Abstract
Purpose
This paper describes the methodology of an ongoing clinical trial of prone positioning in pediatric patients with acute lung injury (ALI). Nonrandomized studies suggest that prone positioning improves oxygenation in patients with ALI/ARDS without the risk of serious iatrogenic injury. It is not known if these improvements in oxygenation result in improvements in clinical outcomes. A clinical trial was needed to answer this question.
Materials and Methods
The pediatric prone study is a multi-center, randomized, non-crossover, controlled clinical trial. The trial is designed to test the hypothesis that at the end of 28 days, children with ALI treated with prone positioning will have more ventilator free days than children treated with supine positioning. Secondary endpoints include the time to recovery of lung injury, organ failure free days, functional outcome, adverse events, and mortality from all causes. Pediatric patients, 42 weeks post-conceptual age to 18 years of age, are enrolled within 48 hours of meeting ALI criteria. Patients randomized to the prone group are positioned prone within 4 hours of randomization and remain prone for 20 hours each day during the acute phase of their illness for a maximum of 7 days. Both groups are managed according to ventilator protocol, extubation readiness testing, and sedation protocols and hemodynamic, nutrition and skin care guidelines.
Conclusions
This paper describes the process, multidisciplinary input, and procedures used to support the design of the clinical trial, as well as the challenges faced by the clinical scientists during the conduct of the clinical trial.
Keywords: Clinical trial, Multisite study, Research methods, Adult Respiratory Distress Syndrome (ARDS), acute lung injury, prone, position
Acute respiratory distress syndrome (ARDS), a diffuse and overwhelming inflammatory reaction of the pulmonary parenchyma to a variety of catastrophic medical conditions, results in high morbidity, mortality and financial burden (1–4). ALI/ARDS is acute in onset and is characterized by arterial hypoxemia resistant to oxygen therapy and diffuse pulmonary infiltrates without evidence of left atrial hypertension (2). ARDS is at the severe end of the ALI spectrum. ALI is defined by a PaO2/FiO2 ratio (PF ratio) ≤ 300 mm Hg whereas ARDS is defined by PF ratio ≤ 200 mm Hg (regardless of positive end-expiratory pressure [PEEP] level).
Although lifesaving, the ventilation strategy that maintains adequate gas exchange in patients with ALI/ARDS may exacerbate lung inflammation and injury (2, 5–6). ALI/ARDS produces a diffuse lung injury that is mechanically heterogeneous at risk for further injury. Specifically, when supine, the reduced volume of the nondependent aerated lung is at risk for alveolar overdistention with conventional ventilator strategies while the interaction of cyclic ventilation and lung injury causes repetitive recruitment and de-recruitment with consequent biomechanical strain in dependent lung regions (7).
Morbidity and mortality from ARDS may be reduced using lung protective ventilation strategies. Prone positioning, as first suggested by Bryan in 1974 (8), is a relatively simple maneuver that improves oxygenation and lung mechanics in adults with severe impairments of gas exchange (9–11). Our preliminary study showed that pediatric patients improved their oxygenation without serious iatrogenic injury after early, repetitive and prolonged prone positioning (12). The improved oxygenation resulting from prone positioning allows a reduction in the intensity of ventilator support, e.g., reduced FiO2 and mean airway pressure which may decrease ventilator associated lung injury (VALI) and facilitate patient recovery. The International Consensus Conference in Intensive Care Medicine: Ventilator-associated Lung Injury in ARDS proposed that prone positioning may prevent VALI but recommended randomized trials to evaluate its short and long-term risk and benefit (6).
To date, two separate clinical trials of prone positioning in adult patients with ALI/ARDS have yielded disappointing results. Gattinoni et al. (13) conducted a multicenter randomized clinical trial in patients with ALI/ARDS comparing conventional treatment (in the supine position) to a strategy of placing patients in a prone position for six or more hours daily for 10 days. Although prone position improved oxygenation, the intervention did not improve patient survival.
Mancebo et al. (14) replicated the Gattinoni study with a mortality endpoint but instituted ventilation protocols in both groups and placed patients prone early in the course of their disease for 20 hours daily. This study closed following an interim analysis without answering their research question because of difficulties with patient accrual. The 25% relative reduction in mortality in the prone group was not sufficient to reach statistical significance.
Our study design addresses the major criticisms of these two papers. Specifically, the current study – the first randomized study of prone positioning in a pediatric population -institutes early entry criteria, a 20-hour protocol, enhanced control in both groups (positional and non-positional protocols) and the outcome of ventilator-free days rather than mortality. We suspect that pediatric patients, whose chest wall compliance is higher than in adults, are more likely to do well with prone positioning making this pediatric study important in the face of disappointing adult trials.
This paper describes the process, multidisciplinary input, procedures, and challenges addressed by a group of pediatric critical care clinical researchers during the conduct of a multisite clinical trial. The nuances of this clinical research study are presented to allow this study to be compared to existing and future pediatric ALI research and assist future pediatric critical care scientists in the conduct of clinical trails.
OVERVIEW
This study is a multicenter, randomized, non-crossover, controlled clinical trial comparing the effects of early, repeated, and prolonged prone positioning with supine positioning in the treatment of 180 children with ALI/ARDS. The trial, which enrolled its first patient in September 2001, is funded by the National Institute of Nursing Research.
ENDPOINTS
Primary outcome
The primary outcome for this study is ventilator free days. This outcome, commonly used by the ARDS network (15), is defined as the number of days from enrollment in the study (Day 1) to Day 28 during which a patient breathes without assistance if the period of unassisted breathing lasted at least 48 consecutive hours. Our definition of assisted breathing includes any positive pressure support > 5 cmH2O even if applied non-invasively (for example, BiPAP).
Ventilator-free days is a composite end point that reflects a difference in mortality and ventilator days among survivors. We recognize that interpretation of composite end-points is often challenging (16). Unfortunately the “cleaner” end-point of mortality is not practical because unacceptably large sample sizes would be required to have adequate statistical power to detect differences in mortality after supine vs. prone positioning. Although epidemiological data remain unpublished, mortality rates in pediatric patients with ARDS are presumed lower than those in adult patients (17). Using the duration of mechanical ventilation is also unacceptable because therapies that decrease mortality may increase the duration of mechanical ventilation. Practicality aside, ventilator free days to Day 28 is a clinically relevant end-point because increasing the number of days a patient is not supported on mechanical ventilation limits iatrogenesis: for example, ventilator associated pneumonia, hazards of immobility, and narcotic exposure.
Secondary outcomes
Secondary endpoints include the time to recovery of lung injury, organ failure free days, functional outcome, adverse events, and mortality from all causes. Time to recovery of lung injury is defined as the number of days from randomization to achieve an oxygenation criterion, i.e., an oxygenation index (OI = [mean airway pressure/ PF ratio] x 100) of 6 or lower for 24 consecutive hours through Day 28. Use of the OI provides an objective measure of the patient’s recovery from ALI/ARDS and capacity to wean from mechanical ventilation (18). Organ failure free days are defined as the number of days from Day 1 to Day 28 in which a patient is without clinically significant non-pulmonary organ dysfunction. Organ dysfunction parameters are derived from the PeLOD score for the neurological, cardiovascular, renal, hematological and hepatic systems (19) (see http://www.sfar.org/scores2/pelod2.html). Differences in cognitive impairment and overall functional health from PICU admission to hospital discharge (or Day 28), are evaluated using the Pediatric Cerebral Performance Category (PCPC) Score and Pediatric Overall Performance Category (POPC) scales (20).
SELECTION OF STUDY SUBJECTS AND THE CONSENT PROCESS
All intubated mechanically ventilated patients in each participating center’s pediatric ICU are screened for study eligibility each day. Patients are considered eligible to participate in the study when all inclusion criteria are confirmed (Table 1) and no exclusion criteria are present (Table 2). Exclusion criteria focus on patients who are likely to be too physiologically unstable to tolerate a change in position, patients who have hypoxemia without ALI/ARDS, and patients who have, or are likely to develop, conditions in which the duration of mechanical ventilation is unlikely to be altered by an improvement in lung function.
Table 1.
Inclusion Criteria
|
Table 2.
Exclusion Criteria
|
Twelve pediatric intensive care units received Institutional Review Board approval to participate in this clinical trial (Appendix 1). After the patient’s eligibility status is confirmed with the care team, parents or legal guardian(s) are asked to provide informed consent for their child’s participation in the study. Several sites have multilingual consents to accommodate diverse patient populations. Given that all patients are sedated on mechanical ventilation, none are able to provide assent.
TREATMENT GROUPS
ASSIGNMENT - Randomization
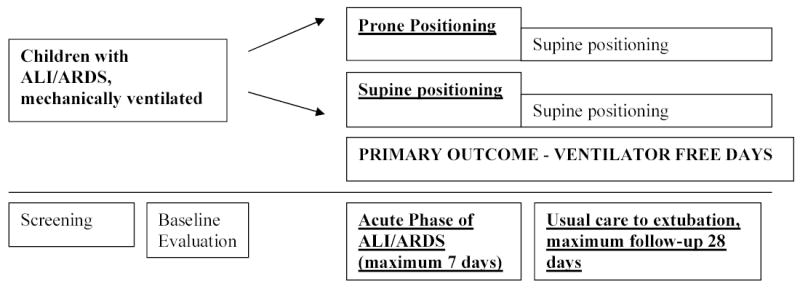
Randomization assignments (using permuted blocks of size 2 and 4, presented in random order) are stratified by center. Each hospital has received serially numbered, opaque, sealed envelopes containing study assignments. Eligible patients are randomized within 48 hours of meeting ALI criteria to either prone or supine positioning for the acute phase of their ALI/ARDS illness for a maximum of 7 days of treatment (Fig. 1).
Figure 1.
Study Schema
STUDY PROCEDURES
The acute phase of illness is defined as the time interval between meeting ALI criteria to meeting extubation readiness testing criteria; specifically, spontaneous breathing, OI < 6, and a decrease and/or plateau in ventilator support over the previous 12 hours (18). The seven day maximum limit was selected because it reflects the acute phase of ARDS illness where endothelial and epithelial cell inflammation and injury are prevalent (21). Also, during our Phase One study, only 10% of patients were positioned prone beyond seven days (12). After 7 days of treatment, all patients who are still intubated receive the standard of care (supine positioning). Other than prone or supine positioning, patients in both groups are managed according to ventilator protocol, extubation readiness testing, and sedation protocols and hemodynamic, nutrition and skin care guidelines during the 28 day period. These explicit decision-support tools are necessary to reduce clinician variation in managing similar clinical situations (22).
INTERVENTIONS
Positioning Protocol
Prone positioning is accomplished by rigid attention to detail that was developed during our Phase One study (12). Individually sized head, chest, pelvic, distal femoral, and lower limb cushions are created using pressure relieving material (covered Eggcrate; Span American Medical Systems, Greenville, SC; or Bendy® Bumper; Medical Ventures; Weymouth, MA). The cushions allow the patient’s abdomen to be supported above the patient’s bed surface and provide skin protection. The chest cushion should measure slightly less than the right-to-left greater tubercle of the upper arm; equal the patient’s anterior-posterior width; and wide enough to cover the patient’s sternum when compressed. The pelvic cushion should measure slightly smaller than the right-to-left iliac crest and be slightly smaller than the patient’s anterior-posterior width. The head pillow should allow the patient’s head to be slightly higher than the chest wedge. A small cushion should be placed under the distal femur to elevate the patient’s knees off the bed. The lower limb cushion should elevate the patient’s toes off the bed. When properly positioned the patient’s body should fold into the cushions, the abdomen should not be compressed and the shoulders should not be hyperextended.
Prone positioning results in cephalad movement of ETT within the trachea. To prevent inadvertent endotracheal tube (ETT) dislodgement, ETT position is checked before prone positioning. The tip of the ETT should be deeper than one third of the total length of the thoracic trachea to prevent it from moving into the cervical trachea when prone (23). To prevent oral pressure ulcers the ETT is secured to the upper lip rather than the corner of the mouth.
Before prone positioning the respiratory therapist checks for the presence of an ETT leak and, if present, inflates the cuff to minimize loss of lung volume. The nurse checks the security of all invasive lines and resecures them as necessary. If the patient is receiving a pharmacologic paralytic agent or if incomplete eyelid closure is present, eye protection is provided to prevent corneal abrasions. Specifically, the nurse cleanses, lubricates, and then covers both eyes with a moisture shield (24).
The site nurse coinvestigator or research assistant in collaboration with the bedside nurse/respiratory therapist team coordinates each turn. Depending on the size of the patient, each turn procedure (supine to prone; prone to supine) involves 2 to 4 individuals including the patient’s nurse and respiratory therapist. During the turn procedure, the respiratory therapist is assigned the primary responsibility of ETT protection. The repositioning technique is reviewed. Infants/toddlers are lifted up, turned 45 degrees, turned prone and then placed on their cushions after the turn. School aged and adolescent patients are turned using the mummy technique. The patient is positioned supine in the center of the bed. The following are layered over the patient: draw sheet or pads, chest and pelvic cushions then full sheet. The sheet is then tucked under the patient’s arms and the sheet under the patient is loosened from the bed frame and brought over the patient’s arms. The patient is then positioned on the side of the bed away from the ventilator, turned 45 degrees toward ventilator, then positioned prone on cushions and new linen.
During each turn the patient’s head is kept in alignment with the body, avoiding hyperextension; the patient’s arms are contained next to the torso; and the patient’s lower legs are crossed with the upper leg pointed in the direction of the turn.
Patients are turned toward the ventilator without disconnecting from the ventilator. If the patient requires ETT suctioning, turning is delayed until the patient is suctioned and has returned to their pre-suctioning ventilator settings. If deemed necessary by the care team, the FiO2 may be manipulated to maintain target SpO2 during repositioning. All other ventilator settings remain constant until the one hour post positioning study blood gas is obtained.
If the patient is supported on HFOV the care team anticipates the need to increase the oscillator’s power to maintain the same PaCO2 while in the prone position. Bronchial drainage may increase while prone so frequency of suctioning is reevaluated.
When prone, the patient’s head pillow is positioned to prevent extreme lateral head rotation. The patient’s upper arms remain at their side and their lower arms are flexed up. The lower limbs are cushioned so that the patient’s knees and toes are positioned off the bed.
Prone positioning includes a 20-hour cyclic rotation from full prone to right lateral/prone to full prone to left lateral/prone then to full prone. When tilted into a lateral prone position, the patient’s dependent lower arm is repositioned against their torso and their non-dependent lower arm is flexed at the elbow and positioned up towards the patient’s head. Ten hours into the prone position the patient’s upper chest and head are lifted so that their head can be turned in the opposite direction. If supported on HFOV, this process is facilitated by the use of flexible tubing.
Unless a change in management is anticipated, procedures that require anterior access to the patient are accomplished during the 4-hour supine period. During the patient’s 20-hour prone period, the care team can reposition a patient supine for an assessment or clinical procedure. The patient is returned prone, to complete their rotation, after the evaluation or procedure is completed.
After Day 7, if withdrawal of prone positioning results in a significant increase in ventilator support (e.g., FiO2 >0.6 persisting for 2 hours or more) the care team can elect to continue prone positioning until supine positioning is tolerated.
Non-Positional Protocols
Drafts of the non-positional protocols and guidelines were developed given the best evidence available in January 2000. These decision-making tools were finalized during the startup investigator meeting using a consensus process involving pediatric critical care nurses, physicians and respiratory therapists. Whereas guidelines provide general principles in management, protocols are detailed and generate patient-specific instructions that can be carried out by different clinicians with little random variation (22).
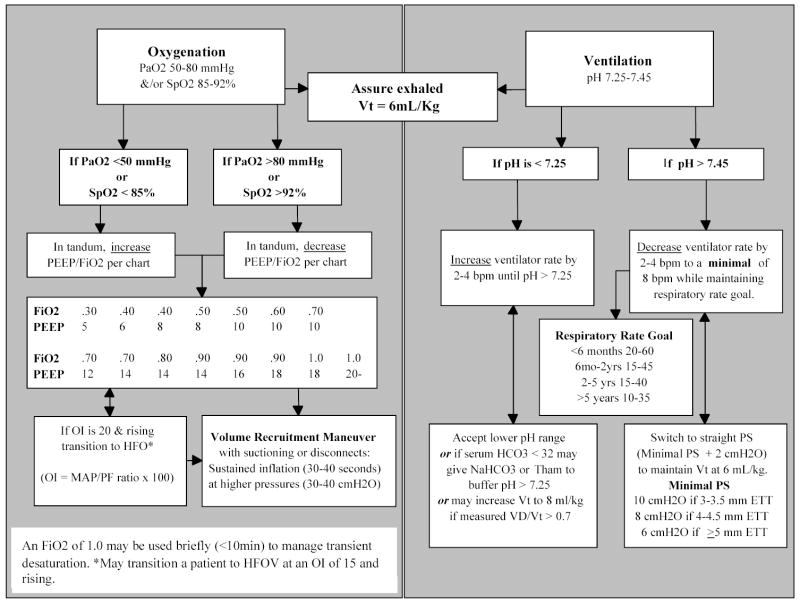
The ventilator protocol reflects the International Consensus Conference in Intensive Care Medicine: Ventilator Associated Lung Injury in ARDS (6) recommendations using a low tidal volume/open lung strategy (15, 25–28) and permissive hypercapnea (29–30) (Figure 2). The goal is adequate oxygenation and ventilation. The protocol recommends a PaO2 range of 50–80 mmHg or a SpO2 range of 85–92% and does not specify a PaCO2 range as long as the pH remains between 7.25 and 7.45. Other than transient maneuvers in response to patient hypotension and/or desaturation, the FiO2 is adjusted according to the ARDSNet PEEP/FiO2 grid (15). One change was made to this grid; an incremental PEEP step of 6 cmH20 was inserted on weaning from 8 cmH20 to 5 cmH20. After pilot testing, clinicians felt the 3 cmH20 PEEP drop to be excessive in pediatric patients. Exhaled tidal volume (Vt), as measured at the airway using a CO2SMO+ monitor (Novametrix Medical Systems Inc; Wallingford, CT, USA), is limited to 6 mL/kg of ideal body weight. The minimum Vt is 4 ml/kg and the maximum Vt is 6 ml/kg unless measured dead space per CO2SMO+ is > 0.7. The head of the bed is elevated 30 degrees in supine patients and placed in a 30 degree reverse trendelenburg position in prone patients to reduce the risk of nosocomial pneumonia (31).
Figure 2.
Ventilator Management
Ideal body weight (IBW) was determined for gender and recumbent length to 3 years of age using the National Center for Health Statistics growth charts. Predicted weight charts for gender/stature beyond 3 years of age do not exist so the predicted body weight table was generated by identifying the 50th percentile weight associated with age then linking that data to the 50th percentile height (32).
Although sites can elect to transition a patient to high frequency oscillatory ventilation (HFOV) when a patient’s OI is 15 and rising, the use of HFOV is mandated with an OI of 20. All centers present during the consensus meeting used HFOV as a ventilation strategy in managing patients with ALI/ARDS. Mandating the use of HFOV at a consistent hypoxic threshold provided the requisite control necessary in the clinical trial. When supported on HFOV the patient’s mean airway pressure is adjusted to maintain optimal lung volume allowing a reduction in FiO2 ≤ 0.6 without clinical or radiological evidence of hyperinflation: no signs of decreased cardiac output and/or the level of the diaphragm visible greater than the ninth rib (28).
No attempt is made to standardize suctioning practices across the study sites. Care is taken to re-recruit lung volume after all ventilator disconnects and after endotracheal suctioning. This is accomplished by volume recruitment maneuvers using sustained inflation (e.g., 30–40 seconds) at higher pressures (e.g., 30–40 cm H20) or, if supported on HFOV, temporary 10% increases in mean airway pressure.
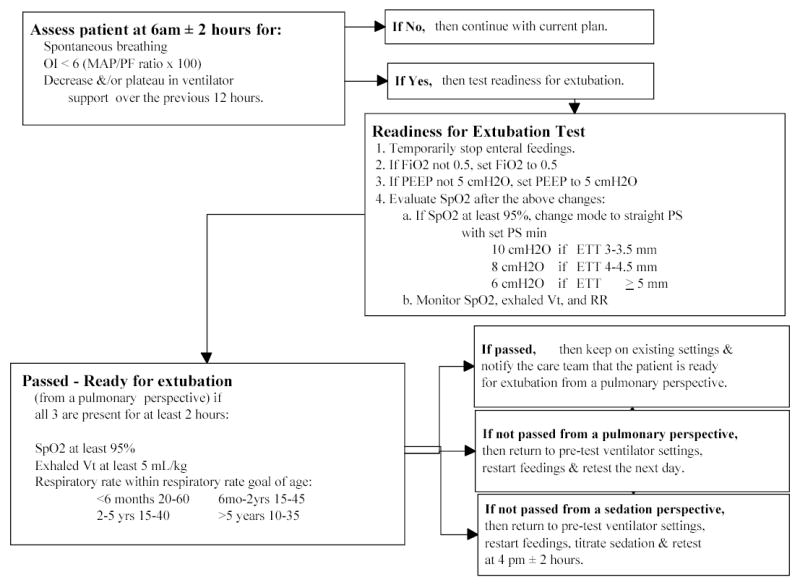
Once the patient demonstrates: (a) spontaneous breathing; (b) OI < 6; and (c) a decrease and/or plateau in ventilator support over the previous 12 hours (18), the acute phase of ALI ends (prone positioning stops) and the patient is tested for extubation readiness (33) (Figure 3). Patients are ready for extubation if they maintain SpO2, Vt, and respiratory rate goals. The patient’s care team is advised of the test results. The action of the care team is noted as extubation may be delayed for non-pulmonary issues. If the patient fails the extubation readiness test because of excessive sedation then sedation is weaned and the patient is retested at 16:00.
Figure 3.
Extubation Readiness Test
Sedation in both groups is managed by the nurse-implemented sedation protocol (34). Essential elements include sedation scoring every four hours using the pediatric Modified Motor Activity Assessment Scale (MMAAS (35–36), pain scoring every fours hours using an age-appropriate pain instrument (FLACC in patients 2 weeks to 7 years of age (37); Individualized Numeric Rating Scale in nonverbal or cognitively impaired patients (INRS; 38)), and the use of benzodiazepine and opioid continuous infusions. The algorithm recommends a level of sedation that matches the patient’s trajectory of illness. In the acute phase, decreases in sedation are not recommended. In the plateau phase, a daily “wake-up” test is performed after which the sedatives are restarted at half of the pre-wake up dose (39) then titrated every eight hours to maintain the prescribed level of comfort. In the ventilator weaning to extubation phase, patients who received opioid infusions for more than 5 days are first weaned from their opioid infusions over 48 hours. The benzodiazepine infusion is converted to intermittent intravenous or enteral dosing. Once the opioid infusion is discontinued, the benzodiazepine is weaned over 10 days. Opioids and benzodiazepines are discontinued without taper in patients receiving these agents for less than 5 days.
The Hemodynamic Management Guideline identifies a fluid conservative strategy with a goal of adequate cardiac output to meet the metabolic needs of the patient (40). The Nutrition Guideline identifies the goal of adequate calories to sustain the metabolic needs of the patient (41). Unless contraindicated (post-operative ileus, intestinal ischemia, and intestinal obstruction), all patients are advanced to full transplyoric enteral nutrition (42). The Skin Care and Pressure Ulcer Guideline identifies the goal of skin integrity. A daily skin assessment is performed and recorded on each patient during the acute treatment phase. Universally accepted prevention strategies are used to prevent pressure ulcers (43–45). Pressure ulcers are staged and managed according to National Pressure Ulcer Advisory Panel recommendations (46–47). Patients developing a new Stage III+ pressure ulcer exit the study.
STUDY VARIABLES
The data set will provide a rich repository of information describing the patient population and trajectory of illness of pediatric patients with ALI/ARDS. At enrollment, patient demographics, baseline assessment, past medical history, ALI/ARDS trigger, and admission functional health and PRISM III are recorded.
During the acute phase up to Day 7, ventilator settings, i-Stat®arterial blood gases, CO2SMO+ exhaled data, MMAAS and pain scores, total sedative dose, neuromuscular blockade and alkalizing agent use, and chest XRay data are collected. Daily measures are obtained when both groups are in the supine position. Patients randomized to prone positioning also have their physiologic values and arterial blood gases assessed after each supine-to-prone turn and before and after each prone-to-supine turn to describe the patient’s response to prone repositioning. To avoid bias, study blood gases are not available to the care team for clinical decision-making. Clinicians are encouraged to obtain data elements according to their clinical judgment.
In addition, daily (to Day 28) vital signs, pulmonary status and respiratory support, extubation readiness, fluid and nutritional balance, organ functions are documented. Data elements are collected only if available and reflect blood studies and values obtained closest to 08:00.
On Day 28, patient status is verified and data are summarized. Specifics include the reason for ending the acute treatment phase, crucial study dates, functional status at discharge, and discharge location. If discharged, parents are directly contacted to verify the patient’s continued recovery. In the event of patient death, the primary and secondary causes of death as specified on the patient’s death certificate are recorded. Protocol deviations and adverse events are monitored throughout the study period and reported to the Data Safety Monitoring Board.
SAMPLE SIZE
The sample size estimates for this study are based conservatively on the anticipated proportions of patients alive and ventilator free at the end of 28 days. We assumed that this will occur in approximately 65% of patients in the supine group and 90% of patients in the prone group, based on our prior studies (12, 18). We also assumed that about 10% of patients may be erroneously positioned or their provider may decide to discontinue a patient’s assigned treatment regimen. Using these assumptions, we calculated a sample size of 90 patients per group. This sample size provides greater than 90% power to detect a probability of 0.65 that the number of ventilator free days for a patient in the experimental group is higher than that of a patient in the control group, using a Wilcoxon rank-sum (Mann-Whitney U) test.
DATA SAFETY MONITORING
The Principal Investigator appointed a four member multidisciplinary independent Data Safety Monitoring Board (DSMB). The DSMB is responsible for monitoring patient safety, protocol adherence, data quality and patient accrual rates. The DSMB receives quarterly reports and participates in twice yearly conference calls. They will also conduct an interim analysis after 50% of patients have completed the study. Group sequential monitoring will be used to stop the study if large treatment differences appear before the end of the study and the method of stochastic curtailing will be used to stop the study early if there is little chance of finding a significant difference between groups. The effect of an O’Brien Fleming rule on the sample size for the study was examined. Assuming a single interim data analysis when half of the patients have completed the study, the null hypothesis is rejected if P < 0.006 (|Z| > 2.74); the null hypothesis is accepted if P > 0.52 (−0.70 < Z < 0.70).
QUALITY CONTROL PROCEDURES
Before enrolling patients, all nurse coinvestigators attended a project initiation meeting and completed a competency-based training program. Study nurses at each site were required to review the Case Report Forms (CRF) and Manual of Operations (MOO), view two videotapes demonstrating prone positioning, and complete and pass a scenario-based post-test. Continued quality is assessed during site visits conducted by a mastered-prepared pediatric critical care nurse auditor. The review includes examination of all study procedures, randomization check, review of completed CRFs and procedures to resolve queries. Research Assistants are observed conducting randomly selected protocol duties. Site visit checklists are used and reports are generated after each visit. All sites enact the quality improvement plan if they do not enroll a patient in 12 weeks. When enacted the plan must be completed in one month and a report of completion sent to the coordinating center. Sites not enrolling a patient in one year are placed on 3 months probation. Sites on probation must enroll a patient or withdraw from the study group.
STUDY DESIGN CHALLENGES
Several unique methodological challenges have presented themselves during the conduct of this clinical trial. These challenges can be considered within three major categories: patient enrollment, controlling bias, and interdisciplinary coordination.
From the onset we predicted a less than 3% incidence of ALI in all intubated mechanically ventilated PICU patients (1, 3). Given this low frequency and the requirement to identify all eligible patients as soon as possible after meeting ALI criteria, we instituted a protocol of daily screening and weekly faxing of screening logs to the coordination center. The coordinating center monitors and projects enrollment numbers (based upon a clinical site’s established enrollment history) that are reviewed quarterly by the Steering and Operations Committees. Given the rapid evolution of critical care therapies, it is critical that data collection be completed in the shortest period of time. Based upon our enrollment projections, an additional eight clinical sites joined and four clinical sites resigned from our study group. The latter did not have a sufficient patient population necessary to maintain competency in study procedures.
Approximately half of all eligible patients are enrolled. The primary reason for this eligibility-enrollment gap is parent refusal. Our parental refusal rate averages 36% to date, but increased from 27% to 53% after implementation of the HIPAA regulations. To address the volume of parent refusals, the Steering and Operations Committee reviewed the nurse-physician team consent process enacted when a parent is emotionally capable of giving informed consent.
The enrollment criteria for our study eventually overlapped with several simultaneous pediatric critical care clinical trials. Sharing schemes were agreed upon by the principal investigators and proposed to the clinical sites to ensure consistent decision making across all sites. We have learned that these processes need constant monitoring to ensure that the agreements are successfully implemented.
Sustaining clinician enthusiasm over a long clinical trial is challenging. Quarterly Steering and Operations Committee conference calls help to bring investigators together to review study progress and collectively problem solve issues and address competing demands.
A priori, we recognized that since the intervention cannot be blinded, we needed objective, independently verifiable outcomes to reduce the chance of reaching a biased conclusion. Although we anticipated many potential sources of bias, one area of concern was bias towards under-reporting of adverse events in the supine group. We address this potential source of bias by intense monitoring of all events by the principal investigator, project manager, study monitor and DSMB.
Finally, mirroring clinical practice, this study has required the unique collaboration of nurses, physicians and respiratory therapists. Many uncompensated hours have been volunteered by clinicians seeking an answer, regardless of outcome, to the question – “Is prone positioning worth doing?” While multidisciplinary clinical research is a priority for the National Institute of Health (http://nihroadmap.nih.gov/overview.asp), this study provided many with their first opportunity to participate in the process. The most difficult aspect, without doubt, was navigating systems designed to support non-clinical research.
In conclusion, this paper presents the design and implementation of a multisite clinical trial in pediatric critical care. The study is presented in detail to allow a comprehensive comparison with other ALI/ARDS clinical studies and to assist future pediatric critical care scientists in the conduct of clinical trails.
Appendix 1: Participating Centers
Children’s Hospital Boston: John H. Arnold MD; John E. Thompson RRT; Michelle LaBrecque RN, MSN. Children’s Memorial Hospital, Chicago: Lauren Sorce RN, MSN; David Steinhorn MD. Primary Children’s Medical Center: Mary Jo Chellis Grant RN, PNP, PhD; Chris Maloney MD. University of California, San Francisco: Jorge Gutierrez MD; Lori D. Fineman RN, MS; Michael A. Matthay MD. Vanderbilt Children’s Hospital: Rick Barr MD; Jade Forlidus, RN, MS; Children’s Medical Center of Dallas: Peter M. Luckett, MD; Shirley Molitor-Kirsch RN, MSN. Children’s Hospital Oakland: Natalie Cvijanovich MD; Lori Wertz RN, MSN. Duke Children’s Hospital: Ira Cheifetz MD; Donna Hamel RRT. University of Virginia Children’s Medical Center: Douglas F. Willson MD; Mary Hostetter, RN, CCRN; Tina Knicely, RN, CCRN. Children’s Hospital St. Louis: Barry P. Markovitz, MD; Rebecca Snider, RN. Penn State Children’s Hospital: Neal J. Thomas, M.D; Lorri Phipps R.N., CPNP. All Children’s Hospital: Bonnie Rice RN, MSN, CCNS; Michelle Smith, M.D.
Footnotes
Address for Reprints: As above; reprints will be ordered.
Financial Support: NIH/NINR RO1NR05336
References
- 1.Royall J, Levin DL. Adult respiratory distress syndrome in pediatric patients. II. Management. J Pediatr. 1988;112:335–347. doi: 10.1016/s0022-3476(88)80310-x. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.Timmons OD, Havens PL, Fackler JC. Predicting death in pediatric patients with acute respiratory failure. Pediatric Critical Care Study Group. Extracorporeal Life Support Organization. Chest. 1995;108:789–797. doi: 10.1378/chest.108.3.789. [DOI] [PubMed] [Google Scholar]
- 4.Artigas A, Bernard GR, Carlet J, et al. The American-European Consensus Conference on ARDS, part 2: Ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1332–1347. doi: 10.1164/ajrccm.157.4.ats2-98. [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS. Mechanical ventilation. American College of Chest Physicians’ Consensus Conference. Chest. 1993;104:1833–1859. doi: 10.1378/chest.104.6.1833. [DOI] [PubMed] [Google Scholar]
- 6.International Consensus Conferences in Intensive Care Medicine. Ventilator-associated Lung Injury in ARDS. Am J Respir Crit Care Med. 1999;160:2118–2124. doi: 10.1164/ajrccm.160.6.ats16060. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Pelosi P, Vitale G, et al. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology. 1991;74:15–23. doi: 10.1097/00000542-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Bryan AC. Comments of a devil’s advocate. American Review of Respiratory Disease. 1974;110:143–144. doi: 10.1164/arrd.1974.110.6P2.143. [DOI] [PubMed] [Google Scholar]
- 9.Fridrich P, Krafft P, Hochleuthner H, et al. The effects of long-term prone positioning in patients with trauma- induced adult respiratory distress syndrome. Anesth Analg. 1996;83:1206–1211. doi: 10.1097/00000539-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Chatte G, Sab JM, Dubois JM, et al. Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med. 1997;155:473–478. doi: 10.1164/ajrccm.155.2.9032181. [DOI] [PubMed] [Google Scholar]
- 11.Pelosi P, Tubiolo D, Mascheroni D, et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157:387–393. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 12.Curley MAQ, Thompson JE, Arnold JH. The Effects of Early and Repeated Prone Positioning in Pediatric Patients with Acute Lung Injury. Chest. 2000;118:156–163. doi: 10.1378/chest.118.1.156. [DOI] [PubMed] [Google Scholar]
- 13.Gattinoni L, Tognoni G, Pesenti A, et al. for the Prone-Supine Study Group. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 14.Mancebo J, Rialp G, Fernandez R, et al. Prone vs. supine position in ARDS. Results of a randomized multicenter trial. Am J Respir Crit Care Med. 2003;167:A180. [Google Scholar]
- 15.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 16.Lauer MS, Topol EJ. Clinical trials--multiple treatments, multiple end points, and multiple lessons. JAMA. 2003;289:2575–2577. doi: 10.1001/jama.289.19.2575. [DOI] [PubMed] [Google Scholar]
- 17.Randolph AG, Meert KL, O’Neil ME, et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334–1340. doi: 10.1164/rccm.200210-1175OC. [DOI] [PubMed] [Google Scholar]
- 18.Curley MAQ, Fackler JC. Weaning from Mechanical Ventilation: Patterns in Pediatric Patients Recovering from Acute Hypoxemic Respiratory Failure. Am J Criti Care. 1998;7:335–345. [PubMed] [Google Scholar]
- 19.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;36:192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 20.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 21.Ware LB, Matthay MA. The acute respiratory distress syndrome. New England J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 22.Morris AH. Treatment algorithms and protocolized care. Current Opinion in Critical Care. 2003;9(3):236–340. doi: 10.1097/00075198-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Marcano BV, Silver P, Sagy M. Cephalad movement of endotracheal tubes caused by prone positioning pediatric patients with acute respiratory distress syndrome. Ped Crit Care Med. 2003;4:186–189. doi: 10.1097/01.PCC.0000059733.05933.04. [DOI] [PubMed] [Google Scholar]
- 24.Cortese D, Capp L, McKinley S. Moisture chamber versus lubrication for the prevention of corneal epithelial breakdown. Am J Crit Care. 1995;4:425–428. [PubMed] [Google Scholar]
- 25.Amato MB, Barbas CS, Medeiros DM, et al. Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1835–1846. doi: 10.1164/ajrccm.152.6.8520744. [DOI] [PubMed] [Google Scholar]
- 26.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 27.Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med. 1998;338:355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 28.Arnold JH, Hanson JH, Toro-Figuero LO, et al. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med. 1994;22:1530–1539. [PubMed] [Google Scholar]
- 29.Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16:372–377. doi: 10.1007/BF01735174. [DOI] [PubMed] [Google Scholar]
- 30.Hickling KG, Walsh J, Henderson S, et al. Low mortality rate in adult respiratory distress syndrome using low- volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22:1568–1578. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- 31.Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomized trial. Lancet. 1999;354:1851–1858. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health and Human Services. Center for Disease Control and Prevention. National Center for Heath Statistics. National Health and Nutrition Examination Survey. CDC Growth Charts: United States, Individual Growth Charts. Revised and corrected 6/8/00. http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/charts.htm.
- 33.Randolph AG, Wypij D, Venkataraman ST, et al. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288:2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 34.Curley MAQ, Dodson BL, Arnold JH. Designing a Nurse-Implemented Sedation Algorithm for Use in a Pediatric Intensive Care Unit – A Preliminary Report. Ped Crit Care Med. 2003;4(3 supp):A158. [Google Scholar]
- 35.Devlin JW, Boleski G, Mlynarek M, et al. Motor activity assessment scale: A valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999;27:1271–1275. doi: 10.1097/00003246-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Oakes L. Caring practices: Providing comfort. In: Curley MAQ, Moloney-Harmon PA, editors. Critical Care Nursing of Infants and Children. Second Edition. Philadelphia PA: WB Saunders Co; 2001. p. 558. [Google Scholar]
- 37.Merkel SI, Voepel-Lewis T. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatric Nursing. 1997;23(3):293–297. [PubMed] [Google Scholar]
- 38.Solodiuk J, Curley MAQ. Evidenced Based Practice: Pain Assessment in Nonverbal Children with Severe Cognitive Impairments – The Individualized Numeric Rating Scale (INRS) J Peds Nsg. 2003;18:295–299. doi: 10.1016/s0882-5963(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 39.Kress JP, Pohlman A, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 40.Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 10: pediatric advanced life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102(8 Suppl):I291–342. [PubMed] [Google Scholar]
- 41.Curley MA, Castillo L. Nutrition and shock in pediatric patients. New Horiz. 1998;6:212–225. [PubMed] [Google Scholar]
- 42.Harrison AM, Clay B, Grant MJ, et al. Nonradiographic assessment of enteral feeding tube position. Crit Care Med. 1997;25:2055–2059. doi: 10.1097/00003246-199712000-00026. [DOI] [PubMed] [Google Scholar]
- 43.Bergstrom N, Allman RM, Alvarez OM, et al. Treatment of Pressure Ulcers. Clinical Practice Guideline. No. 15. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; AHCPR Pub. No. 95–0652. 1994. [Google Scholar]
- 44.Quigley SM, Curley MAQ. Skin integrity in the pediatric population: preventing and managing pressure ulcers. J Soc Pediatr Nurs. 1996;1:7–18. doi: 10.1111/j.1744-6155.1996.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 45.Allman RM, Goode PS, Burst N, et al. Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Advances in Wound Care. 1999;12:22–30. [PubMed] [Google Scholar]
- 46.National Pressure Ulcer Advisory Panel. Pressure ulcer prevalence, cost and risk assessment: Consensus development conference statement. Decubitus. 1989;1989;2:24–28. [PubMed] [Google Scholar]
- 47.National Pressure Ulcer Advisory Panel. Task Force on Darkly Pigmented Skin and Stage I Pressure Ulcers. Stage I Assessment in Darkly Pigmented Skin. 1998 Retrieved February 8, 2004 from the National Pressure Ulcer Advisory Panel Web site: http://www.npuap.org/positn4.htm.