bell-shaped), such that low doses facilitate, but high doses may inhibit responses. Reasons for this may not be complicated: estrogen appears to down-regulate ERα and high doses of BDNF appear to decrease trkB (for references and further discussion, see section V).
There are a number of electrophysiological studies that show a similarity in the effects of estradiol and BDNF on hippocampal glutamatergic transmission. Focusing just on area CA1 and the adult hippocampus, many studies suggest that exogenous estradiol or BDNF can potentiate Schaffer collateral transmission, or facilitate long-term potentiation (LTP). Indeed, the current consensus is that BDNF is critical to the late stage of LTP [34]. Importantly, the studies to date that have examined both estradiol and BDNF in hippocampal slices do not always agree. For example, there are diverse opinions about the ability of estradiol and BDNF to influence GABAergic vs. glutamatergic transmission (Table 2).
Some of the detailed comparisons of estrogen and BDNF action in area CA1 pyramidal cells provide the strongest indication that the two act in either a convergent or concerted fashion. Thus, both estradiol and BDNF exert some of their actions in CA1 by phosphorylating either NR1 or NR2B, two subunits of the NMDA receptor [14, 47–55]. They also have both been shown to influence a specific Ca2+ -dependent potassium current in CA1 pyramidal cells [56–58]. The fact that both estradiol and BDNF have these very specific effects argues against independent effects, although it is not inconceivable that they are simply redundant.
There are even more similarities in action, as shown in Table 2. For example, estradiol and BDNF share robust effects on the structure of dendritic spines and their synapses. Thus, density, complexity and the number of spine synapses is increased by both estrogen and BDNF in hippocampus. It is noteworthy, however, that the exact nature of the changes in morphology may;e distinct: some studies indicate that spine shape or length change whereas other studies report that spine synapses are affected [41, 59–71]. Such data provide some caution to the assumption that estradiol and BDNF have exactly the same effects. However, the conclusion that does appear supported is that there is extensive similarity, perhaps the most of any other pair of neuromodulators that have been studied so intensively. Indeed, the similarities do not stop with those listed above. The rate of dentate gyrus neurogenesis is increased by both estrogen and BDNF [72–75]. The influence of both estrogen and BDNF also applies to glia [76–78], although this is not as well documented as their effects on neurons. In summary, it is remarkable how similar estradiol and BDNF are in their diversity and their mechanisms of action on hippocampal cells. The similarities suggest that estradiol and BDNF share common mechanisms and actions.
III. Mechanisms for estrogen-BDNF interactions in hippocampus
A. Estrogen induces BDNF expression through an ERE element on the BDNF gene
The similarities discussed above suggest that estrogen and BDNF may share common signal transduction pathways, effectors, and may interact to exert their effects. How might this occur? One possibility is that BDNF synthesis is induced by estrogen, and this is supported by studies showing that there is a functional ERE on the BDNF gene [79]. Thus, estrogen can potentially induce brain BDNF mRNA and protein expression. And indeed that does appear to be the case, because ovariectomy reduces BDNF mRNA and estradiol replacement restores it [79–83]. Furthermore, the same appears to be true in male rats, because gonadectomized males showed an increase in BDNF mRNA after estradiol treatment [38].
However, there are some inconsistencies among reports in the literature that argue against a straightforward (i.e. linear) induction of BDNF by estrogen. For example, in some brain regions, estradiol administration does not always restore BDNF levels after ovariectomy [84], although there are some potential explanations for this, such as the fact that retrograde transport may remove BDNF from the structure where it is examined, or the pattern of ER expression determines the ultimate effect on BDNF levels (for discussion, see [84]). Even in hippocampus, some investigators find that the majority of BDNF expression modulated by estrogen is in CA1-CA3 [38, 85] whereas others find it in the dentate gyrus [82, 86].
Other studies do not necessarily find a relationship between elevated estradiol and elevated BDNF, possibly due to the timing required before estrogen can induce BDNF expression [87], which may vary and depend on experimental variables. Indeed, stress can increase or decrease BDNF [88–90], so concurrent estrogen withdrawal or administration could have different effects if an animal is not carefully handled. Prior experience may modify the effects of estradiol of BDNF as it can modify the effects of estradiol itself. Thus, prior behavioral training can modify the changes in spine synapses that estradiol induces [91]. Finally, some doses and regimens of estrogen may simply fail to influence BDNF, as appears to be the case for chronic treatment after ovariectomy [83].
Other studies also exemplify the complexity of estrogen-BDNF interactions. For example, in cultures from embryonic hippocampus, estradiol leads to an increase in dendritic spines, and this is mediated by a reduction in BDNF rather than an elevation [41]. Indeed, in the young animal, the interactions between estrogen and BDNF may be very different from those in adulthood. Studies by Solum and Handa demonstrated this, as well as the risk of using BDNF mRNA to predict BDNF protein levels. They found that in young male rats, gonadectomy reduced BDNF mRNA and a single dose of estradiol could restore it [38]. However, gonadectomy did not reduce BDNF protein; instead BDNF protein levels rose. Moreover, estradiol reduced the elevated levels of BDNF protein to the levels of the intact animal [38].
Estrogen-induced BDNF may also have distinct effects in the young animals vs. adult animals, because targets of BDNF are age-dependent either in expression or function. For example, BDNF down- regulates the expression of the potassium-chloride co-transporter KCC2 [92], which is a major factor in controlling the ability of GABA, acting on GABAA receptors, to exert hyperpolarizing effects on hippocampal pyramidal neurons. By decreasing KCC2 in the adult hippocampus, BDNF can potentially induce depolarizing actions of GABA. But in the immature brain, KCC2 expression is very low until the second postnatal week [92]. In immature neurons, KCC2 is increased by BDNF [93]. This not only is the opposite effect from the mature brain, but it has very different implications, because in the immature brain there are effects on the developing neural circuits that would be unexpected after maturity and circuitry is established.
Due to some of the difficulties in extrapolating from the data using ovariectomized animals or cultured conditions, another approach has been to study BDNF levels across the estrous cycle. Initially this led to counterintuitive results: BDNF mRNA appeared high at times during the estrous cycle when estradiol was not necessarily high [87]. Thus, on the evening of proestrus, (hours after the estrogen surge during the morning or proestrus), BDNF mRNA was not elevated [87, 94].
To address the issue further, studies were undertaken at the time of the proestrous surge (the morning of proestrus), and comparisons made to the same time of day at other times of the estrous cycle. In addition, BDNF protein levels were studied instead of mRNA, because BDNF mRNA does not always predict protein expression. These studies were informative because they showed that on the morning of proestrus, BDNF protein was elevated relative to ovariectomized rats, and it was elevated in the mossy fibers in particular, suggesting that estradiol elevated the level of BDNF in the same areas where it is normally expressed, rather than ectopic locations (Figure 4, [86]).
Figure 4.
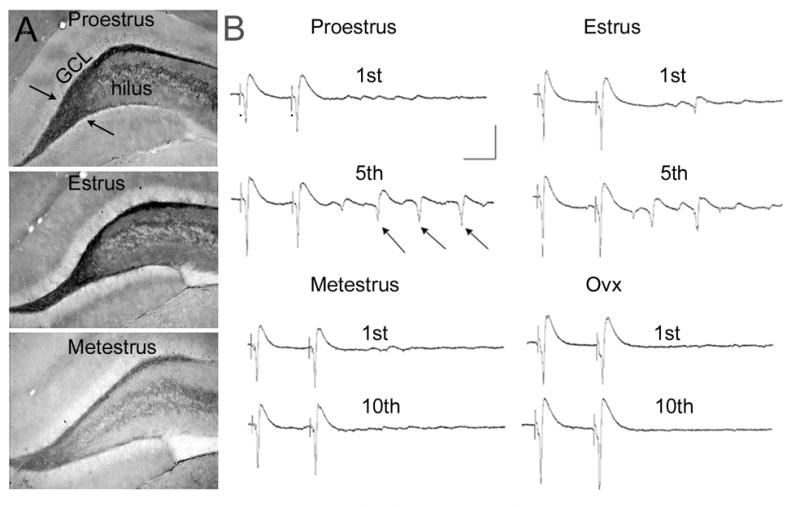
BDNF expression and physiological changes in hippocampal slices across the estrous cycle of the adult female rat.
A. Immunocytochemistry using an antibody to BDNF illustrates increased immunoreactivity in the hilar mossy fibers (arrows) at proestrus and estrus relative to metestrus.
B. Physiological recordings from the CA3 pyramidal cells, targets of the mossy fibers, at proestrus, estrus, metestrus, and after ovariectomy. The recordings represent responses to hilar stimuli (at the dots) triggered in pairs (40 msec interstimulus interval). After the 5th pair, multiple responses (population spikes) occurred in the animals examined on the morning of proestrus (arrows) or estrus, when BDNF in the mossy fibers was elevated. However, even 10 pairs of stimuli failed to evoke multiple population spikes in animals examined on the morning of metestrus or after ovariectomy, when BDNF levels were relatively low. From [86].
Interestingly, BDNF levels were also elevated in the mossy fibers on the morning of estrus, and increased expression in locations of pyramidal cell projections were also apparent at that time, suggesting increased expression in pyramidal cells. Indeed, patches of CA3 pyramidal cell somata were darkly immunoreactive [86]. The high levels of BDNF waned after the morning of estrus, appearing lower on the morning of metestrus (Figure 4; [86]).
Importantly, there were physiological changes in the trisynaptic pathway that occurred in concert with changes in BDNF expression during the estrous cycle. Mossy fiber transmission and Schaffer collateral transmission, the second and third synapse of the trisynaptic circuit, were enhanced (Figure 4; [86]). Interestingly, the changes were analogous to those that are observed after BDNF is superfused onto a normal male hippocampal slice, or the changes detected in transgenic mice that overexpress BDNF [45, 95]. These could be blocked by a trk receptor antagonist [86]. Together, these data supported the hypothesis that physiological levels of estradiol could increase BDNF in hippocampus. In addition, they suggest that there are functional consequences, namely that estradiol can induce functional effects of BDNF.
In summary these studies suggest that estradiol can induce BDNF gene expression under physiological conditions, and non-physiological conditions, i.e. after ovariectomy. More information will be necessary before one can conclude that these effects were dependent on the ERE, however, for reasons discussed further below.
B. Estrogen induces BDNF gene expression by a non-genomic mechanism
Despite the support provided by these studies to the hypothesis that estrogen induces BDNF gene expression by an ERE on the BDNF gene, it has been noted that there are potential problems with the hypothesis. These are based on the fact that the neurons which appear to increase their levels of BDNF during the estrous cycle (the granule cells) do not clearly possess estrogen receptors. Instead, it has been argued that estrogen receptors exist in much higher concentration on GABAergic interneurons juxtaposed to the granule cells which express BDNF [27]. A key element to this hypothesis is that neural activity (e.g., depolarization, action potential discharge) appears to increase BDNF [96], although exactly how much neuronal activity and under what conditions has never been precisely defined. Published studies to date have found that a high frequency electrical stimulus can induce BDNF gene expression in vitro [97, 98], suggesting that only a modest increase in neuronal activity is sufficient to activate the mechanisms that increase levels of BDNF.. However, it may be that a particular frequency, as well as intensity, is necessary; this simply is unclear at the present time. It is important to consider, because estrogen receptor activation of GABAergic neurons may lead to the type of “activity” that induces BDNF gene expression. On the basis of this possibility, an alternate hypothesis for estrogen-BDNF interactions based on the ERE was proposed. It was suggested that estrogen increases BDNF expression indirectly, by acting on GABAergic neurons, which in turn disinhibit the adjacent BDNF-expressing neurons. This hypothesis is attractive because,there is a constant barrage of spontaneous GABAergic input on dentate gyrus granule cells that is thought to help keep these neurons quiescent [99, 100].
However, there are some considerations that suggest caution before accepting this hypothesis. First, regarding expression of estrogen receptors on granule cells, there may be little evidence of ERα expression, but there is evidence of ERβ expression [23, 30]. In addition, although one may question evidence of nuclear receptors, there is evidence of cytosolic receptors in diverse areas of granule cells using electron microscopy [19, 23]. It is also important to bear in mind that, depending on the endocrine state, estrogen receptors may be more or less amenable to immunocytochemical evaluation [19, 20], so they could be missed. And indeed the studies that show few estrogen receptors in granule cells were conducted at a time when they were least likely to be obvious, i.e. times besides the morning of proestrus.
Another important issue is whether estrogen, acting on GABAergic neurons expressing ER, would disinhibit granule cells and this would lead to activity-dependent BDNF expression. One question is what estradiol would do to GABAergic neurons; this is not entirely clear. But even if it would hyperpolarize them, potentially leading to decreased spontaneous release of GABA, granule cells may not depolarize and fire action potentials. One reason for skepticism is based on the fact that the ER-expressing GABAergic neurons may be those which co-express NPY, and they primarily innervate distal dendritic regions of granule cells. Distal effects may do little to facilitate somatic action potential generation. However, some studies suggest that parvalbumin-immunoreactive GABAergic interneurons express ERs, and they would be more likely to innervate proximal processes of granule cells, and hence influence somatic inhibition [28]. Interestingly, some studies suggest that these cells are BDNF-sensitive [101].
In summary, it is important to recognize that the interactions between estrogen and BDNF in hippocampus have not yet been definitively identified. There are two attractive hypotheses, each with a foundation of support, but several potential caveats. One is that estrogen induces BDNF gene expression by acting at an ERE on the BDNF gene. The second is that estrogen acts on GABAergic interneurons which express ER, and by disinhibition of principal cells, BDNF is increased in the principal cells by activity-dependent expression.
IV. NPY as a modulator of estrogen-BDNF interactions
A. BDNF induction of NPY synthesis suggests a molecular cascade: Estrogen-BDNF-NPY
Besides the considerations above, there are other factors that make the hippocampal effects of estrogen and BDNF complex. However, they also may explain some of the data in the literature about estrogen and BDNF that are currently hard to reconcile. The fact is that estrogen and BDNF influence other neuromodulators that in turn may exert the actions that most laboratories consider direct effects of estrogen. One example is NPY.
BDNF can induce NPY expression in hippocampal neurons. NPY has robust effects in hippocampus, including depression of synaptic transmission, neuroprotection, and NPY also exerts anticonvulsant actions [102]. NPY facilitates neurogenesis in the dentate gyrus [103, 104]. Thus, estrogen may lead to increased BDNF expression that in turn elevates levels of NPY. The result of NPY induction could be a decrease in, for example, the pro-excitatory effects of estrogen and BDNF.
A. Induction of NPY by BDNF
In light of the robust effects of NPY in hippocampus, it becomes important to define the conditions that are required for BDNF to induce NPY expression. With this information, one would potentially be able to predict when estrogen and BDNF have effects that are influenced by increased expression of NPY.
Although the mechanisms that underly the induction of NPY by BDNF have not been elucidated in vivo, insights have been gained in vitro. Thus, in cultured neurons, BDNF appears to induce NPY expression in a trkB- and ERK-dependent manner [105–107]. This appears to be dependent on activity as well as BDNF, because GABAA receptor blockade of cultured neurons induces NPY expression by a trk-dependent mechanism, but the opposite occurs if AMPA receptors are blocked [108]. In addition, cultures from BDNF knockout mice do not show upregulation of NPY protein [109].
This implicates an action of BDNF, which is released and binds to trkB receptors that are situated on NPY-expressing neurons, leading to MAP kinase activation and NPY expression. Interestingly, it appears that a more substantial increase in NPYprotein occurs if BDNF exposure continues over hours in culture [106], suggesting that brief exposure to BDNF would be unlikely to have dramatic effects on the levels of NPY. Taken together, these data suggest that NPY protein will be induced only in trkB-expressing cells of hippocampus, and that rapid effects (requiring less than hours) of estrogen or BDNF would only be influenced by NPY weakly. This information alone is interesting, because it is consistent with some reports that describe how NPY increases after experimental manipulations in vivo. Thus, after acute seizures, NPY expression increases in GABAergic neurons of the dentate gyrus. This could be due to a rapid seizure-induced increase in BDNF in granule cells, followed by BDNF release onto GABAergic neurons, many of which are innervated by granule cells. In contrast, recurrent spontaneous seizures over days or weeks appear to be necessary to induce NPY expression in granule cells [110]. The increase in granule cell NPY may require prolonged BDNF elevation, as predicted by the in vitro studies.
B. NPY expression and action in the dentate gyrus
In contrast to what is known about the mechanisms underlying BDNF induction of NPY, a great deal has been elucidated concerning the normal expression and actions of NPY in the dentate gyrus-CA3 region. These studies are useful to review, because they indicate a new strategy that could be used to predict how NPY would modulate the actions of estrogen and BDNF.
In the adult male rat, NPY is expressed as shown schematically in Figure 5. Immunocytochemistry in Figure 5 shows the distribution of NPY protein in neurons and fibers in the normal adult male rat, which is virtually identical to the normal adult female rat. NPY is normally expressed in a subset of GABAergic neurons in the different hippocampal subfields, and within the dentate gyrus these neurons have somata either in the hilar region or on the border of the hilus and granule cell layer. The majority of these neurons send their axon to the distal dendrites of granule cells, located in the outer molecular layer. There they make a variety of contacts on incoming afferents, granule cell dendrites, and other processes [29]. NPY-expressing cells use GABA as a classical transmitter, and are thought to be inhibitory. NPY is presumably released preferentially after high frequency stimulation, like other neuropeptides [102].
Figure 5.
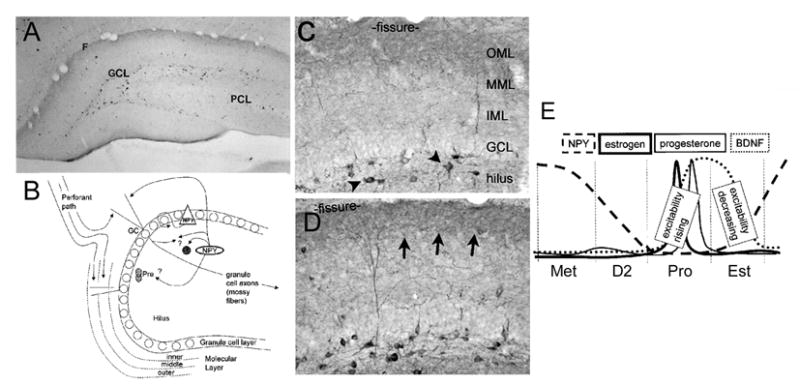
Neuropeptide Y (NPY) changes during the estrous cycle of the adult female rat.
A. A representative section from an adult male rat stained with an antibody to NPY shows numerous immunoreactive neurons in the hilar region, and additional staining in the outer molecular layer, reflecting the major axonal projection of these cells.
B. A schematic illustration of the normal location and axonal projections of GABAergic neurons of the dentate gyrus that co-express NPY. There is strong evidence for their molecular layer projection and hilar collaterals [29], but less evidence that they directly innervate the dentate gyrus progenitors (PRE) or mossy fibers. However, NPY exerts robust actions on progenitors and mossy fiber transmission (see text for further discussion).
C-D. At higher magnification, a comparison is shown of NPY immunoreactivity in the dentate gyrus of a normal adult female rat that was euthanized on the morning of proestrus (C) relative to a section processed concurrently from another animal that was euthanized on the morning of metestrus (D). The arrows indicate increased immunoreactivity in the axon plexus of the hilar NPY-immunoreactive GABAergic interneurons at metestrus.
E. A schematic illustrates a hypothesis for the changes in excitability of the CA3 pyramidal cells in response to hilar stimulation during the estrous cycle. Following the proestrus surge of estradiol, which appears to be followed by an increase in mossy fiber BDNF, which lasts for the duration of proestrus and at least the morning of estrus (Figure 4; [86]). There is a subsequent decline in BDNF and elevation in NPY in GABAergic neurons of the dentate hilus. It is suggested that the elevation in NPY in this interneuronal population limits the ability of hilar stimulation to produce repetitive activity in CA3 pyramidal cells on the morning of metestrus (Figure 4). This may be due not just to the hilar NPY neurons, but possibly also the NPY neurons of area CA3. Together, they potentially release NPY, which decreases glutamate release from mossy fibers [239, 240].
NPY-expressing neurons in the dentate gyrus also send collaterals to other areas of the dentate gyrus, where they presumably act on other cells, such as other types of GABAergic neurons, possibly mossy cells, and the progenitors in the subgranular zone [111]. NPY is also thought to influence mossy fiber transmission, and the hilar collaterals may serve that purpose by axo-axonic contacts on mossy fiber boutons.
There are several effects of NPY in the dentate gyrus. One is to inhibit mossy fiber transmission by a presynaptic action on glutamate release. The depression of neurotransmission is a likely explanation for the anticonvulsant actions of NPY, and for the neuroprotective actions [102].
Another action of NPY is to regulate gated inward-rectifying potassium (GIRK) channels on hilar neurons [112], and this also may be an effect that contributes to the anticonvulsant and neuroprotective effects of NPY, since it may influence when GABA is released from hilar
GABAergic neurons. NPY also decreases calcium entry into N-type calcium channels on granule cells [102]. By doing so, it may limit their activity or have other effects, which decrease their efficacy to discharge their targets, again providing a potential mechanism for anticonvulsant actions. Finally, NPY facilitates neurogenesis of granule cells by Y1 receptors on progenitors [111]. These effects could contribute to the maintenance of the granule cell population over time, which would maintain normal hippocampal function.
C. Evidence that estrogen may modulate NPY via BDNF
What is the evidence that estrogen and BDNF influence NPY in hippocampus? Studies to date suggest that estrogen does regulate NPY levels, and independent studies suggest the same for BDNF. The majority of studies are correlative. For example, the induction of seizures leads to an elevation of BDNF, as well as NPY. Temporally, the elevation of BDNF occurs earlier than the increase in NPY [102], but there is not proof that BDNF necessarily caused the change in NPY [113–115]. Administration of exogenous estradiol [26] or BDNF [116, 117] increases hippocampal NPY, but indirect mechanisms can not be ruled out.
An important confound to the experiments which analyze changes in BDNF and NPY is that we know activity can increase the expression of both BDNF and NPY. Therefore, if studies are conducted that do not clarify whether activity increased, one is not clear whether the activity itself led to the change, or whether the substance used to modify expression led to the change.
In light of this problem, we have begun to study NPY without using seizures as a stimulus, and without exogenous estradiol or BDNF administration. We simply asked whether the normal fluctuations in estradiol during the rat estrous cycle, which are followed by elevated BDNF, would be followed in turn by elevated NPY. Our approach used immunocytochemistry, because this allowed us a chance to detect changes in numerous cells that normally express NPY versus those that normally do not. Our findings are summarized in Figure 5C–E. In that figure are micrographs showing representative sections from animals euthanized on the morning of proestrus and metestrus (for Methods, see [86]). The focus is on the dentate gyrus, where the NPY-expressing neurons are abundant, and their axonal projections are particularly dense in one area, the outer molecular layer (Figure 5). Relative to proestrus, there was an increase in NPY expression at metestrus in the molecular layer axon plexus (Figure 5C–D). These data support the hypothesis that estrogen can induce BDNF gene expression and this may in turn elevate levels of NPY. This tri-molecular cascade correlates with the changes in physiology observed throughout the cycle: heightened excitability at proestrus and estrus, and relative low excitability at metestrus (Figure 4; [86]). Although correlative, it is tempting to speculate that the rise in estrogen-induced BDNF is responsible for the increase in excitability and this is curtailed by the subsequent elevation in NPY. Thus, NPY may serve as an endogenous control mechanism on the pro-excitatory effects of estradiol and BDNF.
D. Implications
1. Explanation of disparate findings in the literature concerning the effects of estrogen and BDNF
These observations raise the possibility that many discordant findings in past studies of estrogen and BDNF could be due to its induction of NPY. Thus, estrogen and BDNF may lead to increased excitation in hippocampus unless NPY is induced, when NPY could dampen excitation. Indeed the hippocampus seems well-designed for such a system, because ERα exists on GABAergic neurons that express NPY, and BDNF released onto NPY neurons increases NPY levels. Furthermore, NPY release onto hippocampal neurons appears to decrease glutamatergic synaptic transmission between principal cells.
In summary, estrogen and BDNF are likely to be neuroprotective when they induce NPY expression. If NPY were not induced, one would predict that estrogen and BDNF would be primarily excitatory. Under such conditions, estrogen and BDNF could facilitate excitotoxicity and seizures. By considering the levels of all three contributors (estrogen, BDNF and also NPY), instead of one or two, many inconsistencies in the literature might be resolved (Table 2).
2. Neurogenesis
Another important implication is based on the fact that estrogen, BDNF and NPY have all been shown to facilitate neurogenesis of dentate gyrus granule cells. Why would all three appear to have this effect? One possible explanation is that they may work in a sequence so that all stages of neurogenesis are supported. In other words, estrogen may influences the initial division of precursors, and by inducing BDNF estrogen would lead to further support for the next stages of neurogenesis, such as maturation and survival. Subsequent induction of NPY may continue support for even later stages of development of newly-born granule cells, such as the stages involving process outgrowth and synaptogenesis.
However, NPY does not appear to facilitate late stages of neurogenesis. Instead, it appears to influence proliferation [111]. These data suggest that estrogen, BDNF, and NPY may work in a complementary fashion rather than a sequential order. Regardless, it is important to recognize that manipulations which alter estrogen or BDNF, such as pharmacological treatment, may have an impact on neurogenesis by changing NPY levels in hippocampus.
3. Non-hippocampal functions
The cascade of estrogen-BDNF-NPY has relevance not only within the hippocampus, but also outside the hippocampus. Indeed, estrogen, BDNF, and NPY are all known to play essential roles in amygdala and hypothalamic function, to name just two CNS areas of many. The implications are schematically illustrated in Figure 6.
Figure 6.
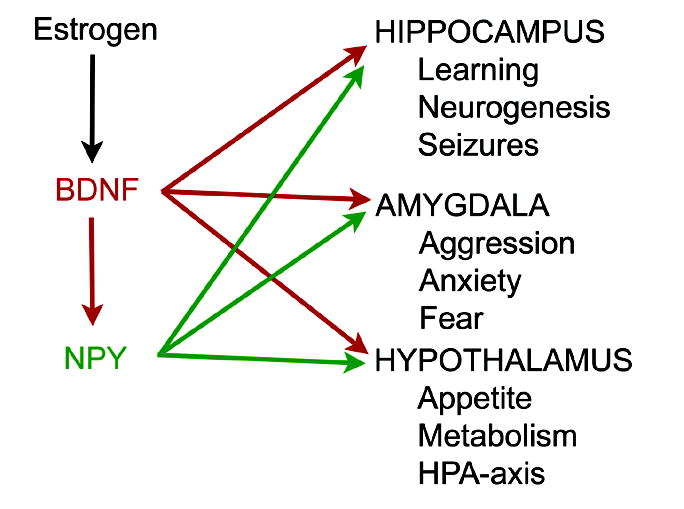
A schematic illustration of the potential for estrogen to exert actions via BDNF and NPY. Although estrogen potentially has direct effects on target structures like the hippocampus, as well as others such as the amygdala and hypothalamus, some of the effects previously attributed to direct actions could be due to the ability of estrogen to induce BDNF synthesis. Further modulation of the effects of BDNF could be due to subsequent induction of NPY expression by BDNF.
The concept diagrammed in Figure 6 is that some of the effects of estrogen that have been previously attributed to direct effects could be mediated, at least in part, by BDNF and NPY. For example, the influence of estrogen on appetite is likely to be due to its actions on hypothalamic neurons. It is possible that estradiol influences BDNF expression in hypothalamus, and this in turn alters NPY which in turn regulates appetite. This hypothesis makes inherent sense given the critical role of NPY in appetite control. BDNF also has been shown to influence feeding behavior, and it may do so by its ability to alter the level of NPY.
Another example is the influence of estradiol on the functions of the amygdala, functions that relate to aggression, anxiety, fear and emotional behavior. It is possible that estrogen has direct actions on amygdala neurons that are responsible for these effects because the amygdala is rich in estrogen-concentrating neurons [22, 118]. However, as depicted in Figure 6, it may also act indirectly, by inducing BDNF. Furthermore, the actions of estradiol that are mediated by its induction of BDNF could be further modified by subsequent induction of NPY.
V. Implications for neurological and psychiatric disease
A. Alzheimer’s disease
As suggested by many investigators, estrogen and neurotrophins such as NGF and BDNF may be relevant to the etiology of Alzheimer’s disease (AD; [119, 120]). Clinical studies have shown that reduced BDNF may be an early marker of the disease, since both proBDNF and BDNF decline prior to the end stages of AD. BDNF may be even more important than cholinergic changes previously thought to predict AD, because the decrease in BDNF precedes changes in choline-acetyltransferase [121]. There is still more information that is needed however, because some studies suggest that BDNF actually increases initially, and only declines at a later time in the progression of the disease [122]. However, the early increase in BDNF was measured from serum, not brain; studies that have examined brain specifically have shown that BDNF declines in the temporal lobe at early stages in the disease [123]. This may simply be due to the fact that serum BDNF does not necessarily reflect brain BDNF levels. Notably, a decrease in temporal lobe BDNF occurred at times when other areas did not show a decline in BDNF levels, suggesting that hippocampal BDNF could be critical, in accordance with the known memory functions associated with temporal structures like hippocampus [123].
The hypothesis that BDNF is implicated in AD has been supported by experimental approaches, such as exposure of laboratory animals to peptides implicated in AD. It appears that exposure to amyloid β (Aβ) inhibits trk signalling by interference with docking proteins like Shc. Docking proteins are important intermediaries between trk activation and intracellular signalling pathways. Low levels of Aβ specifically inhibit the ERK and phosphoinositide 3-kinase (PI3K)/Akt arms of BDNF signal transduction [124]. This has led to the suggestion that impairment of neurotrophin signalling may contribute to AD [124]. BDNF may be the most important neurotrophin, because BDNF is required to maintain tau in a dephosphorylated state, and once tau is phosphorylated, AD pathology usually develops [125].
How may estrogen-BDNF interactions relate to AD? Given the decline in BDNF in AD, the potential specificity of the decline in BDNF to temporal structures like hippocampus, and the decline in estradiol in women with age (and of course complete loss of serum estradiol after menopause), one logical hypothesis is that Alzheimer’s disease is due to the reduction of estrogen-induced BDNF synthesis in hippocampus. Indeed, genetic studies to date do not provide robust evidence that there is a genetic component (a contribution of BDNF polymorphisms to the disease [126–130]), suggesting estrogen-BDNF interactions may be intact, but there simply may be too little estrogen to adequately maintain BDNF levels, leading to reduced signaling. This hypothesis is supported by the evidence that estradiol administration restores cognitive function in ovariectomized animals and in animal models of AD [124].
However, continuous estrogen replacement does not appear to be effective in women, not just in AD but to relieve the symptoms of postmenopausal cognitive decline. Why might this be the case? There is substantial evidence that estrogen downregulates ERα in hippocampus [10, 22, 25]. Although there are indications that this is not always true [131], and indeed there may be a redistribution rather than an absolute loss [21, 22], this makes it unlikely that estrogen therapy would continue to be effective. As an alternate approach, rational drug design would suggest BDNF therapy, not only because of its potential to activate the signaling pathways normally attributed to estrogen, but it also would avoid the health risks associated with estrogen therapy [132]. And in contrast to estrogen, BDNF can increase its receptor, at least at low doses [133], potentially leading to enhanced efficacy. However, high doses of BDNF appear to either decrease or internalize trkB [133], and moreover might activate p75. Therefore, a low dose of a trkB ligand like BDNF could provide a promising therapeutic approach for AD. The same may be true for depression (see below).
B. Depression
Despite intense research efforts, depression remains a psychiatric disorder defined by a complex array of contributing factors and potential mechanisms. Notably, several studies have implicated low levels of BDNF in depression [134, 135]. It is also true that women are more susceptible to depression than men [136, 137]. One approach that highlighted the gender difference well was a study of twins, showing a higher incidence of depression in the female sibling [138]. In addition to these studies, the susceptibility of women to depression during the premenstrual period of their menstrual cycle and the syndrome of postpartum depression suggest a relationship between fluctuations in steroids such as estrogen and depression.
Given the common hypotheses for depression involve the serotonergic or other systems, it can be argued that there may be little reason to suggest that a defective relationship between estrogen and BDNF explains depressive illness. Indeed, it may be most relevant to focus on the dysregulation between stress and serotonin and BDNF [135, 139]. Nevertheless, it is also possible that estrogen regulation of BDNF is relevant, and particularly in hippocampus. Thus, ERα levels may be decreased in depression. Studies to date have shown that ERα levels appear to decline in the dentate gyrus in mental disorders, such as schizophrenia [140]. In depression, amygdala ERα was abnormally low, although the hippocampus was not examined [141]. It is tempting to speculate that estrogen-dependent induction of BDNF may be defective in depression. If true, estrogen treatment would be likely to improve depression, and indeed in certain situations that appears to be the case [142], although it has been suggested that this is due to an estrogen-induced increase in serotonergic function [143], not BDNF. However, it is interesting to note that serotonin may positively modulate BDNF [139].
What might be the mechanism for estrogen and BDNF to improve depression by actions in hippocampus? It might be that hippocampal plasticity and dentate gyrus neurogenesis are key, as recent studies suggest. Thus, antidepressants are thought to act by increasing dentate neurogenesis [144]. Plasticity may be required as well [135]. Indeed, BDNF and trkB have become new targets for the development of novel antidepressant drugs [145].
C. Epilepsy
Estrogen and BDNF, and indeed NPY, have been a focus of considerable attention among investigators interested in epilepsy because of the long history of studies linking each to seizures, not only in humans, but in animal models of epilepsy. However, it has been difficult to understand how each relates to seizures, and it may be because each is typically studied independently. For example, it has been known for decades that estrogen can be proconvulsant, but it has also be shown that this is not necessarily the case in all studies, and moreover that estrogen can be protective.
How can estrogen be proconvulsant (sometimes) and neuroprotective (sometimes)? The answer may lie in its ability to induce BDNF synthesis and hence NPY expression. Thus, estrogen may have limited effects on seizure threshold until it induces BDNF gene expression, and if examined at a time when NPY levels had subsequently increased, estrogen may lead to neuroprotective effects. However, estrogen-induced effects are examined at the time when BDNF is elevated, but not yet NPY, proconvulsant effects would be predicted. This prediction is schematically illustrated in Figure 6.
Figure 7 suggests that BDNF, particularly when increased by estrogen, could lead to seizures. Indeed, BDNF infusion can lead to seizures, although not universally (possibly because it induces NPY synthesis). There is now considerable evidence that BDNF increases excitability, lowers seizure threshold, facilitates epileptogenesis, and is elevated in humans with temporal lobe epilepsy [146]. Moreover, as shown in Figure 8, an initial seizure that might arise due to BDNF actions, might initiate positive feedback, because activity increases BDNF synthesis. One would predict that, were BDNF levels to increase sufficiently, a cycle of repeated seizures might occur [35].
Figure 7.
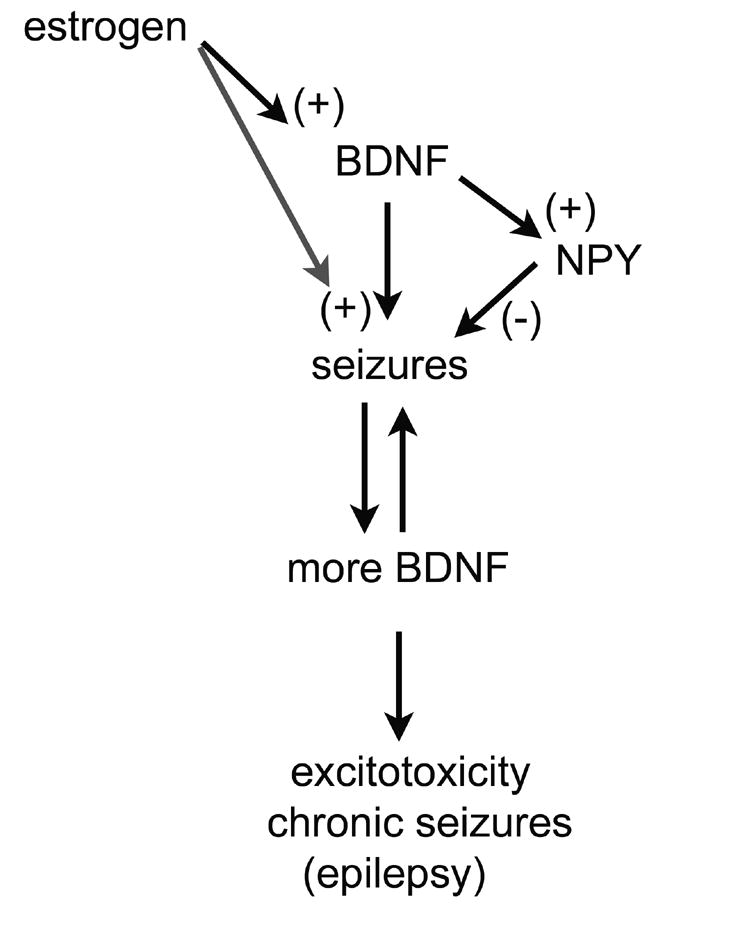
The effects of BDNF on seizure threshold and epilepsy.
Given that BDNF can facilitate many different excitatory pathways that can contribute to seizures, and that other evidence suggests BDNF decreases seizure threshold and in fact can induce seizures, the diagram shown starts with the hypothesis that BDNF could be proconvulsant. If so, a positive feedback may develop, because seizures upregulate BDNF expression. If sufficient, such a positive feedback loop may lead to repeated seizures and seizure-induced excitotoxicity, the hallmark of temporal lobe epilepsy. In addition, estrogen may facilitate this, because estrogen induces BDNF gene expression, and this may have relevance to hormone-sensitive seizures in women with epilepsy (discussed further in the text). However, because BDNF can induce NPY synthesis, a natural brake on this process is present normally, and may serve to prevent epileptogenesis, or simply be responsible for the long interictal periods in many individuals with epilepsy. This hypothesis illustrates the interesting implications of steroid hormone-growth factor interactions, but also emphasizes their complexity.
Estrogen may facilitate this pathway by inducing BDNF synthesis, or it may complement this pathway by exerting effects that also enhance excitability, independent of BDNF actions (Figure 8). Thus, it has been suggested that women with epilepsy, who have an increase in seizures during the periovulatory period of their menstrual cycle (Catamenial Epilepsy, Type I; [147]) might do so because of the rapid rise in estradiol before ovulation. It is also possible, although less commonly discussed, that seizures that occur during the perimenstrual period could be due to an elevation of estradiol, because estradiol does increase during the luteal phase and this could elevate BDNF. Alternatively, and more widely accepted, is the view that waning progesterone at the time of menses leads to a disinhibition, and this lack of inhibition leads to increased seizure frequency [147].
One of the hallmarks of women with epilepsy, and indeed most individuals with epilepsy, is that they do not have epilepsy all the time. In fact this may be due to inherent control mechanisms that are able to inhibit the underlying proconvulsant tone. One potential endogenous brake could be NPY (Figure 8). Thus, estrogen and BDNF may facilitate seizures, but these seizures eventually stop, and this could be due to the rise in NPY levels induced by BDNF. This hypothesis has some basis: in the dentate gyrus of rodents with recurrent seizures (epilepsy), NPY infusion stops seizures [102, 148]. In addition. BDNF and NPY are strongly expressed in the mossy fiber pathway of these animals. This pathway is a major branch of the trisynaptic circuit that is thought to be the main avenue for seizure propagation in limbic circuits. Indeed, without it, seizures may not proceed into hippocampus and therefore would be less likely to generalize. Thus, the expression of BDNF and NPY in the mossy fiber pathway may modulate seizure propagation. BDNF may promote it, NPY may reduce it. Indeed, BDNF does appear to facilitate seizure-like activity due to mossy fiber actions in animal models of epilepsy [149], and in similar epileptic rats NPY is tonically released and inhibits mossy fiber transmission in rats with chronic seizures [150]. Thus, the cascade of estrogen-BDNF-NPY may regulate seizures much more than previously considered.
VI. Summary
As detailed in this overview of estrogen and BDNF actions and interaction in hippocampus, steroid hormone-growth factor interactions are complex not only from the endocrinological point of view, but also from a neurobiological perspective.. Nevertheless, this is area of substantial potential importance for understanding normal CNS function and the neurological and psychiatric disorders that are known to be influenced by steroid hormones. For the example of estrogen and BDNF in hippocampus, there is evidence for an interrelationship that provides insight into fundamental mechanisms of hippocampal structure, function, and plasticity. Whether estrogen action can be explained by an ERE-mechanism and BDNF induction or other mechanisms, the precise role of “downstream” modulators such as NPY, and how these principles may be applicable to other parts of the CNS, are questions that can not be definitively answered at this time. Nonetheless, it is clear that studies of estrogen action, and the studies of BDNF in hippocampus, which are topics that have largely been examined in isolation in the past, would greatly benefit from ‘convergence.’
Acknowledgments
Supported by NS 37562, the New York State Department of Health, and the Helen Hayes Hospital Foundation. We thank Annmarie Curcio and Catherine Snodgrass for secretarial assistance.
References
- 1.Stancel GM, Gardner RM, Kirkland JL, Lin TH, Lingham RB, Loose-Mitchell DS, Mukku VR, Orengo CA, Verner G. Interactions between estrogen and EGF in uterine growth and function. Adv Exp Med Biol. 1987;230:99–118. doi: 10.1007/978-1-4684-1297-0_6. [DOI] [PubMed] [Google Scholar]
- 2.Ignar-Trowbridge DM, Pimental M, Teng CT, Korach KS, McLachlan JA. Cross talk between peptide growth factor and estrogen receptor signaling systems. Environ Health Perspect. 1995;103:35–38. doi: 10.1289/ehp.95103s735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendez P, Cardona-Gomez GP, Garcia-Segura LM. Interactions of insulin-like growth factor-I and estrogen in the brain. Adv Exp Med Biol. 2005;567:285–303. doi: 10.1007/0-387-26274-1_12. [DOI] [PubMed] [Google Scholar]
- 4.Toran-Allerand CD, Singh M, Setalo G. Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 5.Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 7.Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- 8.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 9.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 10.Iivonen S, Heikkinen T, Puolivali J, Helisalmi S, Hiltunen M, Soininen H, Tanila H. Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen alpha and beta mRNA levels in ovariectomized female mice. Neuroscience. 2006;137:1143–1152. doi: 10.1016/j.neuroscience.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- 13.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci USA. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpkins JW, Wang J, Wang X, Perez E, Prokai L, Dykens JA. Mitochondria play a central role in estrogen-induced neuroprotection. Curr Drug Targets CNS Neurol Disord. 2005;4:69–83. doi: 10.2174/1568007053005073. [DOI] [PubMed] [Google Scholar]
- 16.Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 17.Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- 18.Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 19.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 20.Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced n-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendrocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- 21.Adams MM, Fink SE, Shah RA, Janssen WB, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- 23.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves S. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 24.Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- 25.Weiland NG, Orikasa C, Hayashi S, McEwen BS. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–612. doi: 10.1002/(sici)1096-9861(19971201)388:4<603::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: A potential role for neuropeptide Y. Neuroscience. 2005;136:357–369. doi: 10.1016/j.neuroscience.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 27.Blurton-Jones M, Kuan PN, Tuszynski MH. Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J Comp Neurol. 2004;468:347–360. doi: 10.1002/cne.10989. [DOI] [PubMed] [Google Scholar]
- 28.Nitsch R, Soriano E, Frotscher M. The parvalbumin-containing nonpyramidal neurons in the rat hippocampus. Anat Embryol (Berl) 1990;181:413–425. doi: 10.1007/BF02433788. [DOI] [PubMed] [Google Scholar]
- 29.Deller T, Leranth C. Synaptic connections of neuropeptide Y (NPY) immunoreactive neurons in the hilar area of the rat hippocampus. J Comp Neurol. 1990;300:433–447. doi: 10.1002/cne.903000312. [DOI] [PubMed] [Google Scholar]
- 30.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 31.Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- 32.Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen Receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 33.Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 34.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24:47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- 36.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tongiorgi E, Armellin M, Giulianini PG, Zucchini S, Paradiso B, Steward O, Cattaneo A, Simonato M. Brain-derived neurotrophic factor mRNA and protein are targeted to discrete dendritic laminas by events that trigger epileptogenesis. J Neurosci. 2004;24:6842–6852. doi: 10.1523/JNEUROSCI.5471-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19:8009–8026. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dougherty KD, Milner TA. p75NTR immunoreactivity in the rat dentate gyrus is mostly within presynaptic profiles but is also found in some astrocytic and postsynaptic profiles. J Comp Neurol. 1999;407:77–91. doi: 10.1002/(sici)1096-9861(19990428)407:1<77::aid-cne6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 41.Murphy DD, Cole NB, Segal M. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc Natl Acad Sci USA. 1998;95:11412–11417. doi: 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin S, Jones M, Simpson E, Buuse MVd. Impaired spatial reference memory in aromatase-deficient (ArKO) mice. Neuroreport. 2003;14:1979–1982. doi: 10.1097/00001756-200310270-00020. [DOI] [PubMed] [Google Scholar]
- 43.Luine V. Steroid hormone modulation of hippocampal dependent spatial memory. Stress. 1997;2:21–36. doi: 10.3109/10253899709014735. [DOI] [PubMed] [Google Scholar]
- 44.Lacreuse A, Verreault M, Herndon JG. Fluctuations in spatial recognition memory across the menstrual cycle in female rhesus monkeys. Psychoneuroendocrinology. 2001;26:623–639. doi: 10.1016/s0306-4530(01)00017-8. [DOI] [PubMed] [Google Scholar]
- 45.Croll SD, Suri C, Compton DL, Simmons MV, Yancopoulos GD, Lindsay RM, Wiegand SJ, Rudge JS, Scharfman HE. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegler DR, Gallagher M. Spatial memory in middle-aged female rats: assessment of estrogen replacement after ovariectomy. Brain Res. 2005;1052:163–173. doi: 10.1016/j.brainres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 48.Bi R, Foy MR, Thompson RF, Baudry M. Effects of estrogen, age, and calpain on MAP kinase and NMDA receptors in female rat brain. Neurobiol Aging. 2003;24:977–983. doi: 10.1016/s0197-4580(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 49.Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamani MR, Levy WB, Desmond NL. Estradiol increases delayed, N-methyl-D-aspartate receptor-mediated excitation in the hippocampal CA1 region. Neuroscience. 2004;129:243–254. doi: 10.1016/j.neuroscience.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 51.Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. J Neurophysiol. 1999;81:1404–1411. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- 52.Sun JH, Yan XQ, Xiao H, Zhou JW, Chen YZ, Wang CA. Restoration of decreased N-methyl-d-asparate receptor activity by brain-derived neurotrophic factor in the cultured hippocampal neurons: involvement of cAMP. Arch Biochem Biophys. 2001;394:209–215. doi: 10.1006/abbi.2001.2547. [DOI] [PubMed] [Google Scholar]
- 53.Levine ES, Kolb JE. Brain-derived neurotrophic factor increases activity of NR2B-containing N-methyl-D-aspartate receptors in excised patches from hippocampal neurons. J Neurosci Res. 2000;62:357–362. doi: 10.1002/1097-4547(20001101)62:3<357::AID-JNR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crozier RA, Black IB, Plummer MR. Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learn Mem. 1999;6:257–266. [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar A, Foster TC. 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- 57.Kramar EA, Lin B, Lin CY, Arai AC, Gall CM, Lynch G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J Neurosci. 2004;24:5151–5161. doi: 10.1523/JNEUROSCI.0800-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carrer HF, Araque A, Buno W. Estradiol regulates the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. J Neurosci. 2003;23:6334–6344. doi: 10.1523/JNEUROSCI.23-15-06338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience. 2004;127:983–988. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 60.Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 61.Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- 62.Leranth C, Shanabrough M, Redmond DE. Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 63.Woolley CS, McEwen BS. Estadiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danzer SC, Feng G, MacNamara JO. BDNF expression in dentate granule cell giant mossy fiber boutons following status epilepticus, Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2003. Online. (2003) Program No. 477.475. [Google Scholar]
- 68.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 69.Hartmann M, Brigadski T, Erdmann KS, Holtmann B, Sendtner M, Narz F, Lessmann V. Truncated TrkB receptor-induced outgrowth of dendritic filopodia involves the p75 neurotrophin receptor. J Cell Sci. 2004;117:5803–5814. doi: 10.1242/jcs.01511. [DOI] [PubMed] [Google Scholar]
- 70.McLean-Bolton M, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol. 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 73.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ormerod BK, Lee TT, Galea LA. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 75.Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 76.Desmond NL, Levy WB. Ovarian steroidal control of connectivity in the female hippocampus: an overview of recent experimental findings and speculations on its functional consequences. Hippocampus. 1997;7:239–245. doi: 10.1002/(SICI)1098-1063(1997)7:2<239::AID-HIPO10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Rev. 2005;48:273–286. doi: 10.1016/j.brainresrev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 78.Rose CR, Blum R, Pichler B, Lepier A, Kafitz KW, Konnerth A. Truncated TrkB-T1 mediates neurotrophin-evoked calcium signalling in glia cells. Nature. 2003;426:74–78. doi: 10.1038/nature01983. [DOI] [PubMed] [Google Scholar]
- 79.Sohrabji F, Miranda RCG, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Fowler CD, Young LJ, Yan Q, Insel TR, Wang Z. Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles. J Comp Neurol. 2001;433:499–514. doi: 10.1002/cne.1156. [DOI] [PubMed] [Google Scholar]
- 82.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14:1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 83.Cavus I, Duman RS. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Bio Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- 84.Jezierski MK, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Brain Res Mol Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 85.Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendrocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gibbs RB. Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res. 1998;787:259–268. doi: 10.1016/s0006-8993(97)01511-4. [DOI] [PubMed] [Google Scholar]
- 88.Franklin TB, Perrot-Sinal TS. Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology. 2006;31:38–48. doi: 10.1016/j.psyneuen.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 89.Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- 90.Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 91.Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, Maclusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-C1-cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/C1- c-transporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 94.Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- 95.Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- 96.Gall CM. Regulation of brain neurotrophin expression by physiological activity. Trends Pharmacol Sci. 1992;13:401–403. doi: 10.1016/0165-6147(92)90123-n. [DOI] [PubMed] [Google Scholar]
- 97.Thompson KJ, Orfila JE, Achanta P, Martinez JL., Jr Gene expression associated with in vivo induction of early phase-long-term potentiation (LTP) in the hippocampal mossy fiber-Cornus Ammonis (CA)3 pathway. Cell Mol Biol (Noisy-le-grand) 2003;49:1281–1287. [PubMed] [Google Scholar]
- 98.Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 99.Otis TS, Staley KJ, Mody I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 1991;545:142–150. doi: 10.1016/0006-8993(91)91280-e. [DOI] [PubMed] [Google Scholar]
- 100.Mody I, Otis TS, Staley KJ, Kohr G. The balance between excitation and inhibition in dentate granule cells and its role in epilepsy. Epilepsy Res Suppl. 1992;9:331–339. [PubMed] [Google Scholar]
- 101.Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- 102.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 103.Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger AG, Gray WP. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- 104.Howell OW, Scharfman HE, Herzog H, Sundstrom LE, Beck-Sickinger AG, Gray WP. Neuropeptide Y is neuroproliferative for post-natal hippocampal precursor cells. J Neurochem. 2003;86:646–659. doi: 10.1046/j.1471-4159.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 105.Barnea A, Roberts J. Induction of functional and morphological expression of neuropeptide Y (NPY) in cortical cultures by brain-derived neurotrophic factor (BDNF): evidence for a requirement for extracellular-regulated kinase (ERK)-dependent and ERK-independent mechanisms. Brain Res. 2001;919:57–69. doi: 10.1016/s0006-8993(01)02999-7. [DOI] [PubMed] [Google Scholar]
- 106.Barnea A, Roberts J, Croll SD. Continuous exposure to brain-derived neurotrophic factor is required for persistent activation of TrkB receptor, the ERK signaling pathway, and the induction of neuropeptide Y production in cortical cultures. Brain Res. 2004;1020:106–117. doi: 10.1016/j.brainres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 107.Wirth MJ, Patz S, Wahle P. Transcellular induction of neuropeptide Y expression by NT4 and BDNF. Proc Natl Acad Sci USA. 2005;102:3064–3069. doi: 10.1073/pnas.0404712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marty S, Onteniente B. BDNF and NT-4 differentiate two pathways in the modulation of neuropeptide protein levels in postnatal hippocampal interneurons. Eur J Neurosci. 1999;11:1647–1656. doi: 10.1046/j.1460-9568.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 109.Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vezzani A, Schwarzer C, Lothman EW, Williamson J, Sperk G. Functional changes in somatostatin and neuropeptide Y containing neurons in the rat hippocampus in chronic models of limbic seizures. Epilepsy Res. 1996;26:267–279. doi: 10.1016/s0920-1211(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 111.Gray WP, Scharfman HE. NPY and neurogenesis. In: Zukowska Z, Feuerstein G, editors. NPY Family of Peptides in Inflammation, Immune Disorders, Angiogenesis, and Cancer. Birkhauser-Verlag; Switzerland: 2006. in press. [Google Scholar]
- 112.Paredes MF, Greenwood J, Baraban SC. Neuropeptide Y modulates a G protein-coupled inwardly rectifying potassium current in the mouse hippocampus. Neurosci Lett. 2003;340:9–12. doi: 10.1016/s0304-3940(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 113.Reibel S, Larmet Y, Carnahan J, Marescaux C, Depaulis A. Endogenous control of hippocampal epileptogenesis: a molecular cascade involving brain-derived neurotrophic factor and neuropeptide Y. Epilepsia. 2000;41(S6):S127–133. doi: 10.1111/j.1528-1157.2000.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 114.Reibel S, Vivien-Roels B, Le BT, Larmet Y, Carnahan J, Marescaux C, Depaulis A. Overexpression of neuropeptide Y induced by brain-derived neurotrophic factor in the rat hippocampus is long lasting. Eur J Neurosci. 2000;12:595–605. doi: 10.1046/j.1460-9568.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 115.Scharfman HE, Goodman JH, Sollas AL, Croll SD. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol. 2002:201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- 116.Poulsen FR, Jahnsen H, Blaabjerg M, Zimmer J. Pilocarpine-induced seizure-like activity with increased BDNF and neuropeptide Y expression in organotypic hippocampal slice cultures. Brain Res. 2002;950:103–118. doi: 10.1016/s0006-8993(02)03009-3. [DOI] [PubMed] [Google Scholar]
- 117.Croll SD, Wiegand SJ, Anderson KD, Lindsay RM, Nawa H. Regulation of neuropeptides in adult rat forebrain by the neurotrophins BDNF and NGF. Eur J Neurosci. 1994;6:1343–1353. doi: 10.1111/j.1460-9568.1994.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 118.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 119.Simpkins JW, Green PS, Gridley KE, Singh M, deFiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer’s disease. Am J Med. 1997;103:19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 120.Fahnestock M, Garzon D, Holsinger RM, Michalski B. Neurotrophic factors and Alzheimer’s disease: are we focusing on the wrong molecule? J Neural Transm Suppl. 2002;62:241–252. doi: 10.1007/978-3-7091-6139-5_22. [DOI] [PubMed] [Google Scholar]
- 121.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 122.Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. 2005;16 doi: 10.1007/s00702-005-0397-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 123.Lee J, Fukumoto H, Orne J, Klucken J, Raju S, Vanderburg CR, Irizarry MC, Hyman BT, Ingelsson M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 124.Cotman CW. The role of neurotrophins in brain aging: a perspective in honor of Regino Perez-Polo. Neurochem Res. 2005;30:877–881. doi: 10.1007/s11064-005-6960-y. [DOI] [PubMed] [Google Scholar]
- 125.Elliott E, Atlas R, Lange A, Ginzburg I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 Kinase signalling mechanism. Eur J Neurosci. 2005;22:1081–1089. doi: 10.1111/j.1460-9568.2005.04290.x. [DOI] [PubMed] [Google Scholar]
- 126.Matsushita S, Arai H, Matsui T, Yuzuriha T, Urakami K, Masaki T, Higuchi S. Brain-derived neurotrophic factor gene polymorphisms and Alzheimer’s disease. J Neural Transm. 2005;112:703–711. doi: 10.1007/s00702-004-0210-3. [DOI] [PubMed] [Google Scholar]
- 127.Bian JT, Zhang JW, Zhang ZX, Zhao HL. Association analysis of brain-derived neurotrophic factor (BDNF) gene 196 A/G polymorphism with Alzheimer’s disease (AD) in mainland Chinese. Neurosci Lett. 2005;387:11–16. doi: 10.1016/j.neulet.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 128.Bodner SM, Berrettini W, vanDeerlin V, Bennett DA, Wilson RS, Trojanowski JO, Arnold SE. Genetic variation in the brain derived neurotrophic factor gene in Alzheimer’s disease. Am J Med Genet B Neuropsyiatr Genet. 2005;134:1–5. doi: 10.1002/ajmg.b.30154. [DOI] [PubMed] [Google Scholar]
- 129.Nacmias B, Piccini C, Bagnoli S, Tedde A, Cellini E, Bracco L, Sorbi S. Brain-derived neurotrophic factor, apolipoprotein E genetic variants and cognitive performance in Alzheimer’s disease. Neurosci Lett. 2004;367:379–383. doi: 10.1016/j.neulet.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 130.Vepsalainen S, Castren E, Helisalmi S, Iivonen S, Mannermaa A, Lehtovirta M, Hanninen T, Soininen H, Hiltunen M. Genetic analysis of BDNF and TrkB gene polymorphisms in Alzheimer’s disease. J Neural. 2005;252:423–428. doi: 10.1007/s00415-005-0667-5. [DOI] [PubMed] [Google Scholar]
- 131.Rune GM, Wehrenberg U, Prange-Kiel J, Zhou L, Adelmann G, Frotscher M. Estrogen up-regulates estrogen receptor alpha and synaptophysin in slice cultures of rat hippocampus. Neuroscience. 2002;113:167–175. doi: 10.1016/s0306-4522(02)00152-5. [DOI] [PubMed] [Google Scholar]
- 132.Fumagali F, Racagni G, Riva MA. The expanding role of BDNF: a therapeutic target for Alzheimer’s disease? Pharmacogenomics J. 2006;6:8–15. doi: 10.1038/sj.tpj.6500337. [DOI] [PubMed] [Google Scholar]
- 133.Haapasalo A, Sipola I, Larsson K, Akerman KE, Stoilov P, Stamm S, Wong G, Castren E. Regulation of TRKB surface expression by brain-derived neurotrophic factor and truncated TRKB isoforms. J Biol Chem. 2002;277:43160–43167. doi: 10.1074/jbc.M205202200. [DOI] [PubMed] [Google Scholar]
- 134.Russo-Neustadt A, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des. 2005;11:1495–1510. doi: 10.2174/1381612053764788. [DOI] [PubMed] [Google Scholar]
- 135.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 136.Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ. Gender differences in depression: findings from the STAR*D study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 137.Chotai J, Smedh K, Johnasson C, Nilsson LG, Adolfsson R. An epidemiological study on gender differences in self-reported seasonal changes in mood and behaviour in a general population of northern Sweden. Nord J Psychiatry. 2004;58:429–437. doi: 10.1080/08039480410006052. [DOI] [PubMed] [Google Scholar]
- 138.Takkinen S, Gold C, Pedersen NL, Malmberg B, Nilsson S, Rovine M. Gender differences in depression: a study of older unlike-sex twins. Aging Ment Health. 2004;8:187–195. doi: 10.1080/13607860410001669714. [DOI] [PubMed] [Google Scholar]
- 139.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 140.Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Ribinow DR, Kleinman JE, Weickert CS. Alteration in estrogen receptor alpha mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol Psychiatry. 2005;58:812–824. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 141.Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 142.Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine. 2005;28:235–242. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- 143.Rybaczyk LA, Bashaw MJ, Pathak DR, Moody SM, Gilders RM, Holzschn DL. An overlooked connection: serotonergic mediation of estrogen-related physiology and pathology. BMD Womens Health. 2005;5:12. doi: 10.1186/1472-6874-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirements of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:85–89. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 145.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 146.Scharfman HE. Brain-derived neurotrophic factor and epilepsy-a missing link? Epilepsy Curr. 2005;5:83–88. doi: 10.1111/j.1535-7511.2005.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 148.Richichi C, Lin EJ, Stefanin D, Colella D, Ravizza T, Grignaschi G, Veglianese P, Sperk G, During MJ, Vezzani A. Anticonvulsant and antiepileptogenic effects mediated by adeno-associated virus vector neuropeptide Y expression in the rat hippocampus. J Neurosci. 2004;24:3051–3059. doi: 10.1523/JNEUROSCI.4056-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scharfman HE, Goodman JH, Sollas AL. Actions of brain-derived neurotrophic factor in slices from rats with spontaneous seizures and mossy fiber sprouting in the dentate gyrus. J Neurosci. 1999;19:5619–5631. doi: 10.1523/JNEUROSCI.19-13-05619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tu B, Timofeeva O, Jiao Y, Nadler JV. Spontaneous release of neuropeptide Y tonically inhibits recurrent mossy fiber synaptic transmission in epileptic brain. J Neurosci. 2005;25:1718–1729. doi: 10.1523/JNEUROSCI.4835-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim JS, Kim HY, Kim JH, Shin HK, Lee SH, Lee YS, Son H. Enhancement of rat hippocampal long-term potentiation by 17 beta-estradiol involves mitogen-activated protein kinase-dependent and -independent components. Neurosci Lett. 2002;332:65–69. doi: 10.1016/s0304-3940(02)00902-3. [DOI] [PubMed] [Google Scholar]
- 152.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- 154.Zheng WH, Quirion R. Comparative signaling pathways of insulin-like growth factor-1 and brain-derived neurotrophic factor in hippocampal neurons and the role of the PI3 kinase pathway in cell survival. J Neurochem. 2004;89:844–852. doi: 10.1111/j.1471-4159.2004.02350.x. [DOI] [PubMed] [Google Scholar]
- 155.Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell Mol Neurobiol. 2002;22:663–674. doi: 10.1023/a:1021848706159. [DOI] [PubMed] [Google Scholar]
- 156.Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang L, Rubinow DR, Xaing G, Li BS, Chang YH, Maric D, Barker JL, Ma W. Estrogen protects against beta-amyloid-induced neurotoxicity in rat hippocampal neurons by activation of Akt. Neuroreport. 2001;12:1919–1923. doi: 10.1097/00001756-200107030-00030. [DOI] [PubMed] [Google Scholar]
- 159.Sawai T, Bernier F, Fukushima T, Hashimoto T, Ogura H, Nishizawa Y. Estrogen induces a rapid increase of calcium-calmodulin-dependent protein kinase II activity in the hippocampus. Brain Res. 2002;950:308–311. doi: 10.1016/s0006-8993(02)03186-4. [DOI] [PubMed] [Google Scholar]
- 160.Blanquet PR, Mariani J, Derer P. A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cyclic AMP-responsive transcription factor in the rat hippocampus. Neuroscience. 2003;118:477–490. doi: 10.1016/s0306-4522(02)00963-6. [DOI] [PubMed] [Google Scholar]
- 161.Liu J, Fukunaga K, Yamamoto H, Nishi K, Miyamoto E. Differential roles of Ca(2+)/calmodulin-dependent protein kinase II and mitogen-activated protein kinase activation in hippocampal long-term potentiation. J Neurosci. 1999;19:8292–8299. doi: 10.1523/JNEUROSCI.19-19-08292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Panickar KS, Guan G, King MA, Rajakumar G, Simpkins JW. 17beta-estradiol attenuates CREB decline in the rat hippocampus following seizure. J Neurobiol. 1997;33:961–967. [PubMed] [Google Scholar]
- 163.Iida N, Namikawa K, Kiyama H, Ueno H, Nakamura S, Hattori S. Requirement of Ras for the activation of mitogen-activated protein kinase by calcium influx, cAMP, and neurotrophin in hippocampal neurons. J Neurosci. 2001;21:6459–6666. doi: 10.1523/JNEUROSCI.21-17-06459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 166.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cordoba Montoya DA, Carrer HF. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- 169.Good M, Day M, Muir JL. Cyclical changes in endogenous levels of oestrogen modulate the induction of LTD and LTP in the hippocampal CA1 region. Eur J Neurosci. 1999;11:4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 170.Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- 171.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 172.Kang H, Schuman EM. Long-lasting neurotrophin enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 173.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 174.Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 177.Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- 178.Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems. Behav Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- 181.El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J Cell Mol Med. 2004;8:537–544. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signalng in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mizuno M, Yamada K, Je J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem. 2003;10:108–115. doi: 10.1101/lm.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Koponen E, Voikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castren E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26:166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 187.Galea LA, Lee TT, Kostaras X, Sidhu JA, Barr AM. High levels of estradiol impair spatial performance in the Morris water maze and increase “depressive-like” behaviors in the female meadow vole. Physiol Behav. 2002;77:217–225. doi: 10.1016/s0031-9384(02)00849-1. [DOI] [PubMed] [Google Scholar]
- 188.Maclusky NJ, Luine VN, Hajsvan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 189.Woolley CS. Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–180. 181–187. doi: 10.1002/0470870818.ch13. [DOI] [PubMed] [Google Scholar]
- 190.Chakravarthy S, Saiepour MH, Bence M, Perry S, Hartman R, Couey JJ, Mansvelder HD, Levelt CN. Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus. Proc Natl Acad Sci U S A. 2006;103:1071–1076. doi: 10.1073/pnas.0506305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gustafsson E, Lindvall O, Kokaia Z. Intraventricular infusion of TrkB-Fc fusion protein promotes ischemia-induced neurogenesis in adult rat dentate gyrus. Stroke. 2003;34:2710–2715. doi: 10.1161/01.STR.0000096025.35225.36. [DOI] [PubMed] [Google Scholar]
- 194.Larsson E, Mandel RJ, Klein RL, Muzyczka N, Lindvall O, Kokaia Z. Suppression of insult-induced neurogenesis in adult rat brain by brain-derived neurotrophic factor. Exp Neurol. 2002;177:1–8. doi: 10.1006/exnr.2002.7992. [DOI] [PubMed] [Google Scholar]
- 195.Sudo S, Wen TC, Desaki J, Matsuda S, Tanaka J, Arai T, Maeda N, Sakanaka M. Beta-estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neurosci Res. 1997;29:345–354. doi: 10.1016/s0168-0102(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 196.Chen J, Adachi N, Liu K, Arai T. The effects of 17beta-estradiol on ischemia-induced neuronal damage in the gerbil hippocampus. Neuroscience. 1998;87:817–822. doi: 10.1016/s0306-4522(98)00198-5. [DOI] [PubMed] [Google Scholar]
- 197.Wang Q, Santizo R, Baughman VL, Pelligrino DA, Iadecola C. Estrogen provides neuroprotection in transient forebrain ischemia through perfusion-independent mechanisms in rats. Stroke. 1999;30:630–637. doi: 10.1161/01.str.30.3.630. [DOI] [PubMed] [Google Scholar]
- 198.Shughrue PJ, Merchenthaler I. Estrogen prevents the loss of CA1 hippocampal neurons in gerbils after ischemic injury. Neuroscience. 2003;116:851–861. doi: 10.1016/s0306-4522(02)00790-x. [DOI] [PubMed] [Google Scholar]
- 199.Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol. 2004;287:H1501–H1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- 200.Cimarosti H, Siqueira IR, Zamin LL, Nassif M, Balk R, Frozza R, Dalmaz C, Netto CA, Salbego C. Neuroprotection and protein damage prevention by estradiol replacement in rat hippocampal slices exposed to oxygen-glucose deprivation. Neurochem Res. 2005;30:583–589. doi: 10.1007/s11064-005-2693-1. [DOI] [PubMed] [Google Scholar]
- 201.He Z, He YJ, Day AL, Simpkins JW. Proestrus levels of estradiol during transient global cerebral ischemia improves the histological outcome of the hippocampal CA1 region: perfusion-dependent and-independent mechanisms. J Neurol Sci. 2002;193:79–87. doi: 10.1016/s0022-510x(01)00648-7. [DOI] [PubMed] [Google Scholar]
- 202.Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775–5781. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Beck T, Lindholm D, Castren E, Wree A. Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J Cereb Blood Flow Metab. 1994;14:689–692. doi: 10.1038/jcbfm.1994.86. [DOI] [PubMed] [Google Scholar]
- 204.Larsson E, Nanobashvili A, Kokaia Z, Lindvall O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1999;19:1220–1228. doi: 10.1097/00004647-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 205.Kiprianova I, Freiman TM, Desiderato S, Schwab S, Galmbacher R, Gillardon F, Spranger M. Brain-Derived neurotrophic factor prevents neuronal death and glial activation after global ischemia in the rat. J Neurosci Res. 1999:21–27. doi: 10.1002/(SICI)1097-4547(19990401)56:1<21::AID-JNR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 206.Pringle AK, Sundstrom LE, Wilde GJ, Williams LR, Iannotti F. Brain-derived neurotrophic factor, but not neurotrophin-3, prevents ischemia-induced neuronal cell death in organotypic rat hippocampal slice cultures. Neurosci Lett. 1996;211:203–206. doi: 10.1016/0304-3940(96)12745-2. [DOI] [PubMed] [Google Scholar]
- 207.Saarelainen T, Lukkarinen JA, Koponen S, Grohn OH, Jolkkonen J, Koponen E, Haapasalo A, Alhonen L, Wong G, Koistinaho J, Kauppinen RA, Castren E. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons shown increased susceptibilty to cortical injury after focal cerebral ischemia. Mol Cell Neurosci. 2000;16:87–96. doi: 10.1006/mcne.2000.0863. [DOI] [PubMed] [Google Scholar]
- 208.Nunez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm infant brain injury. Endocrinology. 2003;144:2350–2359. doi: 10.1210/en.2002-220840. [DOI] [PubMed] [Google Scholar]
- 209.Harukuni I, Hurn PD, Crain BJ. Deleterious effect of beta-estradiol in a rat model of transient forebrain ischemia. Brain Res. 2001;900:137–142. doi: 10.1016/s0006-8993(01)02278-8. [DOI] [PubMed] [Google Scholar]
- 210.Rudge JS, Mather PE, Pasnikowski EM, Cai N, Corcoran T, Acheson A, Anderson K, Lindsay RM, Wiegand SJ. Endogenous BDNF protein is increased in adult rat hippocampus after a kainic acid induced excitotoxic insult but exogenous BDNF is not neuroprotective. Exp Neurol. 1998;149:398–410. doi: 10.1006/exnr.1997.6737. [DOI] [PubMed] [Google Scholar]
- 211.Blaha GR, Raghupathi R, Saatman KE, McIntosh TK. Brain-derived neurotrophic factor administration after traumatic brain injury in the rat does not protect against behavioral or histological deficits. Neuroscience. 2000;99:483–493. doi: 10.1016/s0306-4522(00)00214-1. [DOI] [PubMed] [Google Scholar]
- 212.Lahteinen S, Pitkanen A, Koponen E, Saarelainen T, Castren E. Exacerbated status epilepticus and acute cell loss, but no changes in epileptogenesis, in mice with increased brain-derived neurotrophic factor signaling. Neuroscience. 2003;122:1081–1092. doi: 10.1016/j.neuroscience.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 213.Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58:815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 214.Budziszewska B, Leskiewicz M, Kubera M, Jaworska-Feil L, Kajita M, Lason W. Estrone, but not 17 beta-estradiol, attenuates kainate-induced seizures and toxicity in male mice. Exp Clin Endocrinol Diabetes. 2001;109:168–173. doi: 10.1055/s-2001-14841. [DOI] [PubMed] [Google Scholar]
- 215.Veiga S, Azcoitia I, Garcia-Segura LM. Extragonadal synthesis of estradiol is protective against kainic acid excitotoxic damage to the hippocampas. Neuroreport. 2005;16:1599–1603. doi: 10.1097/01.wnr.0000179081.39659.7d. [DOI] [PubMed] [Google Scholar]
- 216.Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41:S30–S35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 217.Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;182:124–134. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 218.Galanopoulou AS, Alm EM, Veliskova J. Estradiol reduces seizure-induced hippocampal injury in ovariectomized female but not in male rats. Neurosci Lett. 2003;342:201–205. doi: 10.1016/s0304-3940(03)00282-9. [DOI] [PubMed] [Google Scholar]
- 219.Papalexi E, Antoniou K, Kitraki E. Estrogens influence behavioral responses in a kainic acid model of neurotoxicity. Horm Behav. 2005;48:291–302. doi: 10.1016/j.yhbeh.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 220.Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 221.Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol. Neuroscience. 2004;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 222.Valente SG, Naffah-Mazzacoratti MG, Pereira M, Silva I, Santos NF, Baracat EC, Cavalheiro EA, Amado D. Castration in female rats modifies the development of the pilocarpine model of epilepsy. Epilepsy Res. 2002;49:181–188. doi: 10.1016/s0920-1211(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 223.Woolley CS. Estradiol facilitates kainic acid-induced, but not flurothyl-induced, behavioral seizure activity in adult female rats. Epilepsia. 2000;41:510–515. doi: 10.1111/j.1528-1157.2000.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 224.Scharfman HE, Goodman JH, Rigoulot MA, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp Neurol. 2005;196:73–86. doi: 10.1016/j.expneurol.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Saberi M, Jorjani M, Pourgholami MH. Effects of chronic estradiol benzoate treatment on amygdala kindled seizures in male rats. Epilepsy Res. 2001;46:45–51. doi: 10.1016/s0920-1211(01)00257-1. [DOI] [PubMed] [Google Scholar]
- 226.Saberi M, Pourgholami MH. Estradiol alters afterdischarge threshold and acquisition of amygdala kindled seizures in male rats. Neurosci Lett. 2003;340:41–44. doi: 10.1016/s0304-3940(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 227.Hom AC, Buterbaugh GG. Estrogen alters the acquisition of seizures kindled by repeated amygdala stimulation or pentylenetetrazol administration in ovariectomized female rats. Epilepsia. 1986;27:103–108. doi: 10.1111/j.1528-1157.1986.tb03510.x. [DOI] [PubMed] [Google Scholar]
- 228.Edwards HE, Burnham WM, Mendonca A, Bowlby DA, MacLusky NJ. Steroid hormones affect limbic afterdischarge thresholds and kindling rates in adult female rats. Brain Res. 1999;838:136–150. doi: 10.1016/s0006-8993(99)01619-4. [DOI] [PubMed] [Google Scholar]
- 229.Xu B, Michalski B, Racine RJ, Fahnestock M. The effects of brain-derived neurotrophic factor (BDNF) administration on kindling induction, Trk expression and seizure-related morphological changes. Neuroscience. 2004;126:521–531. doi: 10.1016/j.neuroscience.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 230.Xe XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 231.Binder DK, Routbort MJ, Ryan TE, Yancopoulos GD, McNamara JO. Selective inhibition of kindling development by intraventricular administration of TrkB receptor body. J Neurosci. 1999;19:1424–1436. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Buterbaugh GG, Hudson GM. Estradiol replacement to female rats facilitates dorsal hippocampal but not ventral hippocampal kindled seizure acquisition. Exp Neurol. 1991;111:55–64. doi: 10.1016/0014-4886(91)90050-m. [DOI] [PubMed] [Google Scholar]
- 233.Larmet Y, Reibel S, Carnahan J, Nawa H, Marescaux C, Depaulis A. Protective effects of brain-derived neurotrophic factor on the development of hippocampal kindling in the rat. Neuroreport. 1995;6:1937–1941. doi: 10.1097/00001756-199510020-00027. [DOI] [PubMed] [Google Scholar]
- 234.Osehobo P, Adams B, Sazgar M, Xu Y, Racine RJ, Fahnestock M. Brain-derived neurotrophic factor infusion delays amygdala and perforant path kindling without affecting paired-pulse measures of neuronal inhibition in adult rats. Neuroscience. 1999;92:1367–1375. doi: 10.1016/s0306-4522(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 235.Reibel S, Larmet Y, Le BT, Carnahan J, Marescaux C, Depaulis A. Brain-derived neurotrophic factor delays hippocampal kinding in the rat. Neuroscience. 2000;100:777–788. doi: 10.1016/s0306-4522(00)00351-1. [DOI] [PubMed] [Google Scholar]
- 236.He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 237.Binder DK, Scharfman HE. Growth Factors and Epilepsy. Nova Sciences. 2005 [Google Scholar]
- 238.Scharfman HE. Epilepsy as an example of neural plasticity. The Neuroscientist. 2002;8:154–173. doi: 10.1177/107385840200800211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Klapstein GJ, Colmers WF. On the presynaptic inhibition by neuropeptide Y in rat hippocampus in vitro. Hippocampus. 1993;3:103–111. doi: 10.1002/hipo.450030111. [DOI] [PubMed] [Google Scholar]
- 240.Guo H, Castro PA, Palmiter RD, Baraban SC. Y5 receptors mediate neuropeptide Y actions at excitatory synapses in area CA3 of the mouse hippocampus. J Neurophysiol. 2002;87:558–566. doi: 10.1152/jn.00532.2001. [DOI] [PubMed] [Google Scholar]


